Introduction
Prostate cancer is the most commonly diagnosed
cancer in men and the second leading cause of cancer death during
the past 5 years according to the cancer statistics (1) reported by the American Cancer Society,
this trend can be traced back even 10 years. For patients with
localized prostate cancer, it is treatable and the 5-year survival
rates could be even 100%. Yet, patients usually die of cancer
metastasis or drug resistance with the 5-year survival rates
lowered to 30–40%. It is urgent to explore the mechanisms involved
in cancer metastasis and drug resistance and develop new strategies
to improve the treatment outcome.
Hypoxia is an environmental stimulus that plays an
important role in cancer development and progression especially in
solid tumors which outgrow local blood supply during the
progression to advanced stages. Hypoxic conditions are widely
present in many human malignancies including breast, prostate,
lung, pancreas, rectum and renal cell cancer (2). The key regulator under hypoxic
conditions is stabilized hypoxia-inducible factor (HIF)-1α which
dimerizes with constitutively expressed HIF-1β and then
translocates into the nucleus where they bind to a specific
sequence, the hypoxia-responsive element (HRE), usually present in
the promoter of several hypoxia-dependent target genes (3).
Epithelial to mesenchymal transition (EMT) was first
used to depict embryonic development, which is characterized by
adherent epithelial cells converting to motile mesenchymal cells.
EMT is now been classified into 3 different subtypes that is EMT
during implantation, embryogenesis and organ development, EMT
associated with tissue regeneration and organ fibrosis, EMT
associated with cancer progression and metastasis (4). It has been reported that EMT existed
in many kinds of human cancers such as pancreatic, breast and colon
cancer (5) and hypoxia alone can
trigger an EMT process with increased invasiveness (5). At the molecular level, EMT is
accompanied by loss of epithelial cell markers, such as cell
adhesion protein E-cadherin, and acquisition of mesenchymal
markers, such as vimentin and N-cadherin. With more and more
research concerning on the role of EMT in cancer progression and
metastasis, a family of transcriptional factors including Snail,
Slug, Twist, Zeb and E47 have emerged as EMT master genes since
they can directly downregulate E-cadherin expression which is a
hallmark of EMT.
As mentioned above, hypoxia alone can trigger EMT in
many solid tumors including hepatoblastoma, pancreatic, colon and
breast cancer (5), yet, the
mechanisms involved in prostate cancer under hypoxic condition
remain unclear. In the present study, we demonstrated that hypoxia
might induce diverse molecular, phenotypic, and functional changes
in prostate cancer cells that are consistent with EMT. We also
showed that cell signaling factors such as HIF-1α, which is thought
to be stabilized under hypoxic environment is involved in EMT and
cancer cell invasive potency. Hypoxia-induced EMT in prostate
cancer cells can be blocked by HIF-1α gene silencing and
reoxygenation after hypoxia partially reverses EMT which is
accompanied by a process named mesenchymal-epithelial reverting
transition (MErT). We conclude that EMT could be induced by a
mechanism that might involve the activation of HIF-1α-dependent
cell signaling in hypoxic prostate cancer cells.
Materials and methods
Cell culture under normal and hypoxic
conditions
Human prostate cancer cell lines PC3 and DU145 were
purchased from the National Platform of Experimental Cell Resources
for Sci-Tech (Beijing, China). The cells were cultured in RPMI-1640
medium supplemented with 10% fetal bovine serum (FBS; Gibco-BRL,
Grand Island, NY, USA) at 37°C under a 5% CO2 condition.
To recapitulate the effects of hypoxia as it occurs in prostate
cancer, we exposed 60–70% subconfluent PC3 cells to hypoxic
conditions (3% O2, 5% CO2 and 92%
N2) for up to 72 h.
Fluorescent immunostaining
Immunofluorescence staining was employed to further
confirm EMT phenotype changes of prostate cancer cells under
hypoxic condition. PC3 and DU145 cells were cultured on coverslips
in 24-well plates at initial density 5×104 cells/well.
At the indicated time-points, culture media were removed from the
cultured cells followed by 3 washings with PBS. Cells were fixed
with 4% polyoxymethylene solution for 20 min and washed with PBS 3
times. Cells were incubated with rabbit anti-human E-cadherin
polyclonal antibody (Santa Cruz Biotechnology, Santa Cruz, CA,
USA), and mouse anti-human vimentin monoclonal antibody (Santa Cruz
Biotechnology) respectively, then their corresponding lumophore
conjugated secondary antibodies. DAPI was used for nuclei staining.
Finally, cells were observed under a fluorescent microscope or a
confocal microscope.
Plasmid construction and
transfections
PcDNA3.1 plasmid (a generous gift from Professor
Dalin He) was digested with endonucleases HindIII and
BglII in order to construct vectors for expression of
HIF-1α-small interfering RNAs (siRNAs). Three chemically
synthesized oligonucleotides encoding HIF-1α-short hairpin siRNAs
that included a loop motif were inserted downstream of the
BglII promoter of the plasmid using DNA Ligation kit (Takara
Biotechnology, Co., Ltd., Dalian, China) and cloned. PC3 cells were
transfected with the above plasmids (4 µg total) using
Lipofectamine™ 2000 (Invitrogen Corp., Carlsbad, CA, USA) according
to the manufacturer's instructions. A scramble siRNA was used as
control.
Quantitative real-time PCR (qRT-PCR)
PC3 and DU145 cells were harvested at the indicated
time-points. Total RNA was extracted by using TRIzol (Invitrogen)
according to the manufacturer's protocol. Reverse transcription was
performed according to the protocol of SuperScript™ First-Strand
Synthesis System for RT-PCR (Invitrogen). Quantitative real-time
PCR was performed using SYBR Premix Ex Taq (Takara Biotechnology)
under conditions recommend by the manufacturer and Applied
Biosystems 7300 Real-Time PCR System (Applied Biosystems, Waltham,
MA, USA) supplied with the analytical software.
Primers were designed for transcription factors that
regulate EMT, including Snail, Slug, Twist, Zeb1, Zeb2 and E47,
GAPDH gene as an internal reference gene, by using web-based
program at www.idtdna.com and synthesized from
integrated DNA Technologies (Coralville, IA, USA). The primers used
for the analysis are shown in Table
I.
 | Table IPrimers used for the analysis. |
Table I
Primers used for the analysis.
| Primers |
|---|
| Snail | Forward:
CCACGAGGTGTGACTAACTATG |
| Reverse:
ACCAAACAGGAGGCTGAAATA |
| Slug | Forward:
AACTACAGCGAACTGGACAC |
| Reverse:
GAGGATCTCTGGTTGTGGTATG |
| Twist | Forward:
AGGCATCACTATGGACTTTCTC |
| Reverse:
GGCCAGTTTGATCCCAGTAT |
| Zeb1 | Forward:
CTTCTCACACTCTGGGTCTTATTC |
| Reverse:
CGTTCTTCCGCTTCTCTCTTAC |
| Zeb2 | Forward:
CTAACCCAAGGAGCAGGTAATC |
| Reverse:
GTGAATTCGCAGGTGTTCTTTC |
| E47 | Forward:
GTCTCGGTCATCCTGAACTTG |
| Reverse:
TTTCCTCTTCTCGCCGTTTC |
| GAPDH | Forward:
GGTGTGAACCATGAGAAGTATGA |
| Reverse:
GAGTCCTTCCACGATACCAAAG |
Immunoblotting with ECL detection
PC3 cells were incubated under hypoxic conditions
for different periods of time (6, 24, 48 and 72 h). After each
indicated incubation period, PC3 cells for protein assay were lysed
in RIPA buffer (1% Triton X-100, 0.5% deoxycholic acid, 0.2% SDS,
150 mM sodium chloride and 2 mM EDTA) with complete protease
inhibitor cocktail tablet (Roche, Mannheim, Germany) and pepstatin
A. After removing cell debris by centrifugation, protein
concentration was determined using BCA method.
Total proteins were separated on 8–12%
polyacrylamide gels and transferred onto 0.45 µm
nitrocellulose in a buffer containing 25 mmol/l Tris-HCL (pH 8.3),
192 mmol/l glycine, 20% methanol and blocked with 5% fat-free dry
milk in PBS for 2 h. The membranes were incubated with primary
antibodies. The following antibodies were used: rabbit anti-human
HIF-1α polyclonal antibody (Santa Cruz Biotechnology), rabbit
anti-human E-cadherin polyclonal antibody (Santa Cruz
Biotechnology), and mouse anti-human vimentin monoclonal antibody
(Santa Cruz Biotechnology). β-actin was used as internal
control.
Cell migration and invasion assays
The invasion assays were performed using Millicell
inserts (Millipore, Billerica, MA, USA) coated with Matrigel (BD
Biosciences, Sparks, MD, USA). Cells (2.5×104) were
seeded per upper chambers in serum-free DMEM whereas the lower
chambers were loaded with DMEM containing 5% FBS. After 12 h, on
the upper chambers non-migrating cells were removed by cotton
swabs, and cells invaded through the Matrigel layer to the
underside of the membrane were stained by crystal violet. The cell
numbers were counted. Cell migration assays were performed
similarly, but without Matrigel.
Statistical analysis
Each experiment was performed at least 3 times. All
values are presented as mean ± SD. The statistics were analyzed by
unpaired, two-tailed t-test. Data were considered to be
statistically significant when P<0.05.
Results
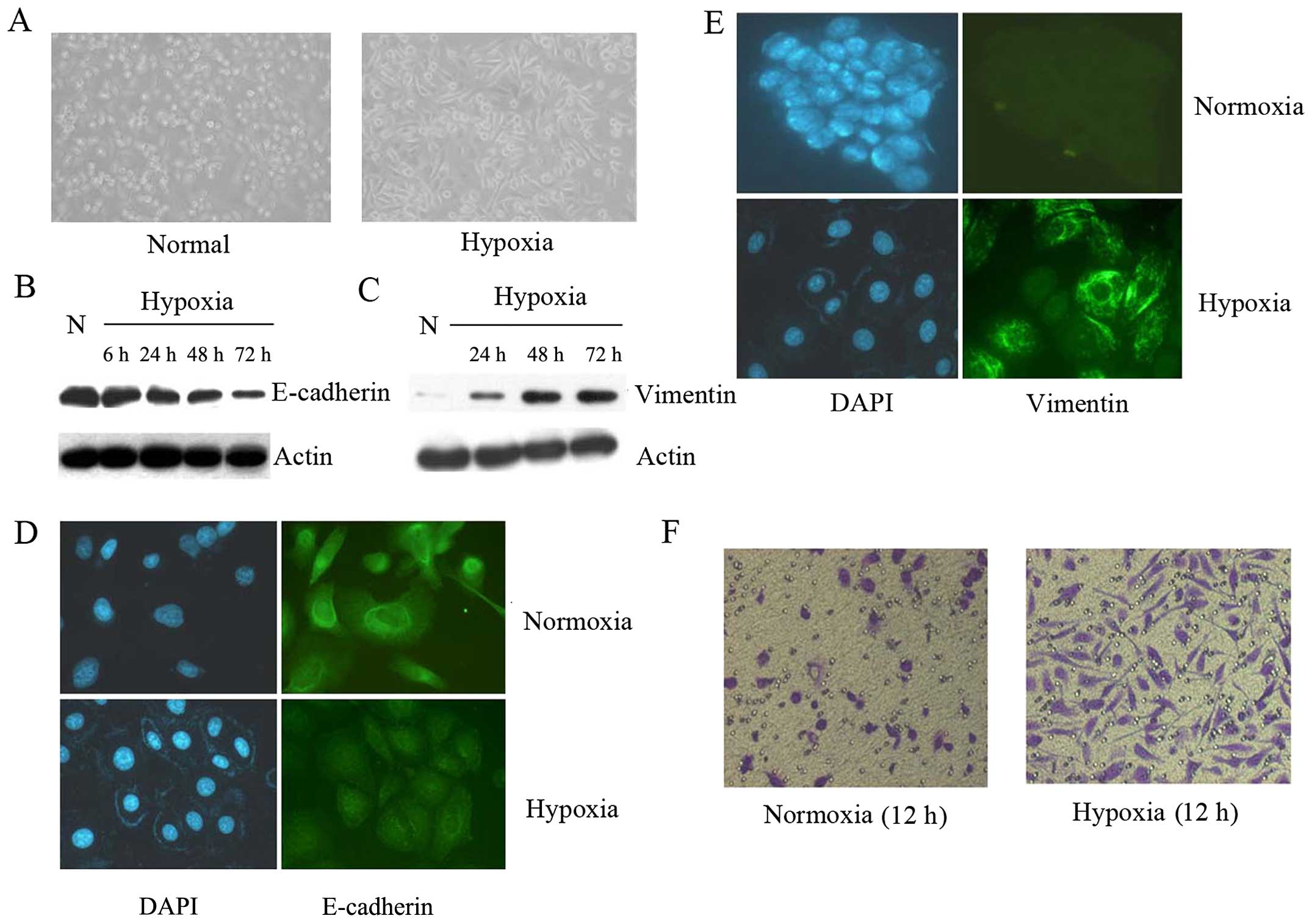
Hypoxia results in the morphologic and
cell biological changes characteristic of EMT in prostate cancer
cells
After 72 h culture at hypoxic conditions (3%
O2, 5% CO2 and 92% N2), PC3 cells started to
loose cell contacts, scattered from cell clusters and acquired a
spindle-shaped and fibroblast-like phenotype which is
characteristic of EMT compared to the normoxic counterparts (95%
air and 5% CO2) (Fig.
1A).
To further confirm whether prostate cancer underwent
EMT, the expression of markers of epithelial and mesenchymal
phenotypes were detected by western blot analysis (Fig. 1B and C). As is shown in Fig. 1B and C, hypoxic PC3 cells underwent
a typical transition manifested by reduced E-cadherin expression
and increased vimentin expression compared with normoxic
control.
Immunofluorescence staining showed that the
expression of E-cadherin decreased (Fig. 1D), but the expression of vimentin
increased (Fig. 1E) when compared
with their counterparts in normoxic environment. Matrigel invasion
assay was also used to assess the cell invasiveness, which
indicated that PC3 cells under hypoxic conditions for 12 h readily
migrated through the Matrigel chamber in a relatively high numbers,
whereas their normoxic partners exhibited a less invasive potency
(Fig. 1F).
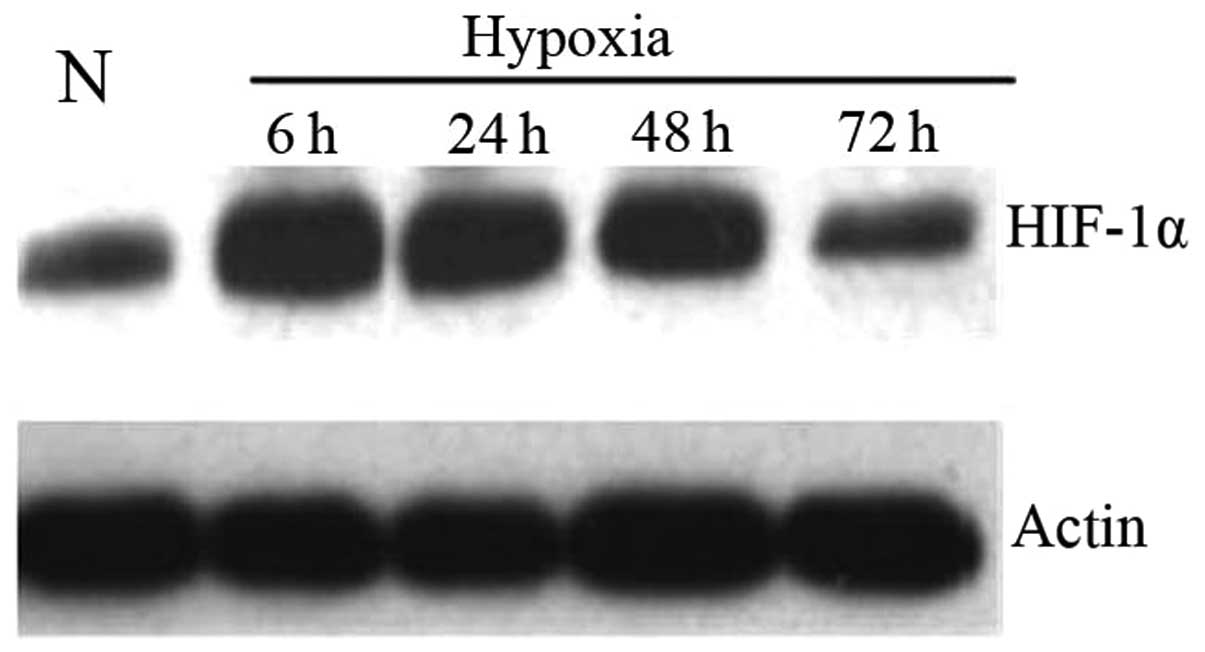
Under hypoxic conditions prostate cancer
cells exhibit heightened HIF-1α expression
HIF-1α was analyzed by western blot analysis to
determine whether the EMT observed in prostate cancer cells was
attributable to heightened HIF-1α activity under hypoxic conditions
(Fig. 2). PC3 cells were incubated
under hypoxic conditions and collected as described before. As
shown by western blot analysis (Fig.
2), HIF-1α protein levels in prostate cancer cells were
upregulated during hypoxia and reached the highest expression at 6
h. The increased HIF-1α protein level was possibly due to the
variation of protein stability under hypoxia condition.
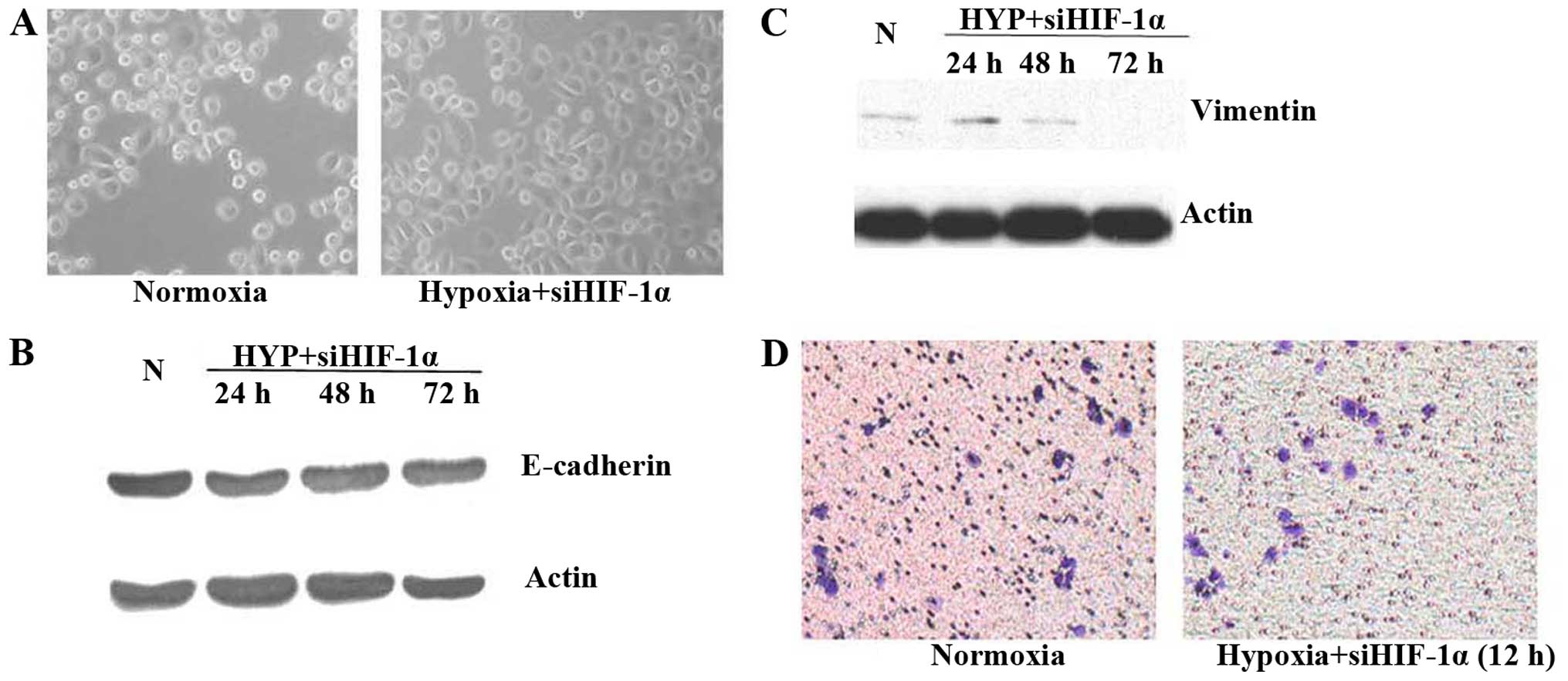
Inhibition of HIF-1α activity in prostate
cancer cells under hypoxic conditions or reoxygenation reverses
EMT
Having established that hypoxia results in elevated
HIF-1α expression in prostate cancer cells, we next investigated
the possibility for inhibition of HIF-1α to attenuate the
mesenchymal characteristics of hypoxic cells. Molecular inhibition
was accomplished by siRNA to downregulate HIF-1α. Inhibition of
HIF-1α activity by siRNA under hypoxic conditions for 48 h did not
cause any significant changes in cell morphology (Fig. 3A). No obvious change in protein
expression was detected by western blot analysis (Fig. 3B and C), consistent with reversion
to an epithelial phenotype. Importantly, inhibition of HIF-1α by
siRNA led to a remarkable decrease in the invasiveness of hypoxic
cells compared with control in Matrigel invasion assay at 12 h
under hypoxic conditions (Fig.
3D).
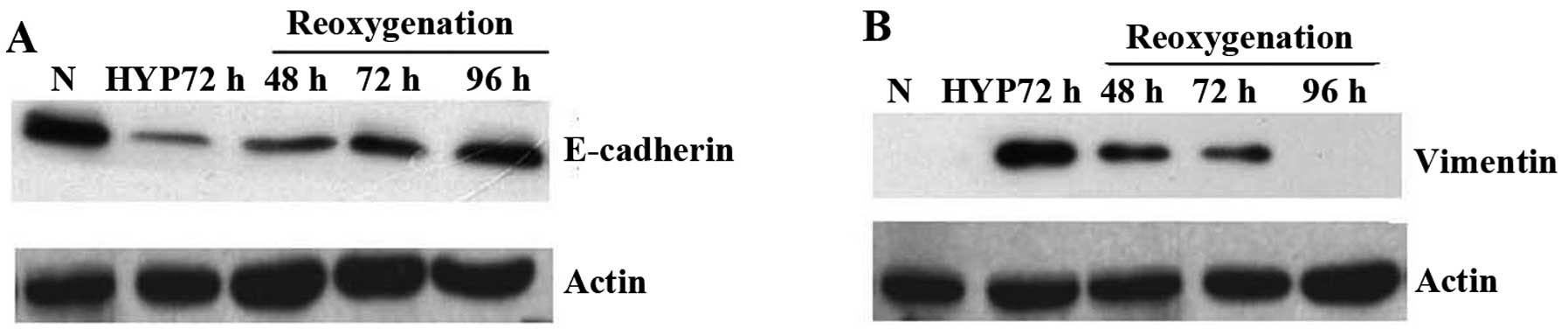
At the same time, we elucidated what would happen
when PC3 cells were re-cultured under normoxic conditions after
exposed to hypoxic conditions for 72 h. Western blot analysis
showed that E-cadherin protein was regained when PC3 cells returned
to normoxia gradually compared to those cultured under hypoxia
(Fig. 4A), whereas expressions of
vimentin gradually lost in contrast to the enhanced expression
under hypoxia condition (Fig. 4B).
These changes occurred after inhibition of HIF-1α activity in PC3
cells indicated that prostate cancer cells might undergo MErT
through reoxygenation after they were exposed in hypoxic
conditions.
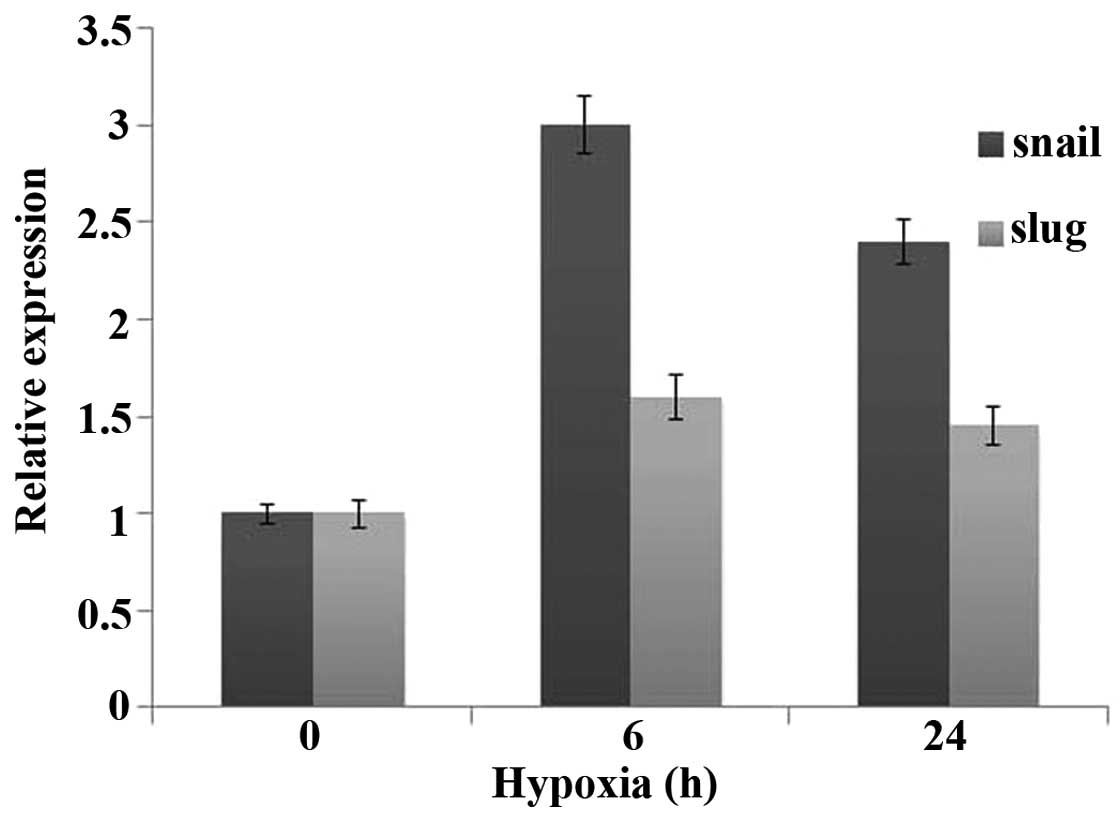
Prostate cancer cells under hypoxic
conditions exhibit upregulation of Snail and Slug, transcriptional
regulators of the EMT program
The gene expression cataract that mediates EMT is
regulated by one or more transcription factors, including Snail,
Slug, Twist, Zeb1, Zeb2 and E47 (6–9) and
these transcription factors are transcriptionally induced by
upstream signals, including hypoxia or HIF-1α. Real-time PCR result
showed that, hypoxic prostate cancer cells exhibited substantial
upregulation of Snail and Slug compared with normoxic cells
(Fig. 5).
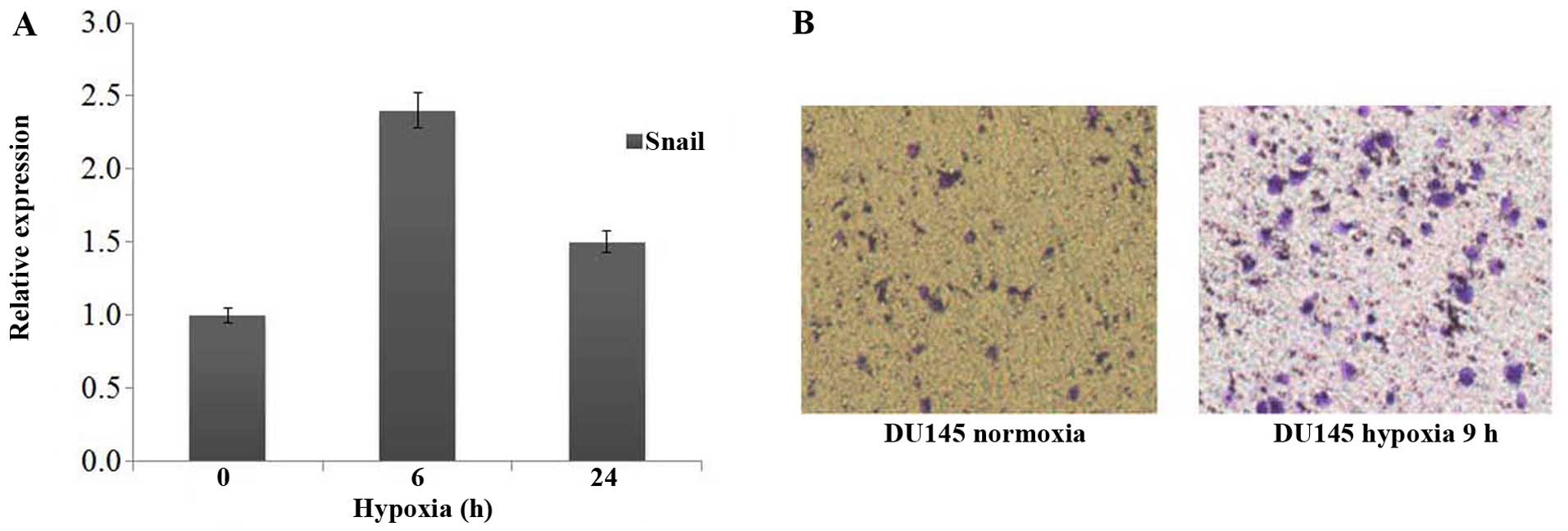
Hypoxic conditions upregulated the
invasive capability of epithelialorigin mesenchymal cells
The DU145 cell line was derived from prostate cancer
brain metastasis, which showed fibroblastic-like phenotype under
normoxia condition. The spindle shape of DU145 cells did not show
any change when they were cultured under hypoxic environment, but
real-time PCR results showed that DU145 cells exhibited substantial
upregulation of Snail as compared with normoxic cells (Fig. 6A). Transwell assay also showed that
Du145 has remarkably increased invasiveness, which related to the
activation of HIF-1α signal pathway and the upregulation of Snail
(Fig. 6B) under hypoxic
conditions.
Discussion
EMT has been defined as a three-part process in
which cells firstly acquire a fibroblast-like morphology, and then
downregulation of epithelial-specific proteins such as E-cadherin
while simultaneously expression of mesenchymal proteins such as
vimentin, and ultimately digest and migrate through extracellular
matrix (ECM) (10). Hypoxia alone
can trigger EMT in many solid tumors including hepatoblastoma,
pancreatic, colon and breast cancer (5). Yet, the mechanisms involved in
prostate cancer under hypoxic conditions remained unclear. A
variety of physiological or pathophysiological conditions can cause
imbalance in oxygen supply and demand. In response to reduction in
oxygen supply, tissues initiate signaling events that trigger the
upregulation of genes that are used to be 'silent' under normoxic
conditions to allow for both short and long-term adaptation to
hypoxia. Many of these events are initiated by the activation of
the hypoxia-inducible factor (HIF) family of transcription factors
of which HIF-1α plays an important role. Thus, we sought to
determine whether the EMT observed in prostate cancer cells under
hypoxic conditions attribute to enhance HIF-1α activity.
HIF-1α was analyzed to determine whether increased
HIF-1α activity result in EMT in prostate cancer cells under
hypoxic conditions. PC3 cells were incubated under hypoxic
conditions for different periods of time (6, 24, 48 and 72 h) and
western blot analysis was used to prove our hypothesis. Having
established that hypoxia lead to elevated HIF-1α expression in
prostate cancer cells, we next investigated the possibility for
inhibition of HIF-1α to attenuate the mesenchymal characteristics
of hypoxic cells. Towards this end, siRNAs were used to inhibit
HIF-1α expression in these hypoxic cells and then compared their
phenotype with their counterparts. Our data indicated changes in
morphology, protein expression and invasion and supported the
notion that hypoxia leads to EMT in prostate cancer cells.
Substantial evidence illustrated that EMT plays
important roles in cancer metastasis and is also responsible for
resistance to conventional chemotherapeutics (11). Tumor microenvironments, including
hypoxia, have been documented as inducing this phenomenon through
upregulation of Twist, Snail, Slug, Zeb1 and Zeb2 according to
different cancer cell types (8,12,13).
Hypoxic microenvironment can be found in central region of solid
tumors and intratumoral hypoxia is conducive to high-grade and high
invasive ability tumor cell screen which might contribute to tumor
malignant progression (14–16). According to epidemiological and
clinical studies, hypoxia and hypoxia-induced signaling pathways
were associated with poor prognosis of patient including prostate
cancer (17), and HIF-1α was
reported to mediate hypoxia and EMT (5,9).
However, the molecular mechanism of hypoxia that induced
aggressiveness of prostate cancer has not been defined
previously.
It was demonstrated that transcription factor HIF-1
is a heterodimer that composed of a constitutively expressed HIF-1β
subunit and a HIF-1α subunit that mediates adaptive responses to
changes in tissue oxygenation (3).
The level of HIF-1α expression is determined not by the rates of
protein synthesis but the protein degradation. In the case of
normoxia, HIF-1α protein is degraded by O2-dependent
prolylhydroxylation, which targets the protein for ubiquitylation
by E3 ubiquitin-protein ligases. Stabilization of HIF-1α is
critical in the transcriptional response to hypoxia. Our
preliminary study suggested that high expression of HIF-1α can
induce prostate cancer LnCap to undergo EMT under normoxia
(18) and inhibition of β-catenin
through shRNA causes a reversal of EMT and metastatic phenotypes
induced by HIF-1α (19).
Some relevant research has already demonstrated EMT
and HIF-1α in the different human PCa cell lines, for example,
DU145, PC-3, PPC-1 and TSU. In these investigations, the majority
of EMT studies in prostate cancer used PC-3 and DU145 human
prostate cancer cells. Our previous research also included
different human PCa cell lines. At the same time, we already
successfully proved that overexpression of hypoxia inducible
factor-1α (HIF-1α) could induce EMT in LNCaP cells, but not in PC3
(18–24). Based on these studies, in the
present study, we simulated the hypoxia condition and explored the
potential mechanism of HIF-1α on EMT in human prostate cancer cell
lines PC3 and DU145.
In the present study, we confirmed that hypoxia
treatment may induce epithelial origin of the prostate cancer cells
PC3 to undergo typical EMT transforming, which includes changes in
cell morphology, decreased expression of E-cadherin, increased
stromal protein vimentin expression, high expression of Snail,
accompanied by increased cell invasion and metastasis. Further
research indicated that inhibition of HIF-1α expression under
hypoxic conditions or reoxygenation reverses EMT which implies that
HIF-1α plays a critical role under hypoxia induced EMT of prostate
cancer and that the EMT process is a means, not an end for cancer
metastasis. Our result is consistent with the study by Yang et
al (8) who also confirmed that
Twist is directly regulated by HIF-1α to induce tumor cell EMT
transformation, while our result indicated that Snail is highly
expressed in the hypoxic microenvironment, and its direct
inhibition of E-cadherin expression in ovarian tumors were reported
(7). Whether Snail mediated EMT
phenomenon induced by activation of HIF-1α under hypoxia, or
hypoxia directly regulates the expression of Snail, or whether
there is a direct transcriptional regulation between HIF-1α and
Snail requires further experimental studies. HIF-1α can also induce
epithelial cell EMT transformation through other signaling
pathways, such as the direct or indirect regulation of vimentin and
fibronectin (25). In addition to
HIF-1α, there are some other transcriptional factors that can
induce EMT phenomenon under hypoxic conditions, such as regulation
of E-cadherin and β-catenin by URG11 (upregulated gene 11)
(26) and regulation of GSK3β
through hypoxia (5) which can
target Snail (27) and further
induce EMT.
Phenomena were reported which described a
mesenchymal to epithelial reverting transition (MErT), where
mesenchymal-like prostate cancer cell lines revert to
epithelial-like, and re-establish cellular adhesion during
colonization when re-express E-cadherin in the liver tumor
microenvironment (28–32). There is a report that hyperoxic
treatment induces MErT in a rat adenocarcinoma model (33), so partial pressure of oxygen alone
can make the tumor cells of epithelial origin conversion between
EMT and MErT and it is of great importance to elucidate the
mechanisms involved in oxygen pressure changes.
We may conclude that EMT is essential for tumor
metastasis and MErT is conducive to the formation of metastasis.
MErT transformation conditions might change when the re-expression
of E-cadherin and the increase in the ability of the intercellular
adhesion is essential. At the same time, we observed that not all
cells underwent conversion of MErT accompanying with the time
extension of reoxygenation, there are still part of the cells
showing interstitial cells long spindle shape and related proteins
were not restored to the level prior to hypoxia, which suggested
that possibly only certain cells undergo some changes, such as
having characteristics of stem cells (30,34–37),
but not the conversion of MErT.
The evidence provides incentives for further
investigation and optimization in establishing the mechanistic role
of how HIF-1α and Snail work in the attenuation of EMT
characteristics and drug resistance under hypoxia and their utility
in the clinical practice in the treatment of prostate cancer for
which there is no effective and curative therapy.
In conclusion, in the present study, we conclude
that EMT could be induced by a mechanism that might involve the
activation of HIF-1α-dependent cell signaling in hypoxic prostate
cancer cells. Our results provide incentives for further
investigation and optimization in establishing the mechanistic role
of how HIF-1α and Snail work in the attenuation of EMT
characteristics and drug resistance under hypoxia and their utility
in the clinical practice in the treatment of prostate cancer for
which there is no effective and curative therapy.
Acknowledgments
We thank Yatong Chen and Tao Peng for their
technical support in research design, acquisition of data, or
analysis and interpretation of data. The present study is supported
by funds from the National Natural Science Foundation of China
(81341066) and the Beijing Health System Special Foundation for
building high-level health personnel.
References
|
1
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2013. CA Cancer J Clin. 63:11–30. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Vaupel P and Mayer A: Hypoxia in cancer:
Significance and impact on clinical outcome. Cancer Metastasis Rev.
26:225–239. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Semenza GL: Targeting HIF-1 for cancer
therapy. Nat Rev Cancer. 3:721–732. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kalluri R and Weinberg RA: The basics of
epithelial-mesenchymal transition. J Clin Invest. 119:1420–1428.
2009. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Cannito S, Novo E, Compagnone A, Valfrè di
Bonzo L, Busletta C, Zamara E, Paternostro C, Povero D, Bandino A,
Bozzo F, et al: Redox mechanisms switch on hypoxia-dependent
epithelial-mesenchymal transition in cancer cells. Carcinogenesis.
29:2267–2278. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Peinado H, Olmeda D and Cano A: Snail, Zeb
and bHLH factors in tumour progression: An alliance against the
epithelial phenotype? Nat Rev Cancer. 7:415–428. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Imai T, Horiuchi A, Wang C, Oka K, Ohira
S, Nikaido T and Konishi I: Hypoxia attenuates the expression of
E-cadherin via up-regulation of SNAIL in ovarian carcinoma cells.
Am J Pathol. 163:1437–1447. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yang MH, Wu MZ, Chiou SH, Chen PM, Chang
SY, Liu CJ, Teng SC and Wu KJ: Direct regulation of TWIST by
HIF-1alpha promotes metastasis. Nat Cell Biol. 10:295–305. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Cheng ZX, Sun B, Wang SJ, Gao Y, Zhang YM,
Zhou HX, Jia G, Wang YW, Kong R, Pan SH, et al: Nuclear
factor-κB-dependent epithelial to mesenchymal transition induced by
HIF-1α activation in pancreatic cancer cells under hypoxic
conditions. PLoS One. 6:e237522011. View Article : Google Scholar
|
|
10
|
Grünert S, Jechlinger M and Beug H:
Diverse cellular and molecular mechanisms contribute to epithelial
plasticity and metastasis. Nat Rev Mol Cell Biol. 4:657–665. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Huber MA, Kraut N and Beug H: Molecular
requirements for epithelial-mesenchymal transition during tumor
progression. Curr Opin Cell Biol. 17:548–558. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Min C, Eddy SF, Sherr DH and Sonenshein
GE: NF-kappaB and epithelial to mesenchymal transition of cancer. J
Cell Biochem. 104:733–744. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Shin SR, Sánchez-Velar N, Sherr DH and
Sonenshein GE: 7,12-dimethylbenz(a)anthracene treatment of a c-rel
mouse mammary tumor cell line induces epithelial to mesenchymal
transition via activation of nuclear factor-kappaB. Cancer Res.
66:2570–2575. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Harris AL: Hypoxia - a key regulatory
factor in tumour growth. Nat Rev Cancer. 2:38–47. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Semenza GL: Defining the role of
hypoxia-inducible factor 1 in cancer biology and therapeutics.
Oncogene. 29:625–634. 2010. View Article : Google Scholar :
|
|
16
|
Le QT, Denko NC and Giaccia AJ: Hypoxic
gene expression and metastasis. Cancer Metastasis Rev. 23:293–310.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Movsas B, Chapman JD, Greenberg RE, Hanlon
AL, Horwitz EM, Pinover WH, Stobbe C and Hanks GE: Increasing
levels of hypoxia in prostate carcinoma correlate significantly
with increasing clinical stage and patient age: An Eppendorf
pO2 study. Cancer. 89:2018–2024. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Jiang YG, Luo Y, He DL, Li X, Zhang LL,
Peng T, Li MC and Lin YH: Role of Wnt/beta-catenin signaling
pathway in epithelial-mesenchymal transition of human prostate
cancer induced by hypoxia-inducible factor-1alpha. Int J Urol.
14:1034–1039. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhao JH, Luo Y, Jiang YG, He DL and Wu CT:
Knockdown of β-Catenin through shRNA cause a reversal of EMT and
metastatic phenotypes induced by HIF-1α. Cancer Invest. 29:377–382.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhao L, Jiang YG, Ma J, Luo Y and Zhao JH:
Characterization of prostate cancer cell lines and their
epithelial-mesenchymal transition in subcutaneous tumors. Zhonghua
Nan Ke Xue. 17:314–317. 2011.In Chinese. PubMed/NCBI
|
|
21
|
Hugo H, Ackland ML, Blick T, Lawrence MG,
Clements JA, Williams ED and Thompson EW: Epithelial-mesenchymal
and mesenchymal-epithelial transitions in carcinoma progression. J
Cell Physiol. 213:374–383. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Thomas R and Kim MH: HIF-1 alpha: A key
survival factor for serum-deprived prostate cancer cells. Prostate.
68:1405–1415. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhong H, Agani F, Baccala AA, Laughner E,
Rioseco-Camacho N, Isaacs WB, Simons JW and Semenza GL: Increased
expression of hypoxia inducible factor-1alpha in rat and human
prostate cancer. Cancer Res. 58:5280–5284. 1998.PubMed/NCBI
|
|
24
|
Luo Y, He DL, Ning L, Shen SL, Li L, Li X,
Zhau HE and Chung LW: Over-expression of hypoxia-inducible
factor-1alpha increases the invasive potency of LNCaP cells in
vitro. BJU Int. 98:1315–1319. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Krishnamachary B, Berg-Dixon S, Kelly B,
Agani F, Feldser D, Ferreira G, Iyer N, LaRusch J, Pak B, Taghavi
P, et al: Regulation of colon carcinoma cell invasion by
hypoxia-inducible factor 1. Cancer Res. 63:1138–1143.
2003.PubMed/NCBI
|
|
26
|
Du R, Huang C, Bi Q, Zhai Y, Xia L, Liu J,
Sun S and Fan D: URG11 mediates hypoxia-induced
epithelial-to-mesenchymal transition by modulation of E-cadherin
and beta-catenin. Biochem Biophys Res Commun. 391:135–141. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhou BP, Deng J, Xia W, Xu J, Li YM,
Gunduz M and Hung MC: Dual regulation of Snail by
GSK-3beta-mediated phosphorylation in control of
epithelial-mesenchymal transition. Nat Cell Biol. 6:931–940. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yates CC, Shepard CR, Stolz DB and Wells
A: Co-culturing human prostate carcinoma cells with hepatocytes
leads to increased expression of E-cadherin. Br J Cancer.
96:1246–1252. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yates C, Shepard CR, Papworth G, Dash A,
Beer Stolz D, Tannenbaum S, Griffith L and Wells A: Novel
three-dimensional organotypic liver bioreactor to directly
visualize early events in metastatic progression. Adv Cancer Res.
97:225–246. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
van der Pluijm G: Epithelial plasticity,
cancer stem cells and bone metastasis formation. Bone. 48:37–43.
2011. View Article : Google Scholar
|
|
31
|
Chaffer CL, Brennan JP, Slavin JL, Blick
T, Thompson EW and Williams ED: Mesenchymal-to-epithelial
transition facilitates bladder cancer metastasis: Role of
fibroblast growth factor receptor-2. Cancer Res. 66:11271–11278.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Elloul S, Vaksman O, Stavnes HT, Trope CG,
Davidson B and Reich R: Mesenchymal-to-epithelial transition
determinants as characteristics of ovarian carcinoma effusions.
Clin Exp Metastasis. 27:161–172. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Moen I, Øyan AM, Kalland KH, Tronstad KJ,
Akslen LA, Chekenya M, Sakariassen PO, Reed RK and Stuhr LE:
Hyperoxic treatment induces mesenchymal-to-epithelial transition in
a rat adenocarcinoma model. PLoS One. 4:e63812009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Hill RP, Marie-Egyptienne DT and Hedley
DW: Cancer stem cells, hypoxia and metastasis. Semin Radiat Oncol.
19:106–111. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Phinney DG: Twist,
epithelial-to-mesenchymal transition, and stem cells. Stem Cells.
29:3–4. 2010. View
Article : Google Scholar
|
|
36
|
Martin A and Cano A: Tumorigenesis: Twist1
links EMT to self-renewal. Nat Cell Biol. 12:924–925. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Zavadil J: A spotlight on regulatory
networks connecting EMT and cancer stem cells. Cell Cycle.
9:2927–2935. 2010. View Article : Google Scholar : PubMed/NCBI
|