Introduction
MicroRNAs (miRNAs), a group of small non-coding RNAs
interacting with the 3′-untranslated region (3-UTR) of targeted
mRNAs, inhibit the expression of target genes by contributing to
the degradation or translational inhibition of target mRNAs
(1). They have been found to be
actively involved in various biological processes including cell
proliferation, apoptosis, differentiation and movement (2,3).
Emerging studies have shown that abnormal expression and function
of miRNAs play important roles in the initiation and progression of
human malignancies (4–6). In addition, miRNAs have been
demonstrated to be promising biomarkers and therapeutic targets of
glioma (7–9). Investigating the expression and
biological function of miRNAs in glioma may contribute to the
identification of novel biomarkers and therapeutic targets for
glioma patients.
Recently, microRNA-520c (miR-520c) was found to play
important roles in various human cancers including diffuse large B
cell lymphoma (10), breast cancer
(11), fibrosarcoma (12) and hepatocellular carcinoma (HCC)
(13). A study on breast cancer
showed that miR-520c promoted cancer cell migration and invasion
in vitro and in vivo by suppression of CD44 (14). In addition, expression of miR-520c
increased proliferation, migration and invasion of HCC cells in
vitro (13). However, miR-520c
abrogated both in vitro cell invasion and in vivo
intravasation of highly invasive MDA-MB-231 cells (11), indicating that miR-520c functions as
a tumor-suppressor in estrogen receptor negative breast cancer.
Previously, Lu et al reported that miR-520c inhibits
glioblastoma U87GM cell migration with decreased activation of
proMMP2 in vitro (15).
However, the clinical significance and biological role of miR-520c
in glioma remain practically unknown.
In the present study, we confirmed that the
expression of miR-520c was decreased in glioma tissues. Low
expression of miR-520c was correlated with poor clinicopathological
features and decreased survival of glioma patients. Our data showed
that miR-520c inhibited the metastatic ability of glioma cells
in vitro. Moreover, transforming growth factor-β receptor
type 2 (TGFBRII) was identified as a downstream target of miR-520c
in glioma.
Materials and methods
Clinical tissues
Fresh human glioma and adjacent non-cancerous
tissues were obtained from 60 patients who underwent therapeutic
removal of gliomas at Taihe Hospital, Hubei University of Medicine.
Patients did not receive any chemotherapy or radiotherapy before
surgical treatment. All clinical specimens were collected and used
after obtaining informed consent from each patient enrolled in the
present study. All specimens were stored in liquid nitrogen for
further investigation. The protocol which involved clinical
specimens in the present study was approved by the Research Ethics
Committee of Hubei University of Medicine.
Cell culture and transfection
Human glioma cell lines including U87 and U251, and
human kidney epithelial cell line (293T) were obtained from the
American Type Culture Collection (ATCC; Manassas, VA, USA). These
cells were cultured in Dulbeccos modified Eagles medium (DMEM) with
10% fetal bovine serum (FBS), 100 U/ml penicillin and 100 mg/ml
streptomycin. All cell cultures were maintained in a humidified
cell incubator with 5% CO2 at 37̊C.
miR-520c and negative control (NC) mimics, miR-520c
and NC inhibitors (anti-NC) were obtained from Genecopoeia
(Guangzhou, China), and were then transfected into glioma cells
with Lipofectamine 2000 following the manufacturers protocol.
Retroviral vectors pMMP-TGFBRII were generated by inserting the
TGFBRII cDNA into pMMP. Retrovirus packaging and transduction were
previously described (16).
Quantitative real-time RT-PCR
(qRT-PCR)
Total RNA from glioma cells was extracted by
miRNeasy Mini kit (Qiagen, Hilden, Germany) and total RNA from
glioma tissues was extracted with TRIzol reagent (Ambion, Thermo
Scientific, Shanghai, China). miR-520c levels in these samples were
assayed using TaqMan MicroRNA assays based on the manufacturer's
protocol (Applied Biosystems, Carlsbad, CA, USA). Real-time PCR of
TGFBRII was performed using an UltraSYBR Mixture (CW0957; CWBIO,
Beijing, China) and an LC480 PCR System (Roche, Indianapolis, IN,
USA). The primers for miR-520c and U6, TGFBRII and GAPDH were
obtained from Genecopoeia. The expression levels of mRNA and
miR-520c were normalized to the endogenous controls GAPDH and U6
respectively, using the 2−ΔΔCt method.
Luciferase reporter assay
To investigate whether miR-520c interacted with the
3′-UTRs of TGFBRII, wild-type (wt) 3′-UTR of TGFBRII predicted to
interact with miR-520c was amplified. Then, the wt 3′-UTR of
TGFBRII and the corresponding vectors (NC, miR-520c mimic, anti-NC,
miR-520c inhibitor or mutant miR-520c) were co-transfected into
293T cells by Lipofectamine 2000. Forty-eight hours after
co-transfection, the cells were lysed and assayed using
Dual-Luciferase® Reporter Assay kit (Promega, Madison,
WI, USA) based on the manufacturer's instructions.
Wound healing assay
Glioma cells transfected with the corresponding
vectors were seeded in 6-well plates to form a single confluent
cell layer. Wounds were made in the confluent cell layer using
100-µl tips. Following wound scratching (0 and 12 h), the width of
the wounds were photographed with a phase-contrast microscope.
Migration and invasion assays
The migratory and invasive abilities of glioma cells
were evaluated with Transwell chambers (BD Biosciences, Franklin
Lakes, NJ, USA). Glioma cells (5–10×104) suspended in
100 µl serum-free medium were seeded into the upper chamber, and
the lower chamber was filled with 20% FBS to induce glioma cell
migration or invasion through the membrane. Matrigel (1:6 dilution;
Becton-Dickinson Labware, Bedford, MA, USA) was added to the upper
chamber for the invasion assay. Twenty-four hours later, cells that
migrated or invaded the Transwell membrane were stained with
crystal violet and the number of cells was counted under a
microscope. TGF-β1 (2.5 ng/ml; R&D Systems, Minneapolis, MN,
USA) was used for inducing glioma cell migration and invasion.
Western blotting
Before protein extraction, glioma cells were washed
with phosphate-buffered saline (PBS) to remove the culture media.
Cellular proteins were obtained from glioma cells using RIPA lysis
buffer and the protein concentrations were assessed using the BSA
method. Cellular proteins (30 µg) were separated by 10% SDS-PAGE,
and were transferred to polyvinylidene fluoride (PVDF) membranes
(Millipore, Bedford, MA, USA). After blocking with 5% non-fat milk
at room temperature for 1 h, the membranes were probed with primary
antibodies at 4̊C overnight. Then, the membranes were incubated
with the corresponding secondary antibodies at room temperature for
2 h. The primary antibodies used in the present study included:
TGFBRII (K105) (#3713; Cell Signaling Technology, Danvers, MA,
USA), CD44 (WL0140; Wanleibio, Shanghai, China) and GAPDH (G8140;
US Biological, Swampscott, MA, USA).
Immunohistochemistry (IHC)
Before IHC staining, glioma tissues were fixed with
4% formalin and embedded with paraffin. Then, the embedded tissues
were cut into 4-µm thick sections and visualized by IHC staining
following the standard protocol in order to evaluate the expression
level of TGFBRII (Cell Signaling Technology) in glioma tissues.
Statistical analysis
All quantitative data are presented as the mean ±
the standard error of the mean (SEM). Statistical analyses
including Pearson Chi-squared test, a two-tailed Student's t-test,
Kaplan-Meier method, the log-rank test and Spearman's correlation
analysis were performed with GraphPad Prism 6 (GraphPad Software
Inc., San Diego, CA, USA). P<0.05 was considered to indicate a
statistically significant result.
Results
Clinical significance of miR-520c
expression in glioma specimens
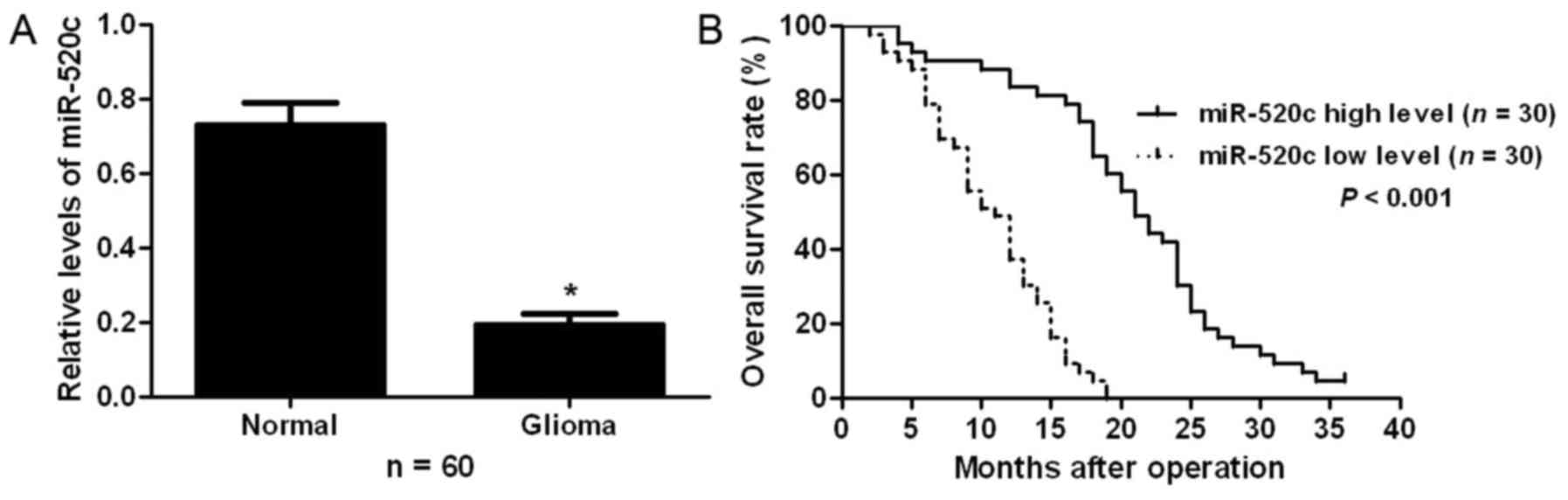
To examine the expression status of miR-520c in
glioma, qRT-PCR was performed for 60 pairs of glioma and adjacent
non-tumor tissues. Our results showed that glioma tissues had
significant decreased expression levels of miR-520c compared with
adjacent non-tumor tissues (P<0.05; Fig. 1A). To clarify the clinical
significance and prognostic value of miR-520c in glioma, all
patients were divided into two groups (miR-520c low-expressing and
miR-520c high-expressing groups) according to the median level of
miR-520c expression. As shown in Table
I, patients with low expression of miR-520c had advanced World
Health Organization (WHO) stage (P=0.024). Furthermore,
Kaplan-Meier analysis showed that patients with low expression of
miR-520c exhibited a significantly decreased overall survival rate
(P<0.001; Fig. 1B). These
results indicate that miR-520c probably plays an oncogenic role in
glioma.
 | Table I.Correlation between the
clinicopathological characteristics and expression of miR-520c in
gliomas. |
Table I.
Correlation between the
clinicopathological characteristics and expression of miR-520c in
gliomas.
|
|
| No. of patients |
|---|
|
|
|
|
|---|
| Characteristics | Total no. of pts.
(n=60) | Low miR-520c | High miR-520c | P-value |
|---|
| Age (years) |
|
|
| 0.301 |
|
<50 | 28 | 12 | 16 |
| ≥50 | 32 | 18 | 14 |
| Gender |
|
|
| 0.592 |
| Male | 38 | 18 | 20 |
|
Female | 22 | 12 | 10 |
| Tumor size (cm) |
|
|
| 0.417 |
|
<5 | 21 | 9 | 12 |
| ≥5 | 39 | 21 | 18 |
| Histologic tumor |
|
|
| 0.698 |
| type |
|
Astrocytic | 42 | 22 | 20 |
|
Oligodendrogial | 7 | 3 | 4 |
|
Oligoastrocytic | 11 | 5 | 6 |
| WHO grade |
|
|
| 0.024a |
| I+II | 18 | 5 | 13 |
|
III+IV | 42 | 25 | 17 |
miR-520c inhibits the migration and
invasion of glioma cells
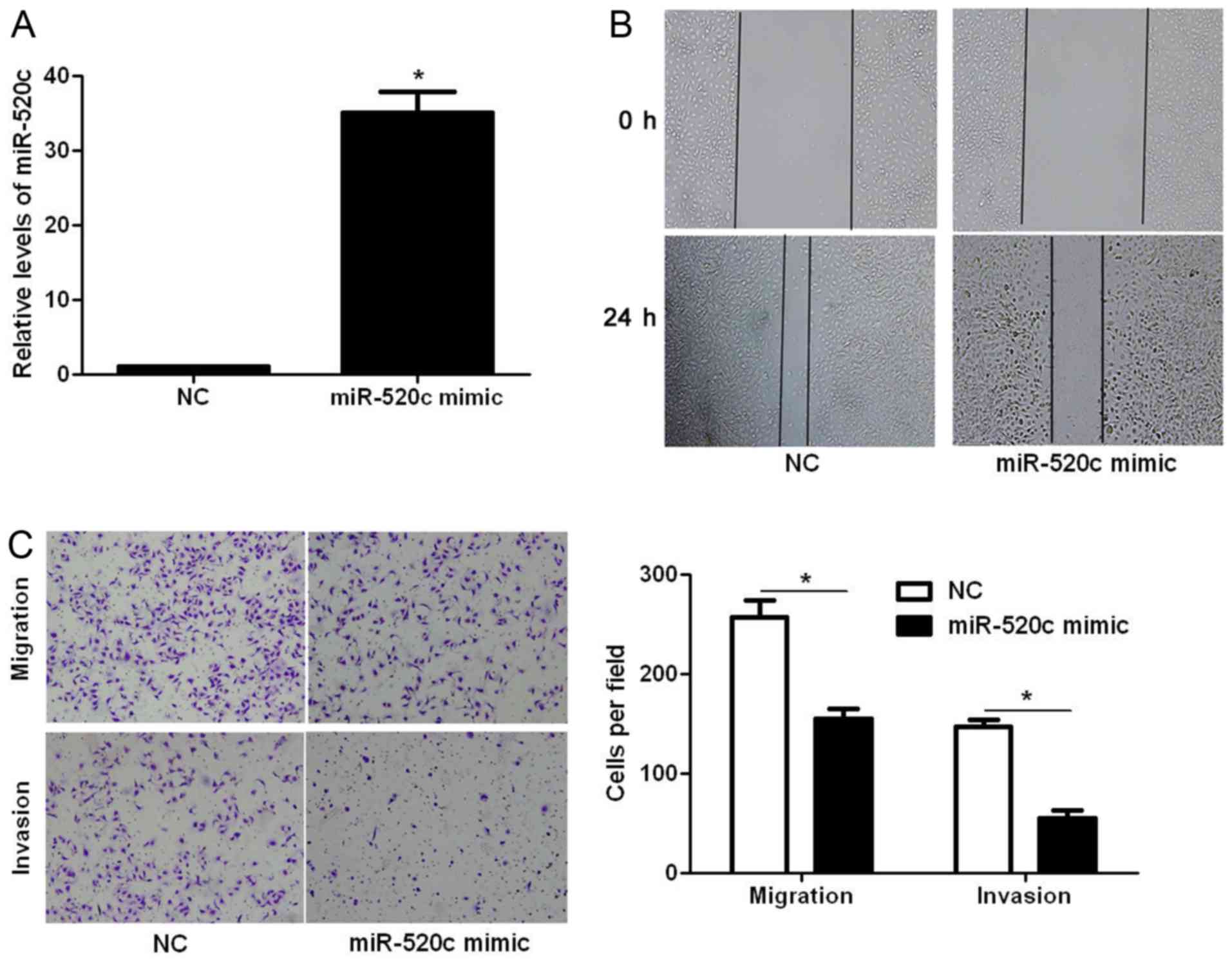
Next, we explored whether miR-520c modulates the
migration and invasion of glioma cells. Transfection of miR-520c
mimic into U251 cells significantly increased the expression level
of miR-520c (P<0.05; Fig. 2A).
The wound healing assays showed that the migration of U251 cells
was significantly decreased after miR-520c overexpression
(P<0.05; Fig. 2B). In addition,
Transwell assays demonstrated that overexpression of miR-520c
prominently suppressed the migration and invasion of U251 cells
(P<0.05, respectively; Fig. 2C).
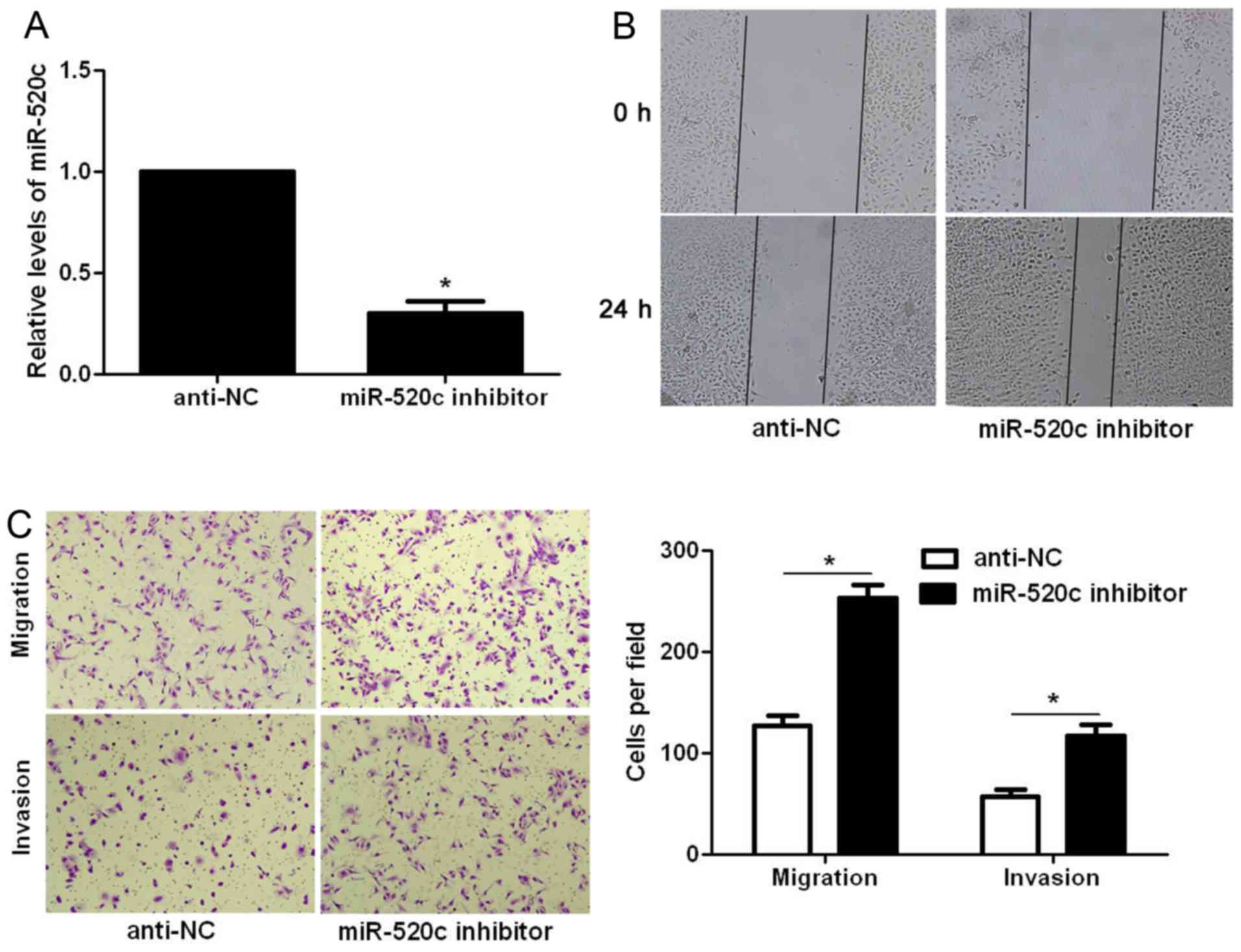
In contrast, miR-520c inhibitor significantly decreased the
expression level of miR-520c in U87 cells (P<0.05; Fig. 3A). Subsequently, miR-520c silencing
significantly facilitated the migration and invasion of U87 cells
(P<0.05, respectively; Fig. 3B and
C). Thus, miR-520c reversed the migratory and invasive
abilities of the glioma cells.
TGFBRII is a downstream target of
miR-520c
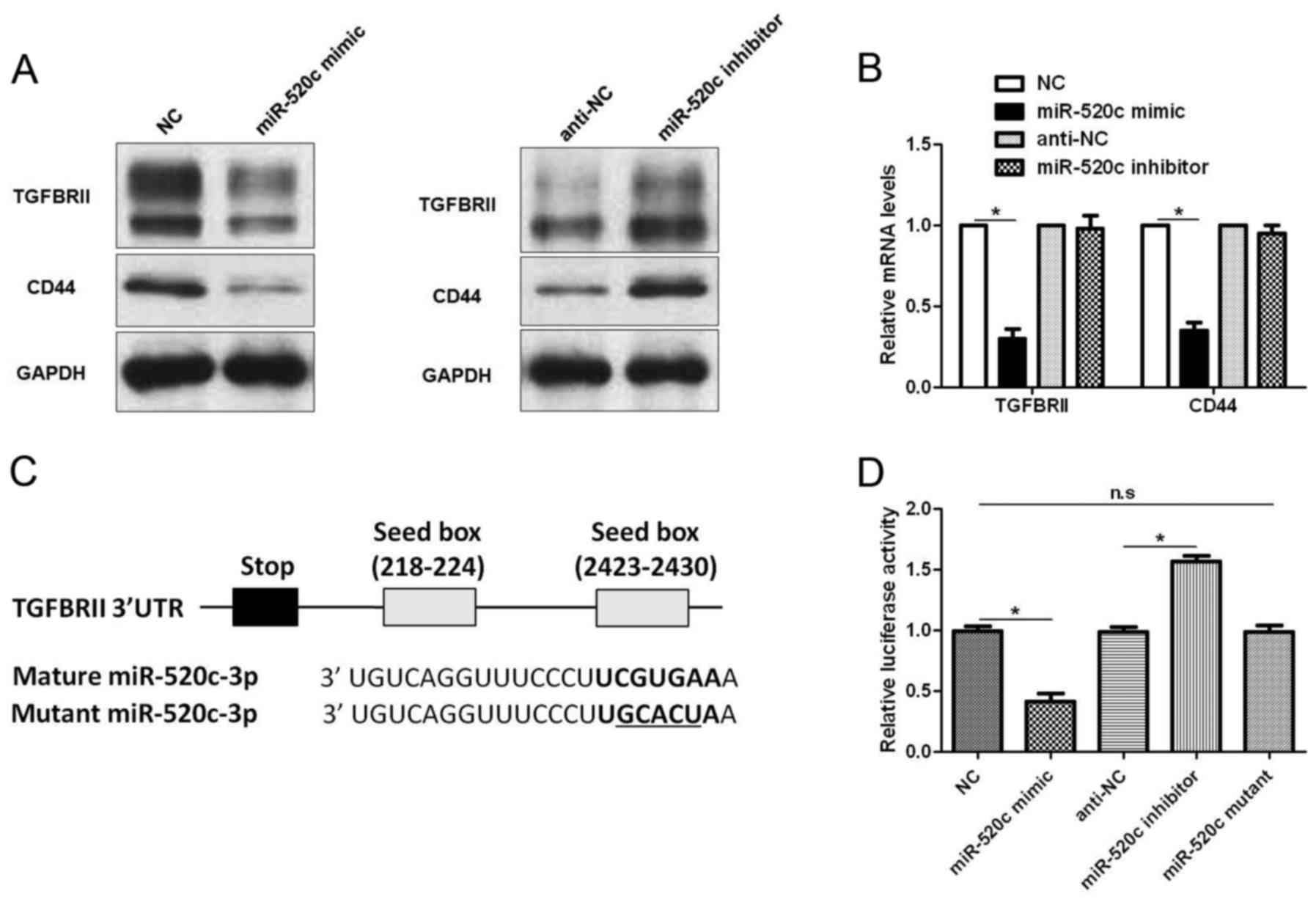
To disclose the underlying molecular mechanisms of
the biological function of miR-520c in glioma cells,
TargetScanHuman 7.1 (http://www.targetscan.org) was used to search for the
downstream target of miR-520c. TGFBRII, a critical regulator of the
TGF-β pathway in glioma (17), was
recognized as a potential downstream target of miR-520c. U251 cells
that were transduced with corresponding vectors were subjected to
immunoblotting for TGFBRII and CD44, a known downstream target of
miR-520c (14). Notably, miR-520c
overexpression decreased the levels of TGFBRII and CD44 protein,
while miR-520c silencing increased the expression of these proteins
(Fig. 4A). Furthermore, qRT-PCR
also confirmed that miR-520c overexpression downregulated the mRNA
levels of CD44 and TGFBRII (P<0.05; Fig. 4B). However, miR-520c knockdown
showed no significant effect on mRNA expression (Fig. 4B). As shown in Fig. 4C, the 3′-UTR of TGFBRII contained
two putative binding sites for miR-520c. Subsequently, we performed
luciferase assay to investigate whether miR-520c could bind to the
putative binding sites in the 3′-UTR of TGFBRII. Alteration of
miR-520c inversely regulated the luciferase activity of TGFBRII
3′-UTR (P<0.05; Fig. 4D), while
mutant miR-520c did not exhibit any influence on the luciferase
activity of TGFBRII 3′-UTR (Fig.
4D). Therefore, these data indicate that TGFBRII is a direct
downstream target of miR-520c in glioma.
An inverse correlation between TGFBRII
and miR-520c is observed in glioma specimens
Glioma and non-tumor tissues were subjected to
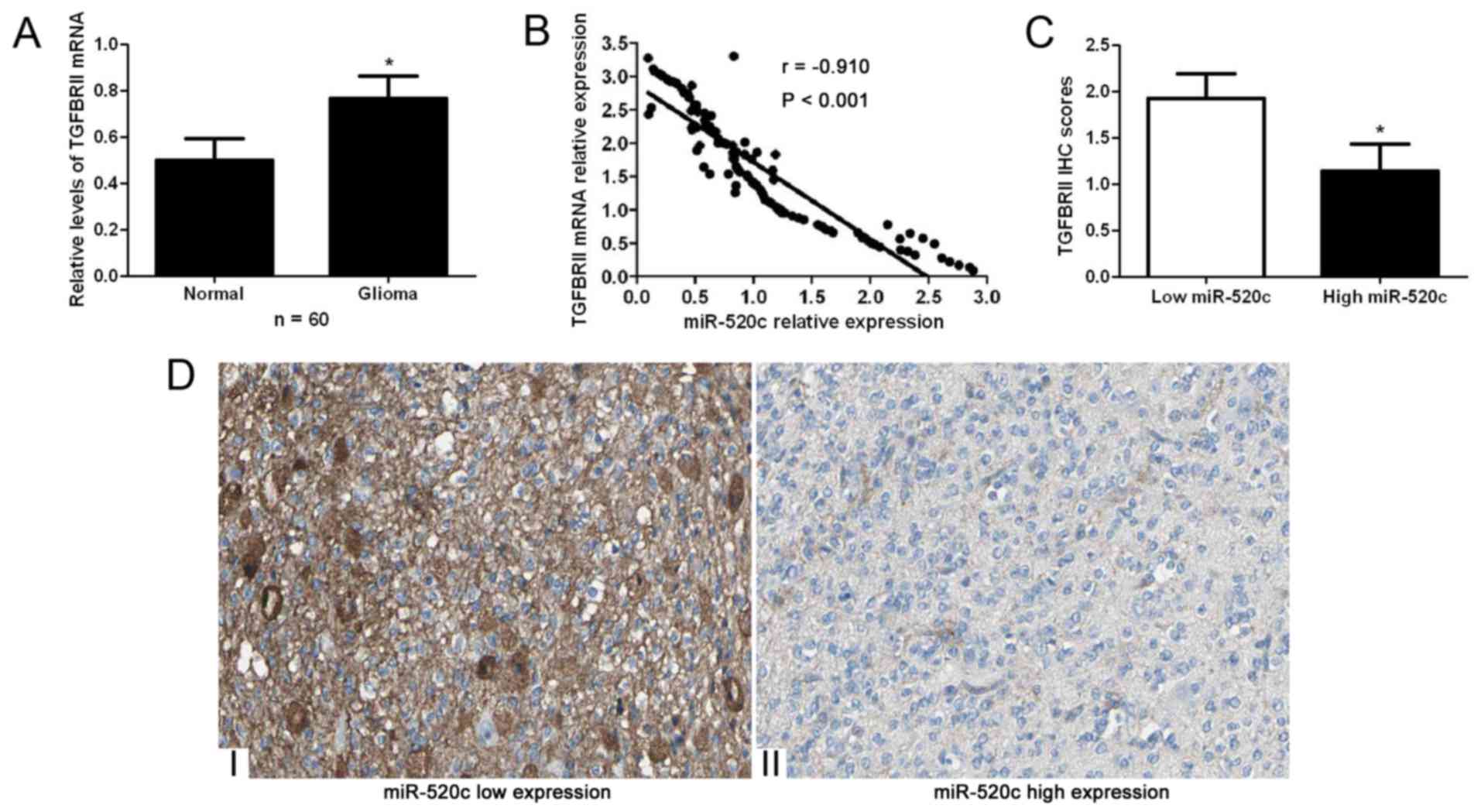
qRT-PCR for TGFBRII mRNA. Our data indicated that the expression
levels of TGFBRII mRNA in glioma tissues were significantly higher
than those in adjacent non-cancerous tissues (P<0.05; Fig. 5A). Spearman's correlation analysis
indicated that miR-520c was strongly correlated with TGFBRII mRNA
expression in the glioma specimens (r=−0.910, P<0.001; Fig. 5B). Moreover, immunohistochemical
staining indicated that miR-520c high-expressing tumors showed weak
staining of TGFBRII, while miR-520c low-expressing tumors showed
strong staining of TGFBRII (P<0.05; Fig. 5C and D).
TGFBRII mediates the functions of
miR-520c in glioma cells
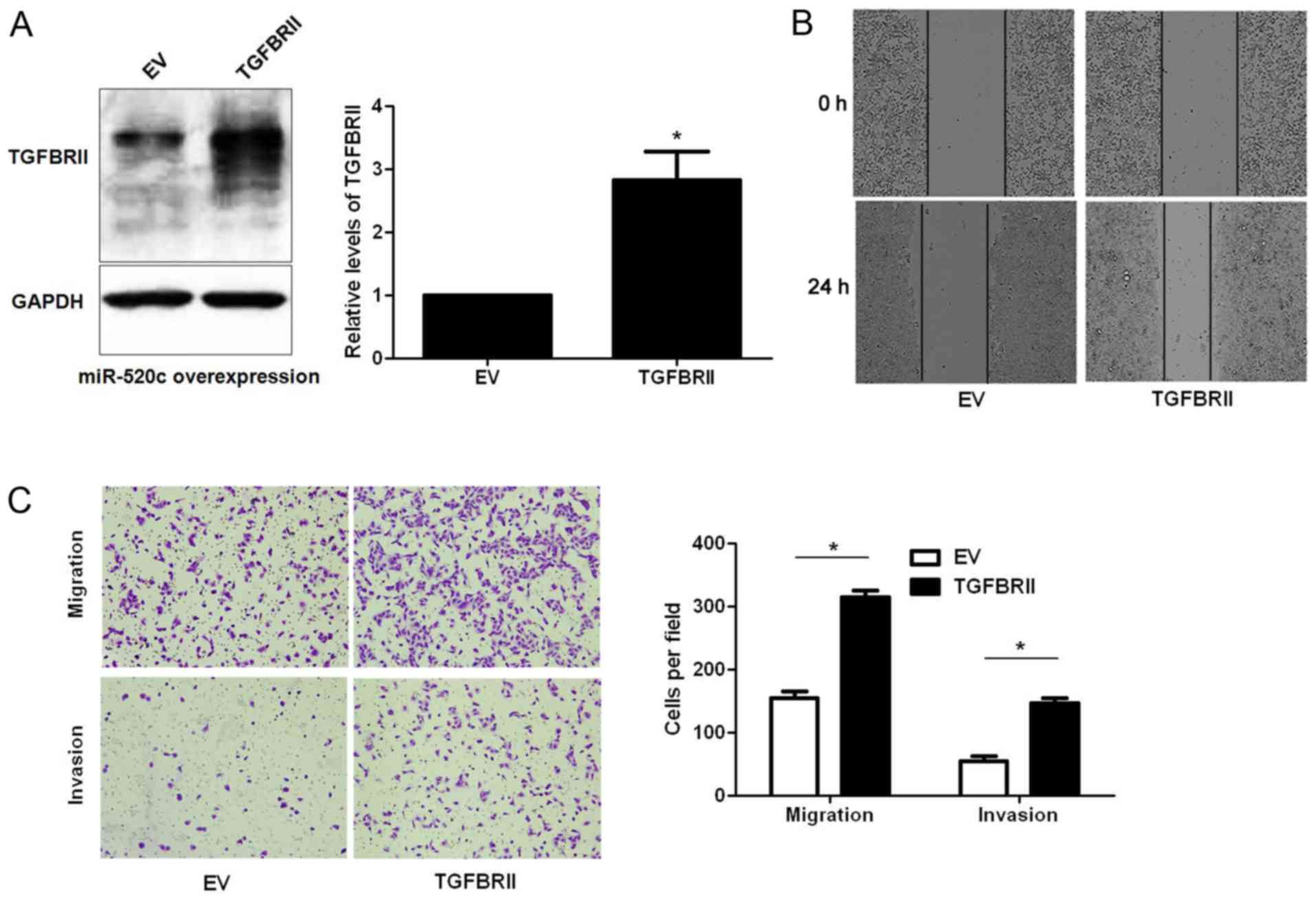
Next, U251 cells with miR-520c overexpression were
infected with empty vector (EV) or TGFBRII retroviruses.
Restoration of TGFBRII expression was confirmed by western blotting
(P<0.05; Fig. 6A). Wound healing
and Transwell assays indicated that TGFBRII restoration abrogated
the effects of miR-520c overexpression in U251 cells with increased
migratory and invasive abilities of glioma cells (P<0.05,
respectively; Fig. 6B and C). Since
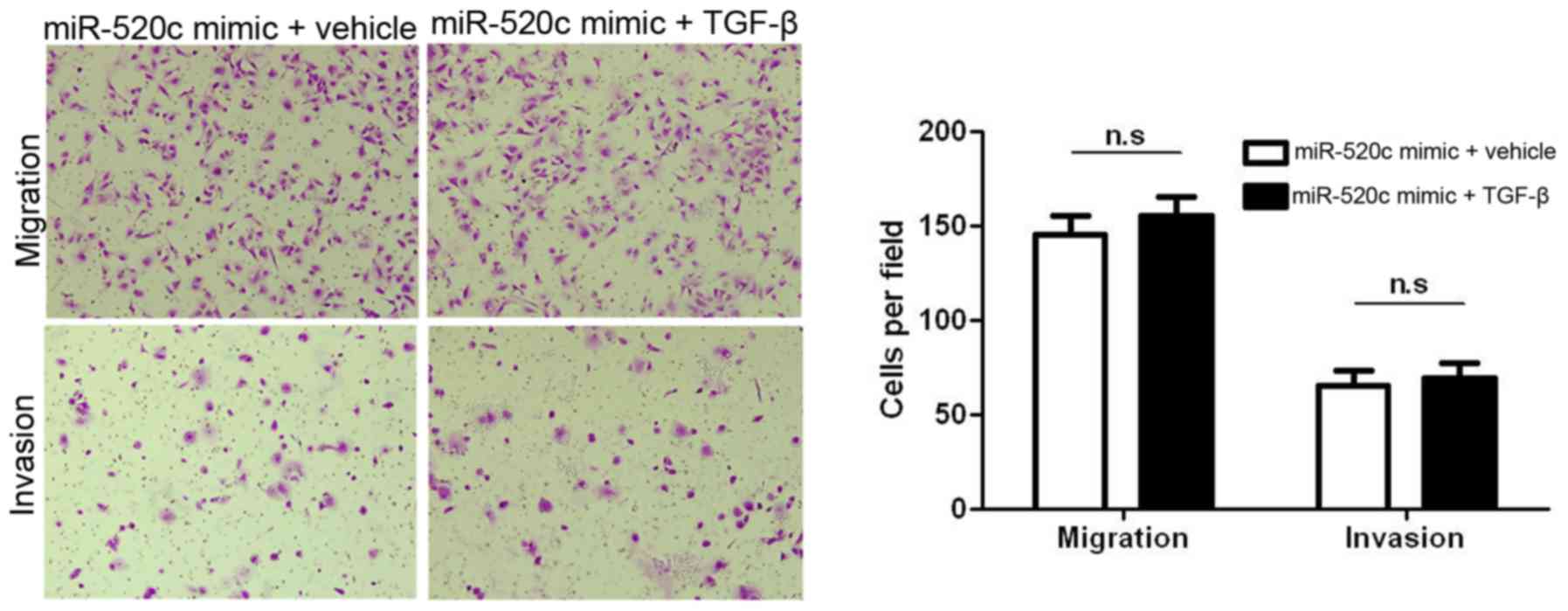
TGFBRII is a target of miR-520c, and TGFBRII is an important
component in TGF-β1-induced migration and invasion,
miR-520c-overexpressing U251 cells were treated with TGF-β1 (2
µg/ml). As expected, the induction of TGF-β1 showed no effect on
miR-520c-overexpressing U251 cells (Fig. 7). Collectively, TGBRII is not only a
target but also a functional mediator of miR-520c.
Discussion
Emerging evidence has confirmed that miRNAs are
actively involved in the pathogensis of glioma (18). In addition, miRNAs have been found
to be critical regulators of the metastasis and
epithelial-mesenchymal transition of glioma cells (19). Due to the important roles of miRNAs
in glioma, miRNAs have been proposed as promising biomarkers and
therapeutic targets of glioma (20). In the present study, miR-520c was
found to be significantly downregulated in glioma tissues. In
addition, the low expression of miR-520c in glioma tissues
conferred advanced WHO stage. More importantly, low expression of
miR-520c was correlated with decreased overall survival of glioma
patients. Therefore, miR-520c plays a tumor-suppressive role in
glioma and potentially serves as a promising biomarker for the
prognosis of glioma patients.
Systemic metastasis is the cause for the
unsatisfactory prognosis of glioma patients (21). Increased migratory and invasive
abilities of glioma cells underlie the systemic metastasis of
glioma (22). Therefore, it is of
great importance to elucidate the molecular mechanisms involved in
the metastasis of glioma cells. In the present study, we found that
miR-520c inhibited the migration and invasion of glioma cells in
vitro, suggesting that miR-520c exerted an antimetastatic role
in glioma. The TGF-β signaling pathway has been reported to be
implicated in tumor metastasis in various human malignancies
(23). The fundamental role of the
TGF-β pathway in promoting cancer progression in multiple stages of
the metastatic process, including epithelial-to-mesenchymal
transition (EMT), is also becoming increasingly clear (23). Notably, the TGF-β-SMAD pathway
confers poor prognosis to glioma patients and promotes cell
proliferation (24). TGFBRII is a
critical component of the TGF-β signaling pathway and TGFBRII
silencing blocked the effects of TGF-β1 in glioma cells (25). In the present study, we found that
miR-520c negatively regulated the abundance of TGFBRII in glioma
cells. In addition, the expression of TGFBRII in glioma tissues was
inversely correlated with miR-520c expression. Moreover, we found
that miR-520c directly interacted with the 3′-UTR of TGFBRII using
a luciferase reporter assay. These data indicate that TGFBRII is a
direct downstream target of miR-520c in glioma. Importantly,
restoration of TGFBRII abolished the effects of miR-520c
overexpression in glioma cells and miR-520c overexpression blocked
TGF-β1-induced migration and invasion of glioma cells. Thus,
TGFBRII is a functional mediator of miR-520c in glioma.
In conclusion, the present study demonstrated that
miR-520c expression was significantly decreased in glioma tissues.
Decreased expression of miR-520c was found to be correlated with
adverse clinical features and poor prognosis of glioma patients. In
addition, miR-520c inhibited migration and invasion of glioma
cells. Furthermore, TGFBRII is a direct functional target of
miR-520c in glioma. These data may result in the discovery of
therapeutic candidates of glioma.
References
|
1
|
Bartel DP: MicroRNAs: Target recognition
and regulatory functions. Cell. 136:215–233. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ambros V: The functions of animal
microRNAs. Nature. 431:350–355. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kloosterman WP and Plasterk RH: The
diverse functions of microRNAs in animal development and disease.
Dev Cell. 11:441–450. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zhang W, Dahlberg JE and Tam W: MicroRNAs
in tumorigenesis: A primer. Am J Pathol. 171:728–738. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Esquela-Kerscher A and Slack FJ: Oncomirs
- microRNAs with a role in cancer. Nat Rev Cancer. 6:259–269. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhang B, Pan X, Cobb GP and Anderson TA:
microRNAs as oncogenes and tumor suppressors. Dev Biol. 302:1–12.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wang BC and Ma J: Role of microRNAs in
malignant glioma. Chin Med J. 128:1238–1244. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wang H, Xu T, Jiang Y, Yan Y, Qin R and
Chen J: MicroRNAs in human glioblastoma: From bench to beside.
Front Biosci. 20:105–118. 2015. View
Article : Google Scholar
|
|
9
|
Brower JV, Clark PA, Lyon W and Kuo JS:
MicroRNAs in cancer: Glioblastoma and glioblastoma cancer stem
cells. Neurochem Int. 77:68–77. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Mazan-Mamczarz K, Zhao XF, Dai B,
Steinhardt JJ, Peroutka RJ, Berk KL, Landon AL, Sadowska M, Zhang
Y, Lehrmann E, et al: Down-regulation of eIF4GII by miR-520c-3p
represses diffuse large B cell lymphoma development. PLoS Genet.
10:e10041052014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Keklikoglou I, Koerner C, Schmidt C, Zhang
JD, Heckmann D, Shavinskaya A, Allgayer H, Gückel B, Fehm T,
Schneeweiss A, et al: MicroRNA-520/373 family functions as a tumor
suppressor in estrogen receptor negative breast cancer by targeting
NF-κB and TGF-β signaling pathways. Oncogene. 31:4150–4163. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Liu P and Wilson MJ: miR-520c and miR-373
upregulate MMP9 expression by targeting mTOR and SIRT1, and
activate the Ras/Raf/MEK/Erk signaling pathway and NF-κB factor in
human fibrosarcoma cells. J Cell Physiol. 227:867–876. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Toffanin S, Hoshida Y, Lachenmayer A,
Villanueva A, Cabellos L, Minguez B, Savic R, Ward SC, Thung S,
Chiang DY, et al: MicroRNA-based classification of hepatocellular
carcinoma and oncogenic role of miR-517a. Gastroenterology.
140:1618–1628.e16. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Huang Q, Gumireddy K, Schrier M, le Sage
C, Nagel R, Nair S, Egan DA, Li A, Huang G, Klein-Szanto AJ, et al:
The microRNAs miR-373 and miR-520c promote tumour invasion and
metastasis. Nat Cell Biol. 10:202–210. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lu S, Zhu Q, Zhang Y, Song W, Wilson MJ
and Liu P: Dual-functions of miR-373 and miR-520c by differently
regulating the activities of MMP2 and MMP9. J Cell Physiol.
230:1862–1870. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Tu K, Li J, Verma VK, Liu C, Billadeau DD,
Lamprecht G, Xiang X, Guo L, Dhanasekaran R, Roberts LR, et al:
Vasodilator-stimulated phosphoprotein promotes activation of
hepatic stellate cells by regulating Rab11-dependent plasma
membrane targeting of transforming growth factor β receptors.
Hepatology. 61:361–374. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ye XZ, Xu SL, Xin YH, Yu SC, Ping YF, Chen
L, Xiao HL, Wang B, Yi L, Wang QL, et al: Tumor-associated
microglia/macrophages enhance the invasion of glioma stem-like
cells via TGF-β1 signaling pathway. J Immunol. 189:444–453. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Xue H, Gao X, Xu S, Zhang J, Guo X, Yan S,
Li T, Guo X, Liu Q and Li G: MicroRNA-Let-7f reduces the
vasculogenic mimicry of human glioma cells by regulating
periostin-dependent migration. Oncol Rep. 35:1771–1777.
2016.PubMed/NCBI
|
|
19
|
Li J, Yuan J, Yuan X, Zhao J, Zhang Z,
Weng L and Liu J: MicroRNA-200b inhibits the growth and metastasis
of glioma cells via targeting ZEB2. Int J Oncol. 48:541–550.
2016.PubMed/NCBI
|
|
20
|
Fan B, Jiao BH, Fan FS, Lu SK, Song J, Guo
CY, Yang JK and Yang L: Downregulation of miR-95-3p inhibits
proliferation, and invasion promoting apoptosis of glioma cells by
targeting CELF2. Int J Oncol. 47:1025–1033. 2015.PubMed/NCBI
|
|
21
|
Usinskiene J, Ulyte A, Bjørnerud A, Venius
J, Katsaros VK, Rynkeviciene R, Letautiene S, Norkus D, Suziedelis
K, Rocka S, et al: Optimal differentiation of high- and low-grade
glioma and metastasis: A meta-analysis of perfusion, diffusion, and
spectroscopy metrics. Neuroradiology. 58:339–350. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Liu J, Zhang J, Huang L, Zhu X, Chen W and
Hu P: XuefuZhuyu Tang exerts antitumor effects by inhibiting glioma
cell metastasis and invasion via regulating tumor microenvironment.
Onco Targets Ther. 9:3603–3612. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Papageorgis P: TGFβ Signaling in tumor
initiation, epithelial-to-mesenchymal transition, and metastasis. J
Oncol. 2015:5871932015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Bruna A, Darken RS, Rojo F, Ocaña A,
Peñuelas S, Arias A, Paris R, Tortosa A, Mora J, Baselga J, et al:
High TGFβ-Smad activity confers poor prognosis in glioma patients
and promotes cell proliferation depending on the methylation of the
PDGF-B gene. Cancer Cell. 11:147–160. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wei F, Wang Q, Su Q, Huang H, Luan J, Xu X
and Wang J: miR-373 inhibits glioma cell U251 migration and
invasion by down-regulating CD44 and TGFBR2. Cell Mol Neurobiol.
36:1389–1397. 2016. View Article : Google Scholar : PubMed/NCBI
|