Introduction
Colon cancer has a high incidence and mortality
rate, and it is the second most common malignancy in the Western
world (1). Despite recent
improvements in treatments, early diagnostic techniques are still
required, and tumor cell metastasis and postoperative recurrence
remain challenges. Thus, early detection and diagnosis is vital for
prognosis, and has great potential to reduce the burden of this
disease (2). Long non-coding RNAs
(lncRNAs) are a class of RNAs whose transcripts are over 200
nucleotides in length, which do not have a protein coding capacity
(3). Studies have shown the
diversity and complexity of lncRNA functions and mechanisms. The
functions of lncRNAs have been ascertained in many cancers,
including gastric cancer, hepatocellular carcinoma, cervical and
lung cancer (4–9). For example, increased lncRNA-ATB
[lncRNA activated by transforming growth factor β (TGF-β)], a
mediator of TGF-β signaling, induced epithelial-mesenchymal
transition (EMT) and invasion, and indicated hepatocellular
carcinoma metastases and poor prognoses, in addition to promoting
organ colonization of disseminated tumor cells (4). In non-small cell lung cancer (NSCLC),
smoking history, infiltration degree, lymph node metastasis, and
distant metastasis are all related with the expression of lncRNA
AFAP1-AS1, and a high expression of lncRNA AFAP1-AS1 may be
associated with the short survival times of NSCLC patients
(6). Dysregulation and aberrant
expression are important causes of tumorigenesis, and promote
relevant tumor progression. Notably, the roles that lncRNAs play
are vital in nearly every aspect of tumor biology. However, the
clinical prognostic significance and potential functions of lncRNAs
are still not understood in colon tumorigenesis.
The lncRNA small nucleolar RNA host gene 1 (SNHG1)
(Gene ID: 23642, HGNC: 32688, MIM: 603222, Location: 11q12.3, Exon
count: 11) has been reported to play a role in cancer
carcinogenesis. Cui et al revealed that in NSCLC, SNHG1
indicated a poor prognosis and promoted NSCLC development via the
SNHG1/miR-101-3p/SOX9/Wnt/β-catenin axis (10). Yan et al further revealed
that SNHG1 directly bound miR-338 and promoted esophageal carcinoma
cell growth by alleviating cell apoptosis of CST3 cells caused by
miR-338 (11). Li et al
determined that SNHG1 acted as a competing endogenous (ce) RNA for
miR-199a-3p in prostate cancer, by inhibiting the activity of
miR-199a-3p, and reducing the suppression of CDK7 by miR-199a-3,
thus promoting cell proliferation and cell cycle progression in
prostate cancer (12). However,
studies in colon cancer on SNHG1 expression, biological function,
and tumor correlation mechanisms are few. We referred to the
Oncomine database, and determined the expression of SNHG1 in human
normal colon tissues, cancerous colon tissues, and colon cancerous
cell lines. We then used cell function tests to identify potential
molecular mechanisms in cells before and after knockdown of SNHG1,
to determine if SNHG1 influenced colon cancer cell proliferation,
apoptosis, migration, and invasion. In addition, possible pathways
involved in the mechanism of colon cancer carcinogenesis were
suggested.
Materials and methods
Database and patient tissue
samples
Oncomine is a bioinformatics database of abundant
DNA microarrays used in collecting, standardizing, and as an
analyzing platform, aimed at facilitating the discovery of the
functions from genome-wide expression analysis (13,14).
We used the Oncomine database (www.oncomine.org) to identify differentially expressed
genes between colon cancer tissues and normal tissues. By searching
‘Gene: SNHG1’; ‘Analysis Type: cancer vs. normal analysis’; ‘Cancer
Type: colorectal cancer’; and setting ‘P value: all’; ‘Fold Change:
all’; ‘Gene rank: all’; ‘Sample Type: Clinical Specimen’; and ‘Data
Type: mRNA’, we obtained seven useable studies as follows: three
out of seven related to the colon, and 1,352 samples in total. We
analyzed SNHG1 differential expression in these three datasets
between normal and colon cancer tissues, then used GraphPad Prism 5
(GraphPad Software, Inc., La Jolla, CA, USA) and SPSS version 19.0
(SPSS, Inc., Chicago, IL, USA) to analyze the statistical
differences. We also randomly acquired 13 colon cancer patient
tissues, who underwent surgical treatment at the First Affiliated
Hospital of Chongqing Medical University from January 2015 to
December 2016. None of the patients involved in this study had
undergone radiation or chemotherapy prior to surgery. Every excised
colon tissue, no matter if cancerous or adjacent normal colon
tissue, was immediately stored in liquid nitrogen, and saved at the
Chongqing Medical University Laboratory. The histopathological
features of cells in these tissue samples were observed by
pathologists at Chongqing Medical University who used standard
methods to diagnose colon cancer. There were no obvious tumor cells
in adjacent non-cancerous tissues, which were included as standard
control samples. The use of the human tissues was approved by the
Ethics Committee of Chongqing Medical University (Chongqing,
China).
Cell culture
The colon cancer cell lines HCT-116, Caco-2 and
HT-29, were obtained from the Molecular Tumor and Epigenetics
Laboratory at the First Affiliated Hospital of Chongqing Medical
University. All the cell lines were cultured in RPMI-1640 medium
(Hyclone™; GE Healthcare Life Sciences, Logan, UT, USA)
supplemented with 10% fetal bovine serum (FBS; Gibco; Thermo Fisher
Scientific, Inc., Grand Island, NY, USA), 100 mg/ml streptomycin
and 100 µg/ml penicillin (both from Beyotime Institute of
Biotechnology, Beijing, China), in an incubator at 37°C with 5%
CO2. The medium was changed each day and cells were
subcultured every 2–3 days.
RNA extraction, reverse transcription
and real-time quantitative PCR (RT-qPCR)
RNAiso Plus (Takara Bio USA, Inc., Mountain View,
CA, USA) was used to isolate total RNA from all tissues and cell
lines following the manufacturer's instructions. All RNA
concentrations and purities were examined with ultraviolet
spectrometry (ND-1000 spectrophotometer; NanoDrop Technologies,
Wilmington, DE, USA). The reverse-transcribed extracted RNA was
produced from cDNA using the GoScript™ Reverse Transcription System
(Promega Corp., Sunnyvale, CA, USA) according to the manufacturer's
instructions, and RT-qPCR was used to compare SNHG1 expression.
RT-qPCR primer sequences were designed by Takara Bio, Inc. (Shiga,
Japan) as follows: SNHG1: 5′-TTGCTGCCTTTCTTACATGATCC-3′ (sense
primer), 5′-AGACACGAAGTGGAGTTATGGGAA-3′ (antisense primer). β-actin
was employed as an internal control. The β-actin primer sequences
were 5′-TCCTGTGGCATCCACGAAACT-3′ (sense primer) and
5′-GAAGCATTTGCGGTGGACGAT-3′ (antisense primer). The concentration
of primers was 10 µmol/l. The RT-qPCR steps were in accordance to
the standard SYBR-Green (BioTool, Kirchberg, Switzerland)
instructions and were performed on a Real-Time PCR System (Applied
Biosystems, Foster City, CA, USA). The thermal cycler protocol was
as follows: step 1, repeated once, 50°C for 2 min; step 2, repeated
once, 95°C for 10 min; step 3, repeated 40 times, 95°C for 15 sec,
and 60°C for 1 min; step 4, repeated once, 95°C for 15 sec, 60°C
for 1 min and 95°C for 15 sec.
Plasmid construction, transfection,
G418 selection
To assess if SNHG1 influenced colon cancer cell
biological functions, we acquired plasmid pRNAT-U6.1/Neo-vector and
pRNAT-U6.1/Neo-sh-SNHG1, which were synthesized by Guangzhou
RiboBio, Co., Ltd. (Guangzhou, China), and transfected them into
colon cancer cells. Sequences were as follows:
GATCCCGAGGACATCAGAAGGTGAATTGATATCCGT TCACCTTCTGATGTCCTCTTTTTTCCAAA
(forward) and AGCTTTTGGAAAAAAGAGGACATCAGAAGGTGAAC
GGATATCAATTCACCTTCTGATGTCCTCGG (reverse). Regarding the
transfections, we plated the cells (Caco-2 and HCT-116 cells) into
6-well plates; transfection was performed at 40% cell density, at
room temperature. Initially 5 µl Lipofectamine™ 2000 reagent
(Invitrogen; Thermo Fisher Sientific, Shanghai, China) was mixed
with 500 µl RPMI-1640 medium (Hyclone; GE Healthcare Life Sciences)
for 5 min, then 4 µg plasmid (vector or sh-SNHG1) (Guangzhou
RiboBio, Co., Ltd.) with 500 µl RPMI-1640 was added for 5 min. Two
500-µl samples were added to 1 ml of the mixture for 20 min, then 1
ml of RPMI-1640 medium was added to a total 2 ml final volume. The
samples were added into every well at 40% cell density. After
culturing the dilution at 37°C containing 5% CO2 for 4–6
h, 2 ml of medium with 10% FBS was used to replace the medium.
Then, 48 h later, we used fluorescence microscopy to observe
plasmid transfection efficiency. When the efficiency reached
30–40%, G418 (Amresco, LLC, Solon, OH, USA) was added into the
medium (G418 at 300 µg/ml for Caco-2 cells and 400 µg/ml for
HCT-116 cells) to select stably transfected cells. The medium
containing G418 was changed every 3 days until the non-transfected
cells died. To further confirm that the plasmids were successfully
transfected into colon cancer cells after G418 selection, RT-qPCR
was performed to detect SNHG1 differential expression between the
vector group and the sh-SNHG1 cell group. The RT-qPCR in this step
was performed as aforementioned.
Cell proliferation assays [Cell
Counting Kit-8 (CCK-8) and clone formation assays]
To assess cell proliferation before and after SNHG1
knockdown, the cells (HCT-116 and Caco-2) were divided into the
HCT-116-vector, HCT-116-sh-SNHG1, Caco-2-vector and Caco-2-sh-SNHG1
groups. They were plated at a density of 5×103
cells/well in triplicate in 96-well plates. The cells were all
stably transfected. After culturing at 0, 24, 48 and 72 h, 10 µl of
CCK-8 reagent (Dojindo Molecular Technologies, Rockville, MD, USA),
was added to each well for 2 h and the OD (optical density) at 450
nm was recorded for growth density. The absorbances at 0, 24, 48
and 72 h were plotted. By comparing each point-in-time OD value the
statistical differences between the vector group and sh-SNHG1
groups were identified.
In another colony formation assay study, the cells
(HCT-116 and Caco-2 cells) were divided into the HCT-116-vector,
HCT-116-sh-SNHG1, Caco-2-vector and Caco2-sh-SNHG1 groups. Each
cell group was plated at 5×102 cells/well in 6-well
plates, and cultured in RPMI-1640 medium with 10% FBS. G418 was
added (G418 300 µg/ml for Caco-2 and 400 µg/ml for HCT-116 cells)
and exchanged for fresh medium every 3 days until single colonies
(≥50 cells) formed. The number of colonies in each group was
counted by eyes and ImageJ version 2.1.4.7 software (National
Institutes of Health, Bethesda, MD, USA) after fixing with
paraformaldehyde and staining with crystal violet. We conducted
statistical analyses to compare the proliferation differences
between the vector and experimental cell groups.
Flow cytometric analyses of the cell
cycle and apoptosis assays
Stably transfected HCT-116 and Caco-2 cells were
cultured at 37°C in 5% CO2 for 48–72 h until 70%
confluence, then harvested with trypsin without EDTA, harvested by
centrifugation (120 × g, 5 min), resuspended in 1 ml of
phosphate-buffered saline (PBS), and 5×106 cells from
each group were used. Binding buffer (500 µl) was used to resuspend
the cells, then 5 µl of Annexin V-fluorescein isothiocyanate
(Annexin V-FITC) and 5 µl of propidium iodide were added and
incubated without light at room temperature for 5–15 min, and
finally analyzed by flow cytometry. The Annexin V-EGFP Apoptosis
Detection kit was purchased from Beyotime Institute of
Biotechnology.
Transwell® migration and
invasion assays
The migration ability of the cells (HCT-116 and
Caco-2 cells) was assessed using 24-well Transwell (Corning 3422;
Corning Inc., Corning, NY, USA) plates. Medium (700 µl) containing
10% FBS was added into the lower chamber, and 200 µl of serum-free
medium with 6×104 transfected cells were added to the
upper chamber. After 48 h, the cells still remaining in the upper
chamber were discarded; cells migrating to the lower chamber were
fixed with paraformaldehyde and stained by hematoxylin and
eosin.
The invasion assays required the stimulation of
cancer cells to migrate in the 24-well Transwell plates. Therefore,
Matrigel (BD Biosciences, San Jose, CA, USA) was diluted 1:7 with
serum-free medium at 4°C according to the manufacturer's
instructions. Then, 100 µl of the diluted Matrigel was added into
the upper chamber at 37°C until the Matrigel had solidified.
Subsequently, 700 µl of medium containing 10% FBS was added to the
lower chamber, and 100 µl of serum-free medium with 8×104 stably
transfected cells were added to the upper chamber (on the surface
of the Matrigel). Each sample was plated in triplicate. After
incubation for 48 h, the stained cells were counted under a light
microscope (magnification, ×200).
Western blotting
RIPA lysis buffer and phenylmethanesulfonyl fluoride
were used to extract cell proteins, and the protein concentrations
were determined using a BCA protein assay kit (all from Beyotime
Institute of Biotechnology). Protein (40 µg) was separated by
electrophoresis (5% stacking gel and 12% separating gel) and
transferred onto PVDF membranes (EMD Millipore, Billerica, MA,
USA). Non-specific binding was blocked with 5% evaporated milk and
the membranes were reacted with primary antibodies overnight at
4°C. The primary antibodies were all obtained from Cell Signaling
Technology (Danvers, MA, USA) and included anti-E-cadherin (1:500
dilution; cat. no. 3195), anti-β-catenin (1:500 dilution; cat. no.
8480), anti-cyclin D1 (1:500 dilution; cat. no. 2978) and
anti-c-Myc (1:500 dilution; cat. no. 13987). The secondary
antibodies (1:3,000 dilution; cat. no. 7074; Cell Signaling
Technology) were incubated for 2 h. They were decided by the
corresponding primary antibodies. After washing the blots with TBST
three times, the target proteins were detected using BeyoECL Plus
reagent (Beyotime Institute of Biotechnology, Shanghai, China)
using a FUSION FX imager instrument (Vilber Lourmat,
Marne-la-Vallée, France). The electrophoresis and blotting were
assessed on the basis of each target protein's molecular
weight.
Immunohistochemistry
We obtained 20 paired colon cancer and corresponding
normal paraffin sections from Chongqing Medical University from
January 2015 to December 2016. Sections were placed in an oven at
60°C for 2 h, dewaxed with water and rinsed with PBS (pH 7.4) 3
times. Then, the sections were dehydrated through a series of
concentration gradient ethanol (100, 95 90, 80 and 70%).
Subsequently, we added 3% hydrogen peroxide to each section and
incubated the sections for 10 min at room temperature. Then, the
sections were rinsed with PBS. The primary mouse monoclonal
antibody E-cadherin (1:100 dilution; cat. no. WL00941), β-catenin
(1:100 dilution; cat. no. WL0962a), cyclin D1 (1:100 dilution; cat.
no. WL01435a) and c-Myc (1:100 dilution; cat. no. WL01781) all
purchased from Wanleibio (Beijing, China) were added to sections
and incubated at 4°C overnight. The following morning, we washed
the sections 3 times with PBS, and the corresponding secondary
antibody (80 µl) was added to each section. The sections were then
incubated for 30 min at room temperature. 3,3′-Diaminobenzidine
tetrahydrochloride (DAB) was used to stain the sections and
hematoxylin was used to counterstain the cell membrane and
cytoplasm for 15 sec. The sections were then immediately rinsed
with water. Finally, the sections were treated with a series of
gradient alcohol, xylene and neutral gum in order to be dehydrated
and sealed. We used PBS instead of a primary antibody as a blank
control, and known positive staining as a positive control.
Immunohistochemical staining of the sections was observed under
light microscope by three pathologists independently.
Assessment of
immunohistochemistry
The evaluations were as follows: i) β-catenin:
strong nuclear staining and cytoplasmic staining of >10% of
cells was considered aberrant positive expression in colon cancer
tissues (15); ii) E-cadherin: a
membranous stain of >60% of cells was considered to be a score
3+; a score 2+ was considered as a stain of 20–60% of cells; a
stain of <20% of cells was considered as a score 1+; a score 0
was used for a 0% stain. A score of 3+ was classified as having
positive expression, and all others were classified as having
decreased expression (16); iii)
c-Myc: strong nuclear staining of >25% of cells was considered
positive expression (17); iv)
cyclin D1: nuclear staining of >5% of cells was considered as
positive expression, while negative expression was considered as a
stain of <5% of cells (18).
Statistical analysis
We used SPSS, version 19.0 software (IBM Corp.,
Armonk, NY, USA), ImageJ version 2.1.4.7 software (National
Institutes of Health, Bethesda, MD, USA) and GraphPad Prism version
5.0 software (GraphPad Software, Inc., La Jolla, CA, USA) to
perform statistical analyses. The independent samples t-test was
applied to intra-group differentiation. The Chi-squared test
analysis was used to assess clinicopathological relationships.
Repeated measures analysis of variance (ANOVA) was used for several
groups. P<0.05 was considered to indicate a statistically
significant difference.
Results
SNHG1 expression is upregulated in
colon cancer tissues compared to adjacent non-cancerous tissues,
and is higher in colon cancer cells than in normal cells
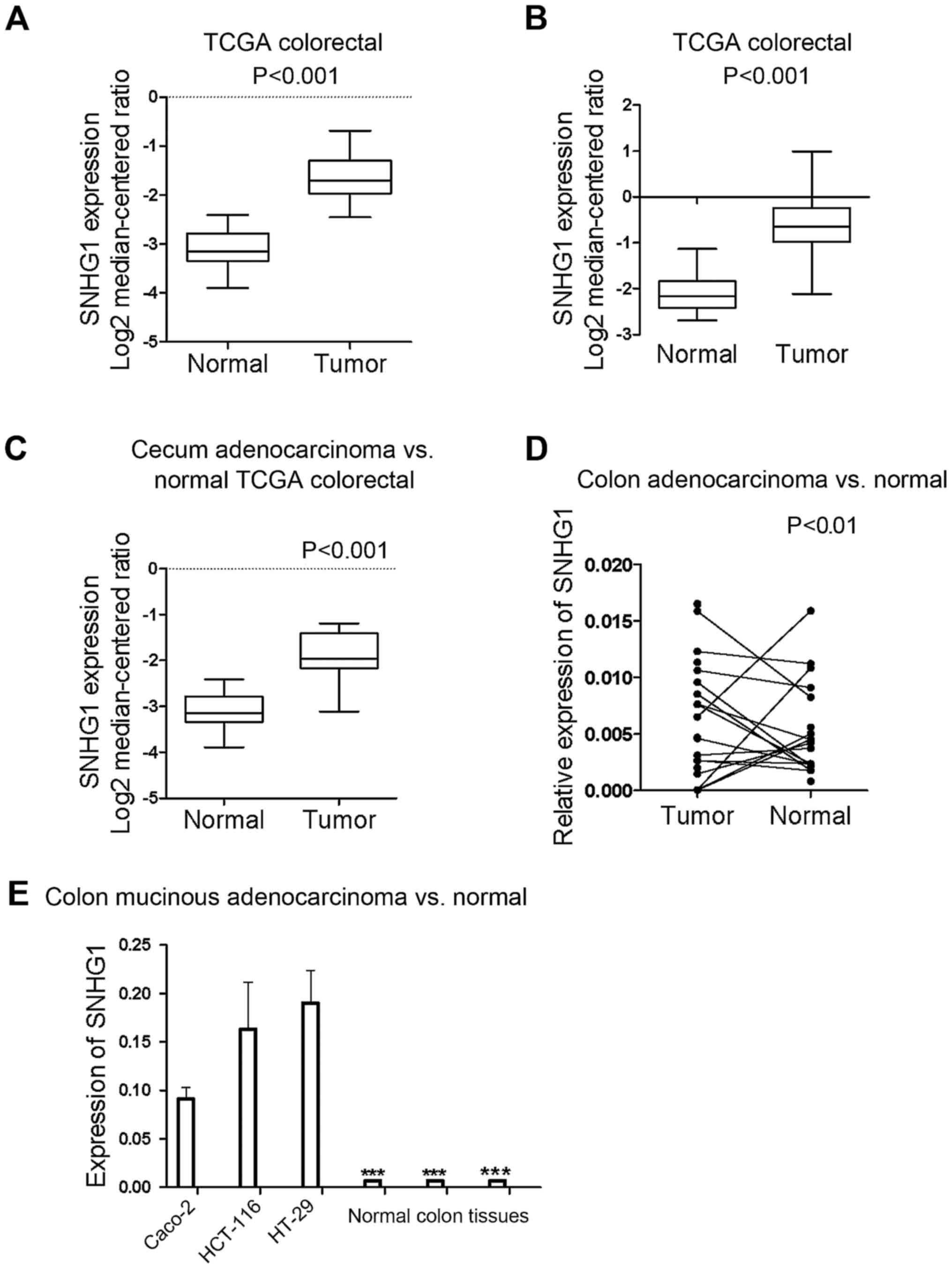
All the samples identified from the Oncomine
database revealed that the expression of SNHG1 in colon cancer
tissues was significantly higher than in normal colon tissues
(P<0.05; P-value for the median-ranked analysis: 2.31E-19). The
sample microarrays of three datasets (TCGA colorectal: cecum
adenocarcinoma vs. normal; colon adenocarcinoma vs. normal; and
colon mucinous adenocarcinoma vs. normal) exhibited respective fold
changes between cancer and normal tissues of 2.697-fold (Fig. 1A; P<0.001), 2.806-fold (Fig. 1B; P<0.001), and 2.241-fold
(Fig. 1C; P<0.001),
respectively. All the dataset microarray results revealed that
SNHG1 expression in colon cancer samples was at least 2-fold higher
than the normal sample levels.
To further ascertain SNHG1 expression differences in
patient tissues, RT-qPCR was used to examine SNHG1 expression in 13
pairs of colon cancer patient tissues compared with adjacent
non-cancer tissues. Since SNHG1 belongs to lnc-RNAs and does not
encode a protein, in tissues and cells it can be measured and
analyzed by RT-qPCR. The results revealed that except for 2 colon
cancer patients, all of the colon cancer cases indicated a
significantly higher expression of SNHG1 in cancer tissues compared
with adjacent non-cancer tissues (Fig.
1D; P<0.01). Table I
contains the clinicopathological characteristics of the 13 patients
with colon cancer.
 | Table I.Clinicopathological characteristics
of the 13 patients with colon cancer. |
Table I.
Clinicopathological characteristics
of the 13 patients with colon cancer.
|
| Sex | Age | Weight | Smoking | TNM stage |
Differentiation |
|---|
|
|
|
|
|
|
|
|
|---|
|
| Female | Male | (years) | (kg) | Yes | No | I | II | III | IV | Middle | Middle-low | Low |
|---|
| Number | 7/13 | 6/13 | 56±14 | 55±7 | 4/13 | 9/13 | 0 | 7 | 4 | 2 | 9 | 3 | 1 |
The differences in the expression of SNHG1 not only
existed in the tissues, but also in different colon cancer cell
lines. RT-qPCR was performed on three colon cancer cell lines
(HCT-116, HT29 and Caco-2) and three control colon tissue sample.
We found that SNHG1 was upregulated in HCT-116, Caco-2 and HT-29
cells compared with the human normal colon tissue (Fig. 1E; P<0.001).
In summary, SNHG1 was upregulated in colon cancer
tissues and cell lines compared to normal tissues and cells. SNHG1
may therefore be an oncogene playing a role in the promotion of
colon cancer development, as has also been reported in NSCLC and
prostate cancer.
Recombinant plasmid is successfully
transfected
The transfection of recombinant plasmids into colon
cancer cells was used to observe if SNHG1 downregulation influenced
cell biological functions. To verify transfection success,
fluorescence cell counting was used to examine plasmid transfection
efficiency, and the results revealed ~30–40% efficiency (Fig. 2). We added G418 to select stably
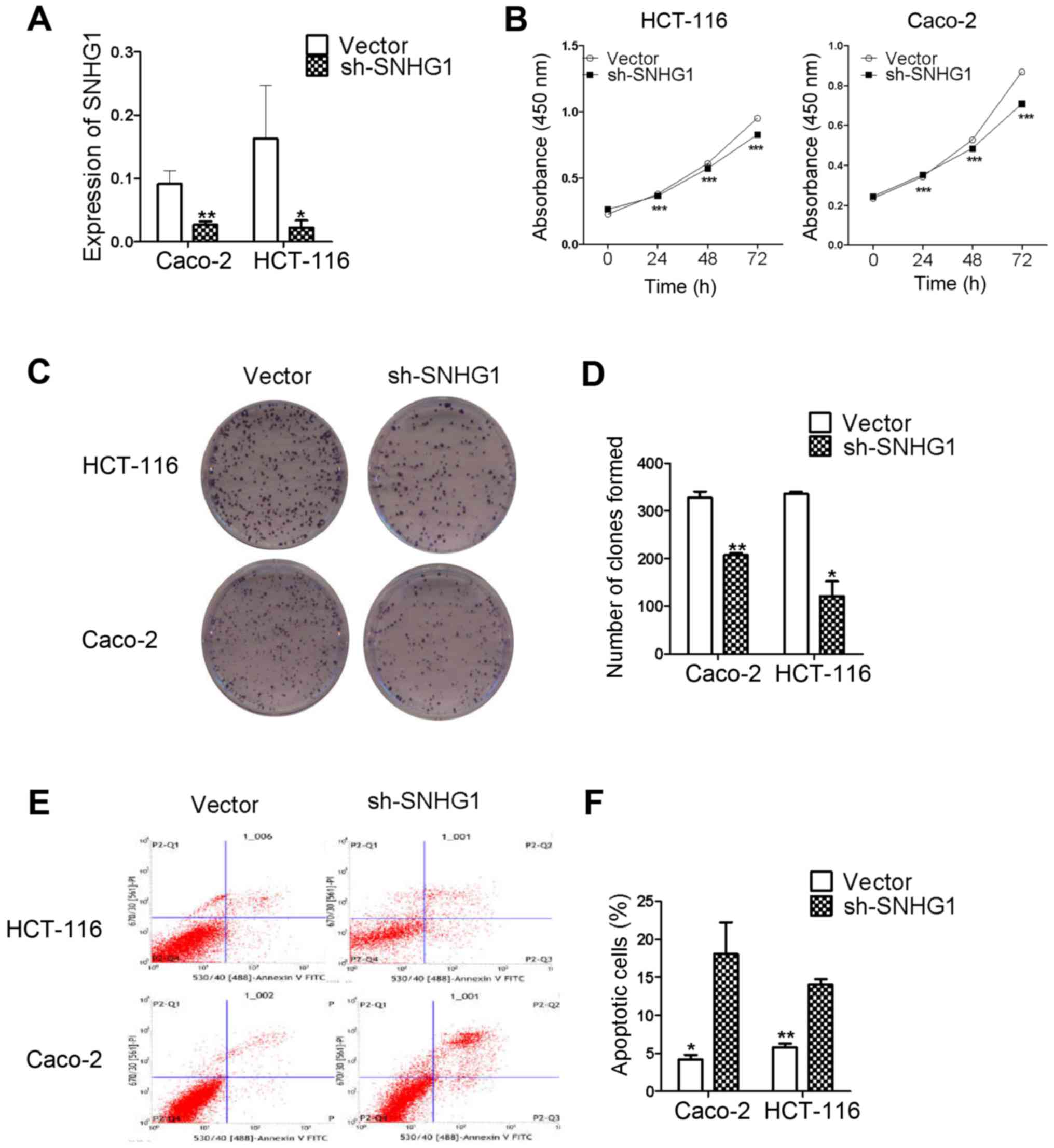
transfected cells, and RT-qPCR detected plasmid transfection in the
stably transfected cells. The results revaled that SNHG1 was
significantly decreased in Caco-2 and HCT-116 cells transfected
with pRNAT-U6.1/Neo-sh-SNHG1 when compared with the
pRNAT-U6.1/Neo-vector transfected cells (Fig. 3A; Caco-2, P<0.01; HCT-116,
P<0.05). These results indicated that recombinant plasmid
transfection decreased SNHG1.
CCK-8 and colony formation assays
reveal that SNHG1 promotes colon cancer cell proliferation
The CCK-8 and colony formation assay results
confirmed that SNHG1 influenced CaCo-2 and HCT-116 cell
proliferation in the sh-SNHG1 transfected group. The CCK-8 results
presented in Table II, revealed
that cell proliferation was slower at 24, 48 and 72 h in the
sh-SNHG1 group compared to the vector group. The results were also
revealed in Fig. 3B for both Caco-2
and HCT-116 cells (Caco-2, P<0.001; HCT-116, P<0.001).
 | Table II.Effect of recombinant plasmids on
cell proliferation. |
Table II.
Effect of recombinant plasmids on
cell proliferation.
| A, Effect of
recombinant plasmids on HCT-116 cell proliferation (means ±
SD) |
|---|
|
|---|
| Time (h) | Vector group | sh-SNHG1 group |
|---|
| 0 | 0.2277±0.0020 | 0.2644±0.0015 |
| 24 | 0.3796±0.0123 | 0.3655±0.0193 |
| 48 | 0.6093±0.0179 | 0.5722±0.0571 |
| 72 | 0.9510±0.0089 | 0.8267±0.0136 |
|
| B, Effect of
recombinant plasmids on Caco-2 cell proliferation (means ±
SD) |
|
| Time
(h) | Vector
group | sh-SNHG1
group |
|
| 0 | 0.2341±0.0091 | 0.2438±0.0057 |
| 24 | 0.3420±0.0112 | 0.3514±0.0051 |
| 48 | 0.5264±0.0131 | 0.4845±0.0146 |
| 72 | 0.8685±0.0106 | 0.7076±0.0260 |
The colony formation assay results further
ascertained the proliferation abilities of Caco-2 and HCT-116
cells. Fig. 3C revealed the colony
formation results of the vector and sh-SNHG1-transfected groups in
the two cell lines. Similar to the results presented in Fig. 3D, the Caco-2 vector group vs. the
sh-SNHG1 group was 328±23 vs. 207±6 (P<0.01), and the HCT-116
vector group vs. the sh-SNHG1 group was 336±7 vs. 122±54
(P<0.05), respectively, which confirmed that colon cancer cell
proliferation was decreased in the sh-SNHG1 group. The results from
both assays demonstrated that SNHG1 played a role in promoting
colon cancer cell proliferation.
Flow cytometry: the effects of SNHG1
on cell apoptosis
Flow cytometric analyses were used to identify
whether SNHG1 was associated with colon cancer cell apoptosis. As
revealed in Fig. 3E and F, the
apoptosis level in the Caco-2 vector group vs. the sh-SNHG1 group
was 4.18±1.03 vs. 18.07±7.19 (P<0.05), and the HCT-116 vector
group vs. the sh-SNHG1 group was 5.78±0.83 vs. 14.08±1.14
(P<0.01). These data further demonstrated that SNHG1 reduced
colon cancer cell apoptosis in both HCT-116 and Caco-2 cells.
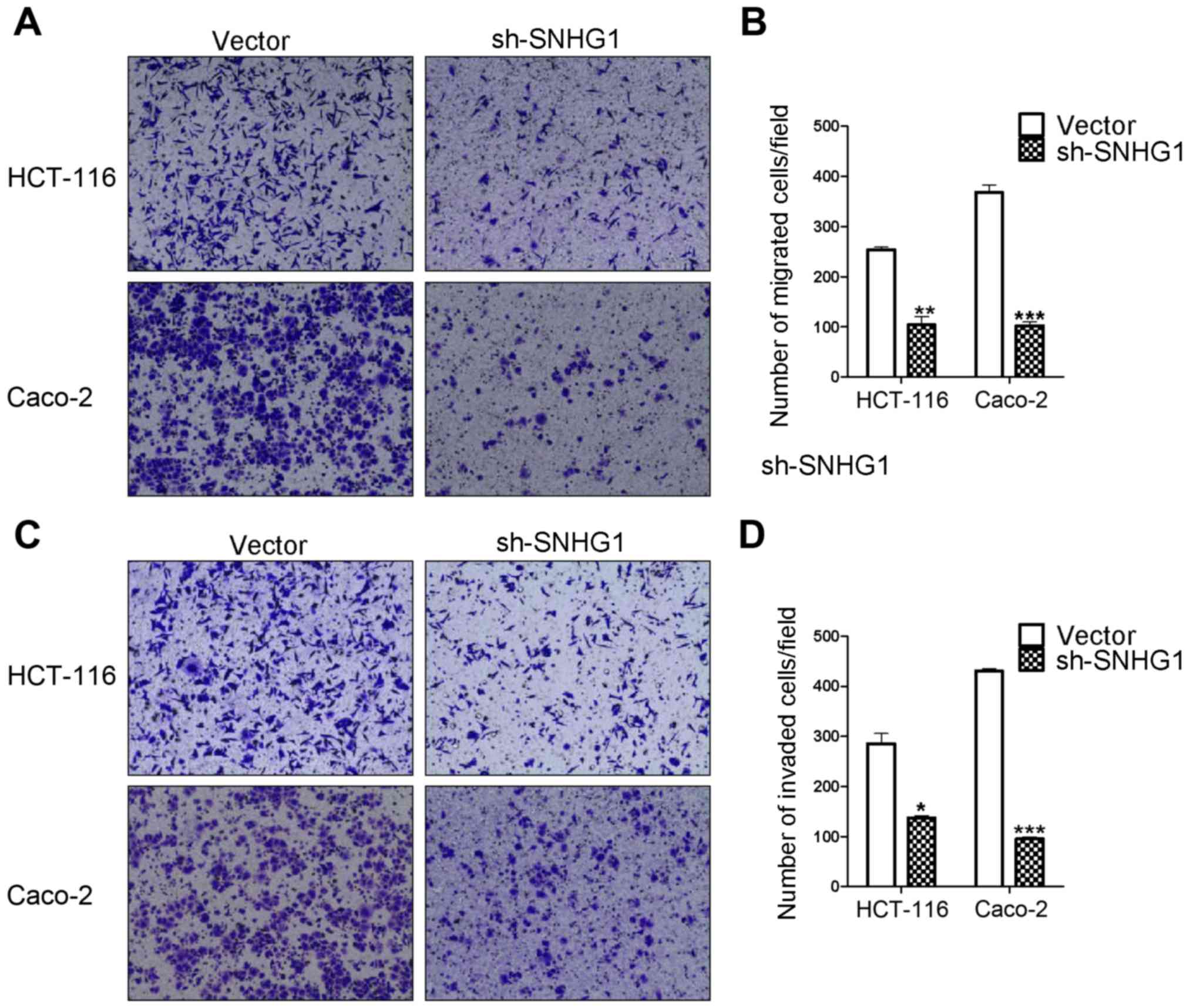
Transwell assay results reveal that
SNHG1 promotes colon cancer cell migration and invasion
abilities
Transwell migration and invasion assays are based on
the medium concentration difference between the upper and lower
side of the chambers. Cells migrate from a nutrient-poor side
chamber to a nutrient-rich side chamber (the lower side). The
migration assay results revealed that the HCT-116 sh-SNHG1 group
cell counts (104±29 cells) were significantly decreased compared to
the HCT-116 vector group (254±8 cells) (P<0.01), in addition,
the cell counts for the Caco-2 sh-SNHG1 group were 102±13 vs. the
CacCo-2 vector group 368±25 (P<0.001). The invasion experiment
results from the HCT-116 sh-SNHG1 group vs. the vector group were
137±8 cells vs. 285±35 (P<0.05) and the CacCo-2 sh-SNHG1 group
vs. the vector group were 95±7 vs. 430±9 (P<0.001). These
results were in agreement with the images displayed in Fig. 4A-D, which revealed that HCT-116- and
Caco-2-vector transfected cells had greater migration and invasion
abilities than the sh-SNHG1-transfected cells, and further
suggested the important carcinogenenic role of SNHG1 in colon
cancer cell migration and invasion.
Key markers of SNHG1 in cell
metastatic diffusion and proliferation
The Transwell results confirmed that SNHG1 could
facilitate colon cancer cell invasion and migration. The CCK-8 and
colony assays revealed that SNHG1 could promote colon cancer cell
proliferation. We next performed immunohistochemistry and western
blot analysis to explore the underlying mechanisms. E-cadherin,
β-catenin, cyclin D1 and c-Myc proteins were detected in these
experiments. The immunohistochemistry experiment revealed that the
expression of β-catenin, cyclin D1 and c-Myc in colon cancer
tissues was significantly higher than that in normal tissues, while
E-cadherin expression was lower in colon cancer tissues compared to
normal tissues (Fig. 5; Table III). In addition, western blotting
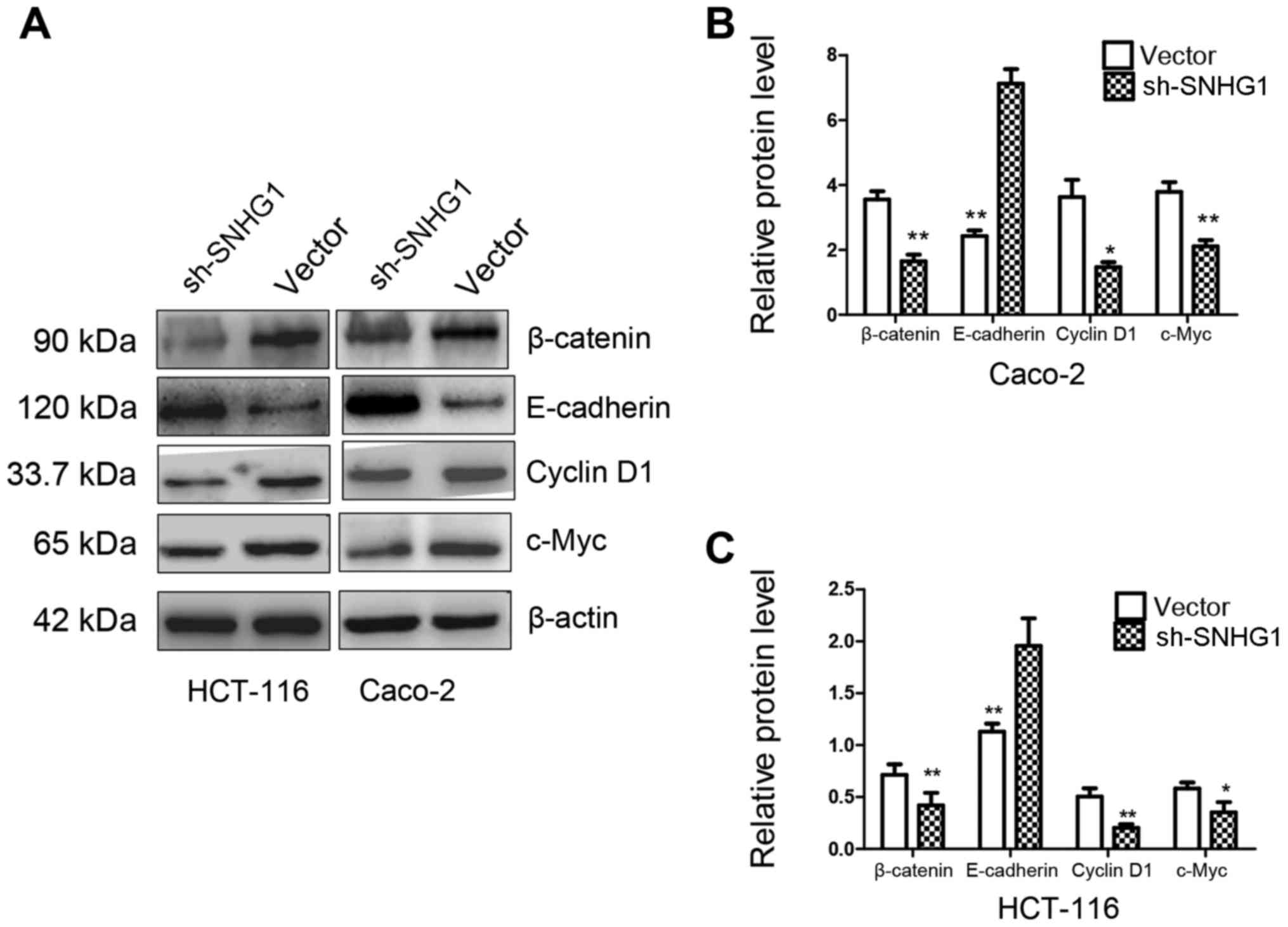
indicated that both in HCT-116 and Caco-2 cell lines with sh-SNHG1
transfection, proteins β-catenin, cyclin D1 and c-Myc were
significantly decreased, while E-cadherin exhibited a higher
expression than the vector group (Fig.
6; P<0.05, P<0.01). These results implied that the trends
observed while investigating the expression of E-cadherin,
β-catenin, cyclin D1 and c-Myc in the sh-SNHG1 group corresponded
to the trends in colon normal tissues, which further confirmed the
carcinogenic characteristics of SNHG1 and suggested that the
Wnt/β-catenin signaling pathway may participate in SNHG1-induced
carcinogenesis.
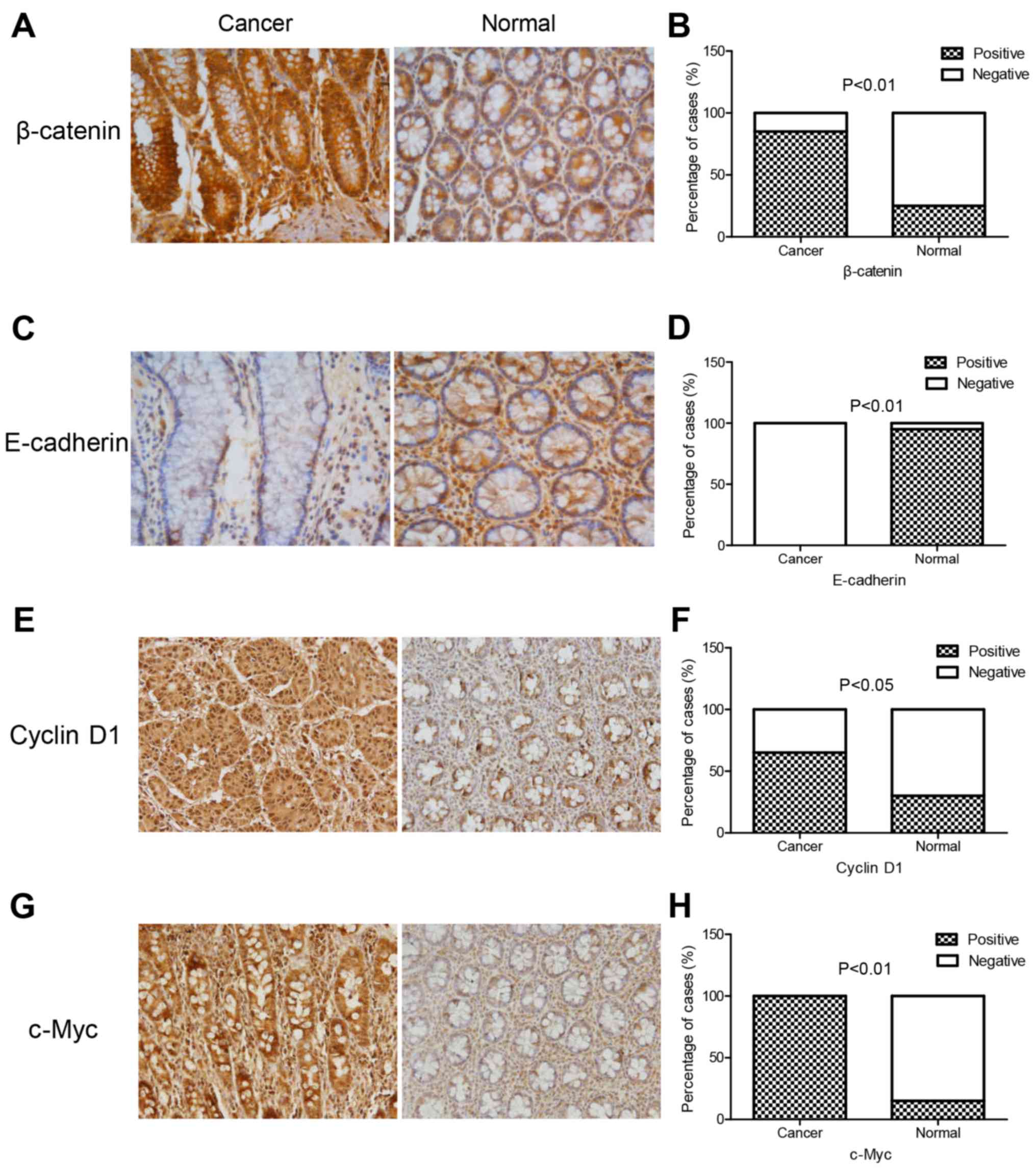 | Figure 5.Immunohistochemical staining of
E-cadherin, β-catenin, cyclin D1 and c-Myc proteins in colon cancer
tissues and normal tissues. The expression of β-catenin, cyclin D1,
and c-Myc in colon cancer tissues was significantly higher than
that in normal tissues, while E-cadherin expression was lower. (A,
C, E and G) β-catenin, E-cadherin, cyclin D1 and c-Myc protein
expression in colon cancer tissues and normal tissues, and (B, D, F
and H) the quantitative analysis of A, C, E and G). |
 | Table III.Expression of β-catenin, E-cadherin,
cyclin-D1 and c-Myc in colon cancer tissues and colon normal
tissues. |
Table III.
Expression of β-catenin, E-cadherin,
cyclin-D1 and c-Myc in colon cancer tissues and colon normal
tissues.
|
| β-catenin
%(n/total) | E-cadherin
%(n/total) | Cyclin D1
%(n/total) | c-Myc
%(n/total) |
|---|
|
|
|
|
|
|
|---|
|
| Positive | Negative | Positive | Negative | Positive | Negative | Positive | Negative |
|---|
| Cancer | 85 (17/20) | 15 (3/20) | 0 (0/20) | 100 (20/20) | 65 (13/20) | 35 (7/20) | 100 (20/20) | 0 (0/20) |
| Normal | 25 (5/20) | 75
(15/20) | 95 (19/20) | 5 (1/20) | 30 (6/20) | 70 (14/20) | 15 (3/20) | 85 (17/20) |
| P-value | P<0.01 | P<0.01 | P<0.05 | P<0.01 |
Discussion
Colon cancer is one of the most common
gastrointestinal malignancies and the third leading cause of
cancer-related mortality among males and females worldwide
(19,20). The high rate of relapse and
unfavorable prognoses suggest a need for novel molecular biomarkers
for the diagnosis and therapeutic treatment of colon cancer to
increase disease survival (21).
lncRNAs have specific expressions in tissues and cell lines
(22), and may have uses in the
dysregulation of cancer cells, including epigenetic effects,
biological effects, and other potential antitumor properties
(23,24). SNHG1 is a novel biomarker that may
play a role in lung cancer, hepatocellular carcinoma, and
neuroblastoma. Its mechanism of action has been reported (25–28).
SNHG1 has a cancer-inducing role on different target genes, but its
regulatory mechanism in colon cancer is unknown.
We used a database (Oncomine) to identify
differentially expressed genes in colon cancer tissues vs. normal
tissues, and it was determined that SNHG1 expression was
statistically higher in cancer than in normal tissues. SNHG1
expression in cancer vs. normal tissues was at least 2-fold higher,
and this was in agreement with the microarray GSE31737 analyses
(19). However, database or chip
accession is always an initial determination, therefore we then
verified SNHG1 expression in colon cancer tissues and normal
tissues using RT-qPCR, and found that except for 2 colon cancer
patients, all of the colon cancer cases indicated a significantly
higher expression of SNHG1 in cancer tissues compared with adjacent
non-cancer tissues, which was consistent with the Oncomine database
and statistically significant. However, this study lacked
sufficient tissue samples from patients to analyze and correlate
the clinical characteristics of patients, such as age, sex, tumor
size, or aggressive stages, therefore more studies with a larger
patient population are necessary for further investigation. Normal
colon tissues consist of various normal colon cells, thus we also
compared the expression of SNHG1 between three colon cancer cell
lines (HCT-116, Caco-2 and HT-29 cells) with normal colon tissues.
It was revealed that SNHG1 was upregulated in colon cancer cells,
which implied that SNHG1 may be a biomarker for colon cancer. This
result was in agreement with the SNHG1 levels in other cancers,
such as in esophageal and lung cancer (10,11).
Knockdown of SNHG1 was used to investigate SNHG1 biological
functions, and the results revealed that SNHG1 promoted cell
proliferation and colony formation, favored cell migration and
invasion abilities, but reduced cell apoptosis in colon cancer
cells. These results demonstrated that SNHG1 played an oncogenic
role in colon cancer, and that suppressing SNHG1 expression and
interfering with its function may be possible new therapeutic
approaches.
The canonical WNT pathway (Wnt/β-catenin signaling)
participates in a variety of cellular processes including embryonic
development, maintenance of tissue homeostasis, and cancer
pathogenesis, among which β-catenin is the key regulatory factor
(29). In Homo sapiens, β-catenin
is a multifunctional protein (30)
consisting of 781 amino acids. When the Wnt pathway is inactivated,
the destruction complex, composed of CSNK1A1, AXIN1, GSK3b and APC
phosphorylates serine residues and stabilizes β-catenin
ubiquitination. Once the Wnt pathway is activated, the
stabilization of β-catenin leads to its translocation from the
cytoplasm to the nucleus, causing its association with
high-mobility group domain factors such as T-cell factor
(Tcf)/lymphocyte enhancer factor, resulting in transcriptional
activation of target genes leading to aberrant cell proliferation,
differentiation, migration, and invasion to causing carcinogenesis
and poor prognoses (30). β-catenin
is a pivotal protein in Wnt/β-catenin signaling (31).
In a recent study, lncRNA SNHG1 and Wnt/β-catenin
signaling were linked to carcinogenesis. SNHG1 promoted NSCLC
progression via miR-101-3p and SOX9 activating the Wnt/β-catenin
signaling pathway (10). In our
study, SNHG1 promoted colon cancer cell aberrant proliferation,
migration, and invasion. In addition, a significant decrease of
β-catenin in the sh-SNHG1 group was observed compared to the vector
group. This suggested that β-catenin played a role in the molecular
mechanism of SNHG1 action, and the Wnt/β-catenin signaling pathway
may be involved. Previous studies have described β-catenin as part
of the E-cadherin complex (32),
and this specific complex may link the actin cytoskeleton to
accommodate cell adhesive abilities, stabilize integrity, and alter
cellular functions. E-cadherin loss is a critical factor of EMT, a
fundamental process that facilitates tumor cell migration and
invasion into surrounding tissues during tumor metastasis (33,34).
If E-cadherin lacks enough β-catenin its target genes or other
affiliated proteins may be affected and this could cause
carcinogenesis, thus, E-cadherin and β-catenin expression may have
opposite effects (29). Our western
blotting results revealed that E-cadherin expression was
significantly higher in the sh-SNHG1 group than the vector group.
β-catenin decreased after SNHG1 was downregulated, which was in
agreement with our Transwell assay results which revealed that
SNHG1 inhibition induced decreased invasion and migration abilities
of colon cancer cells. This further suggested that in the
vector-transfected colon cancer cells, knockdown of SNHG1
influenced EMT and the Wnt/β-catenin pathway to reduce the
migration and invasion of colon cancer cells. In summary, SNHG1 led
to colon cancer invasion and migration possibly via the
Wnt/β-catenin pathway.
To further study this pathway, we detected c-Myc and
cyclin D1, the downstream targets of the Wnt/β-catenin pathway
(35,36), which are involved in Wnt and
β-catenin signaling and are known as cell cycle modulators showing
accumulated expression during accelerated cell proliferation
(37,38). Our western blot analyses revealed
that cyclin D1 and c-Myc decreased in Caco-2 and HCT-116 cells with
SNHG1 downregulation; which was also in agreement with our CCK-8
and colony formation assays. SNHG1 reduced E-cadherin but increased
β-catenin protein levels, cyclin D1 and c-Myc expression,
supporting our hypothesis that SNHG1 promoted colon cancer cell
migration, invasion, and proliferation possibly via the
Wnt/β-catenin pathway. Concurrently, the immunohistochemical
experiment revealed that the expression of β-catenin, cyclin D1 and
c-Myc in colon cancer tissues was significantly higher than in
normal tissues, while E-cadherin expression was lower, which
implied that the trends of those proteins in normal colon tissues
corresponded to the trends of sh-SNHG1 group in the western blots.
Combining the results of two experiments further confirmed the
oncogenic role of SNHG1. Further investigations such as examining
the phosphorylation of key proteins, and inhibiting or positively
stimulating specific pathways, and detecting more sufficient
efficacious pathway signaling factors should be performed in the
future.
The data demonstrated that SNHG1 functioned as an
oncogene in colon cancer and may act via the Wnt/β-catenin pathway
to promote carcinogenesis. SNHG1 could be a potential predictor for
colon cancer patient prognosis and may be a therapeutic target.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used during the present study are
available from the corresponding author upon reasonable
request.
Author's contributions
ZJ and HY designed the study. HY, SW, YJK and CW
performed the experiments. HY and SW wrote the paper. YJK, CW, YX,
YZ and ZJ received and edited the manuscript. All authors read and
approved the manuscript and agree to be accountable for all aspects
of the research in ensuring that the accuracy or integrity of any
part of the work are appropriately investigated and resolved.
Ethics approval and consent to
participate
All experimental protocols were approved by the
Chongqing Medical University Ethics Committee (Chongqing,
China).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Negm OH, Hamed MR, Schoen RE, Whelan RL,
Steele RJ, Scholefield J, Dilnot EM, Kumara Shantha HM, Robertson
JF and Sewell HF: Human blood autoantibodies in the detection of
colorectal cancer. PLoS One. 11:e01569712016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ferlay J, Steliarova-Foucher E,
Lortet-Tieulent J, Rosso S, Coebergh JW, Comber H, Forman D and
Bray F: Cancer incidence and mortality patterns in Europe:
Estimates for 40 countries in 2012. Eur J Cancer. 49:1374–1403.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Fatica A and Bozzoni I: Long non-coding
RNAs: New players in cell differentiation and development. Nat Rev
Genet. 15:7–21. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yuan JH, Yang F, Wang F, Ma JZ, Guo YJ,
Tao QF, Liu F, Pan W, Wang TT, Zhou CC, et al: A long noncoding RNA
activated by TGF-β promotes the invasion-metastasis cascade in
hepatocellular carcinoma. Cancer Cell. 25:666–681. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Peng W and Fan H: Long non-coding RNA
PANDAR correlates with poor prognosis and promotes tumorigenesis in
hepatocellular carcinoma. Biomed Pharmacother. 72:113–118. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Deng J, Liang Y, Liu C, He S and Wang S:
The up-regulation of long non-coding RNA AFAP1-AS1 is associated
with the poor prognosis of NSCLC patients. Biomed Pharmacother.
75:8–11. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Li L, Zhang L, Zhang Y and Zhou F:
Increased expression of LncRNA BANCR is associated with clinical
progression and poor prognosis in gastric cancer. Biomed
Pharmacother. 72:109–112. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chen J, Fu Z, Ji C, Gu P, Xu P, Yu N, Kan
Y, Wu X, Shen R and Shen Y: Systematic gene microarray analysis of
the lncRNA expression profiles in human uterine cervix carcinoma.
Biomed Pharmacother. 72:83–90. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yang Q, Xu E, Dai J, Liu B, Han Z, Wu J,
Zhang S, Peng B, Zhang Y and Jiang Y: A novel long noncoding RNA
AK001796 acts as an oncogene and is involved in cell growth
inhibition by resveratrol in lung cancer. Toxicol Appl Pharmacol.
285:79–88. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Cui Y, Zhang F, Zhu C, Geng L, Tian T and
Liu H: Upregulated lncRNA SNHG1 contributes to progression of
non-small cell lung cancer through inhibition of miR-101-3p and
activation of Wnt/β-catenin signaling pathway. Oncotarget.
8:17785–17794. 2017.PubMed/NCBI
|
|
11
|
Yan Y, Fan Q, Wang L, Zhou Y, Li J and
Zhou K: LncRNA Snhg1, a non-degradable sponge for miR-338, promotes
expression of proto-oncogene CST3 in primary esophageal cancer
cells. Oncotarget. 8:35750–35760. 2017.PubMed/NCBI
|
|
12
|
Li J, Zhang Z, Xiong L, Guo C, Jiang T,
Zeng L, Li G and Wang J: SNHG1 lncRNA negatively regulates
miR-199a-3p to enhance CDK7 expression and promote cell
proliferation in prostate cancer. Biochem Biophys Res Commun.
487:146–152. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Rhodes DR, Yu J, Shanker K, Deshpande N,
Varambally R, Ghosh D, Barrette T, Pandey A and Chinnaiyan AM:
ONCOMINE: A cancer microarray database and integrated data-mining
platform. Neoplasia. 6:1–6. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Rhodes DR, Kalyana-Sundaram S, Mahavisno
V, Varambally R, Yu J, Briggs BB, Barrette TR, Anstet MJ,
Kincead-Beal C, Kulkarni P, et al: Oncomine 3.0: Genes, pathways,
and networks in a collection of 18,000 cancer gene expression
profiles. Neoplasia. 9:166–180. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Jalving M, Heijink DM, Koornstra JJ,
Boersma-van Ek W, Zwart N, Wesseling J, Sluiter WJ, de Vries EG,
Kleibeuker JH and de Jong S: Regulation of TRAIL receptor
expression by β-catenin in colorectal tumours. Carcinogenesis.
35:1092–1099. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Rashed HE, Hussein S, Mosaad H, Abdelwahab
MM, Abdelhamid MI, Mohamed SY, Mohamed AM and Fayed A: Prognostic
significance of the genetic and the immunohistochemical expression
of epithelial-mesenchymal-related markers in colon cancer. Cancer
Biomark. 20:107–122. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ji L, Wei Y, Jiang T and Wang S:
Correlation of Nrf2, NQO1, MRP1, cmyc and p53 in colorectal cancer
and their relationships to clinicopathologic features and survival.
Int J Clin Exp Pathol. 7:1124–1131. 2014.PubMed/NCBI
|
|
18
|
Senol S, Ceyran AB, Kösemetin D, Gobanoglu
B, Aydin D, Duran EA and Leblebici M: Immunohistochemical profile
of tumor pathways and prognostic significance in colon
adenocarcinomas. J Environ Pathol Toxicol Oncol. 36:29–41. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Weijun C, Hailu W, Yushu Z and Zhenqiu C:
Study on expression of long non-coding RNA in colon carcinoma.
Linchuang Zhongliuxue Zazhi. 18:882–886. 2013.
|
|
20
|
Xu X, Chen R, Li Z, Huang N, Wu X, Li S,
Li Y and Wu S: MicroRNA-490-3p inhibits colorectal cancer
metastasis by targeting TGFβR1. BMC Cancer. 15:10232015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Agüero F, Murta-Nascimento C, Gallén M,
Andreu-García M, Pera M, Hernández C, Burón A and Macià F:
Colorectal cancer survival: Results from a hospital-based cancer
registry. Rev Esp Enferm Dig. 104:572–577. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yan X, Hu Z, Feng Y, Hu X, Yuan J, Zhao
SD, Zhang Y, Yang L, Shan W, He Q, et al: Comprehensive genomic
characterization of long non-coding RNAs across human cancers.
Cancer Cell. 28:529–540. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hu X, Feng Y, Zhang D, Zhao SD, Hu Z,
Greshock J, Zhang Y, Yang L, Zhong X, Wang LP, et al: A functional
genomic approach identifies FAL1 as an oncogenic long noncoding RNA
that associates with BMI1 and represses p21 expression in cancer.
Cancer Cell. 26:344–357. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Huang W, Thomas B, Flynn RA, Gavzy SJ, Wu
L, Kim SV, Hall JA, Miraldi ER, Ng CP, Rigo F, et al: DDX5 and its
associated lncRNA Rmrp modulate TH17 cell effector functions.
Nature. 528:517–522. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhang M, Wang W, Li T, Yu X, Zhu Y, Ding
F, Li D and Yang T: Long noncoding RNA SNHG1 predicts a poor
prognosis and promotes hepatocellular carcinoma tumorigenesis.
Biomed Pharmacother. 80:73–79. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
You J, Fang N, Gu J, Zhang Y, Li X, Zu L
and Zhou Q: Non-coding RNA small nucleolar RNA host gene 1 promote
cell proliferation in nonsmall cell lung cancer. Indian J Cancer.
7:99–102. 2014. View Article : Google Scholar
|
|
27
|
Chaudhry MA: Small nucleolar RNA host
genes and long non-coding RNA responses in directly irradiated and
bystander cells. Cancer Biother Radiopharm. 29:135–141. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Sahu D, Hsu CL, Lin CC, Yang TW, Hsu WM,
Ho SY, Juan HF and Huang HC: Co-expression analysis identifies long
noncoding RNA SNHG1 as a novel predictor for event-free survival in
neuroblastoma. Oncotarget. 7:58022–58037. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Rosenbluh J, Wang X and Hahn WC: Genomic
insights into WNT/β-catenin signaling. Trends Pharmacol Sci.
35:103–109. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Cui J, Zhou X, Liu Y, Tang Z and Romeih M:
Wnt signaling in hepatocellular carcinoma: Analysis of mutation and
expression of beta-catenin, T-cell factor-4 and glycogen synthase
kinase 3-beta genes. J Gastroenterol Hepatol. 18:280–287. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Prasetyanti PR, Zimberlin CD, Bots M,
Vermeulen L, Melo FS and Medema JP: Regulation of stem cell
self-renewal and differentiation by Wnt and Notch are conserved
throughout the adenoma-carcinoma sequence in the colon. Mol Cancer.
12:1262013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Greco A, De Virgilio A, Rizzo MI, Pandolfi
F, Rosati D and de Vincentiis M: The prognostic role of E-cadherin
and β-catenin overexpression in laryngeal squamous cell carcinoma.
Laryngoscope. 126:E148–E155. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Guarino M, Rubino B and Ballabio G: The
role of epithelial-mesenchymal transition in cancer pathology.
Pathology. 39:305–318. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Stoyianni A, Goussia A, Pentheroudakis G,
Siozopoulou V, Ioachim E, Krikelis D, Golfinopoulos V, Cervantes A,
Bobos M, Fotsis T, et al: Immunohistochemical study of the
epithelial-mesenchymal transition phenotype in cancer of unknown
primary: Incidence, correlations and prognostic utility. Anticancer
Res. 32:1273–1281. 2012.PubMed/NCBI
|
|
35
|
MacDonald BT, Tamai K and He X:
Wnt/β-catenin signaling: Components, mechanisms, and diseases. Dev
Cell. 17:9–26. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Vlad A, Röhrs S, Klein-Hitpass L and
Müller O: The first five years of the Wnt targetome. Cell Signal.
20:795–802. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Prensner JR, Chen W, Han S, Iyer MK, Cao
Q, Kothari V, Evans JR, Knudsen KE, Paulsen MT, Ljungman M, et al:
The long non-coding RNA PCAT-1 promotes prostate cancer cell
proliferation through cMyc. Neoplasia. 16:900–908. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Yamamoto K, Lee BJ, Li C, Dubois RL,
Hobeika E, Bhagat G and Zha S: Early B-cell-specific inactivation
of ATM synergizes with ectopic CyclinD1 expression to promote
pre-germinal center B-cell lymphomas in mice. Leukemia.
29:1414–1424. 2015. View Article : Google Scholar : PubMed/NCBI
|