Introduction
Cervical cancer is the fourth most common type of
malignant tumour affecting women worldwide (1). Although the mortality rate of patients
with cervical cancer has decreased in recent decades due to radical
surgery and chemoradiotherapy, the treatment of this type of cancer
remains an immense global challenge due to its recurrence, invasion
and metastasis rate (2). Therefore,
effective prevention and treatment strategies for cervical cancer
are required, particularly immunotherapy targeting its
pathogenesis.
Mycobacterium bovis Bacillus Calmette-Guérin
(BCG) is a live attenuated bovine tuberculosis vaccine that is the
first choice for intravesical treatment, and it is the gold
standard for the immunotherapy of superficial bladder cancer
(3–5).
Genitourinary tumours, such as bladder cancer (6) and benign diseases, including condyloma
acuminatum and cervical genital warts, are closely related to human
papillomavirus (HPV) infection (7–9). Clinical
studies have established that BCG immunotherapy exerts a specific
effect on genital warts of the genitourinary system (7,9). R also
identified a certain therapeutic effect on cervical condyloma
acuminate with the local injection of BCG (8). However, whether BCG intervention is an
effective treatment for cervical cancer remains unknown. Patients
with cervical cancer often have severe cellular immune dysfunction.
BCG has been shown to exert a significant therapeutic effect
against cervical intraepithelial neoplasia (CIN II–III in
combination with interferon (IFN) and improves the cellular immune
function of patients with tumours (10). BCG-activated peripheral blood
mononuclear cells also exert a significant killing effect on
high-risk HPV-infected HeLa cells (11). Therefore, BCG intervention for
cervical cancer holds promise as a novel immunotherapy method.
The mechanism of BCG immunotherapy is attributed to
a local non-specific immune response that kills tumour cells: The
interaction of inflammation and cancer (12). Tumour-associated macrophages (TAMs)
play a key role in tumour immunosuppression and progression
(13). Macrophages are differentiated
from bone marrow-derived monocytes and have a high heterogeneity
and plasticity, exerting a number of effects on the
microenvironment (14,15). Macrophages are often classified as the
classically activated M1 type and alternatively activated M2 type
based on cell functions. M1 macrophages are primarily stimulated by
lipopolysaccharide (LPS) or IFN-γ (16) and possess high antigen-presenting
capabilities (17), which are
characterized by the high secretion of pro-inflammatory cytokines,
such as IL-6, IL-12, IL-23 and TNF-α (18), to promote a Th1 response, and
microbicidal and tumoricidal activities (19). M2 macrophages are polarized via IL-4,
IL-10, and IL-13 and exhibit a weak antigen-presenting ability
(20). These cells are known as the
‘repair’ macrophages and are associated with parasite containment,
tissue remodelling, debris elimination, immune tolerance and
regulatory functions, which ultimately lead to the immune escape of
tumour cells (21). The present study
used LPS and IFN-γ to polarize M1 macrophages, and M2 macrophages
were polarized with IL-4.
In the present study, microarray datasets were
downloaded from the Gene Expression Omnibus (GEO) and The Cancer
Genome Atlas (TCGA) and the differentially expressed genes (DEGs)
and functional pathways were analysed between cervical cancer
tissues, high-grade squamous intraepithelial lesion (HSIL) of the
cervix tissues and normal cervical tissues. Gene Ontology (GO) and
Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichment
analyses and protein-protein interaction (PPI) network analyses
were used to elucidate molecular mechanisms underlying the
carcinogenesis and progression of cervical cancer. Survival
analysis revealed that M1 markers played an anti-tumour role, and
M2 markers were involved in the carcinogenesis and invasion of
cervical cancer.
Persistent infection with high-risk HPV is a major
risk factor for cervical cancer (22). The majority of cervical cancers are
associated with a high risk of HPV infection, and HPV16 and 18 are
associated with ~70% of all cervical cancers (23). Immunotherapy stimulates the regression
of certain virus-associated epithelial malignancies, such as
HPV-induced cancers in the cervix (24), head and neck (25) and anus (26). Viral oncoproteins from tumours with
HPV-positive expression are latent candidate tumour degradation
antigens as these proteins are immunologically foreign and are
essentially expressed by cancer cells (27,28).
However, evidence supporting their role in immunotherapy-mediated
tumour degradation is limited.
In the present study, the association of IL-12 and
IL-10 with survival was examined and the survival-based cell cycle
and p53 signalling pathways in cervical cancer were identified
using TCGA and GEO databases. Furthermore, the effects and
molecular mechanisms of action of BCG on the polarization of M1 and
M2 macrophages, and the regulation of the p53/retinoblastoma
(Rb)/E2F transcription factor 1 (E2F1) pathway in HeLa cervical
cancer cells were investigated in order to provide a theoretical
basis for the clinical immunotherapy of cervical cancer.
Materials and methods
Cervical cancer datasets and
pre-processing
Cervical cancer gene expression data that were
publicly available and reported in full clinical annotation were
systematically searched. The microarray datasets, GSE9750 and
GSE7803 (Affymetrix GPL96 platform, Affymetrix Human Genome U133A
Array), and TCGA-cervical squamous cell carcinoma (CESC) were
downloaded from the GEO database (http://www.ncbi.nlm.nih.gov/geo) and UCSC Xena browser
(https://xenabrowser.net/datapages/).
The GSE9750 dataset contains 24 normal cervical tissue samples and
28 cervical cancer tissue samples. The GSE7803 dataset contains 10
normal cervix tissue samples, 7 HSIL tissue samples and 21 cervical
cancer tissue samples. The DEGs, with log fold change (FC) >3
and adj. P-value <0.01, in the mRNA expression profiling sets,
GSE9750 and GSE7803, were selected with GEO2R (http://www.ncbi.nlm.nih.gov/geo/geo2r).
Survival analysis
GEPIA is a recently published analysis tool
containing the RNA sequencing expression data of 8,587 normal
samples and 9,736 tumour samples (http://gepia.cancer-pku.cn). GEPIA provides
differential mRNA expression analysis of tumour/normal tissues and
pathological stages or grades for analysis, patient survival
analysis and correlation analysis (29). The cut-off P-value was 0.05. The
association between mRNA expression and overall survival (OS), or
disease-free survival (DFS) was determined using the ‘Cox PH Model’
with the Kaplan-Meier survival plot. The survival curves of samples
with high and low gene expression were compared using the log-rank
test.
UALCAN (http://ualcan.path.uab.edu) is a comprehensive web
resource based on the TCGA that includes the clinical data of 31
cancer types and the MET500 cohort database (30). In the present study, UALCAN was used
to analyse the mRNA expression levels of IL-12 and IL-10 in primary
CESC tissues and their association with pathological stage and
survival. The association between mRNA expression and pathological
stage was determined using an unpaired Student's t-test (between 2
groups) or one-way analysis of variance (ANOVA) (between multiple
groups) followed by the LSD or Tukey's multiple comparisons post
hoc test. The survival analysis of the UALCAN survival curves was
performed in the same manner as that for the GEPIA data. The
cut-off P-value was 0.05.
Functional and pathway enrichment
analyses of DEGs
The functions of 939 DEGs in the GSE7803/9750
cervical cancer samples were analysed with GO and KEGG in the
Database for Annotation, Visualization, and Integrated Discovery
(DAVID) (https://david.ncifcrf.gov/summary.jsp) (31). GO enrichment analysis was used to
predict the biological processes of 939 DEGs, and KEGG analysis was
used to define the pathways related to the 939 DEGs in the
GSE7803/9750 CESC samples.
PPI network
The PPI network was obtained by using the Search
Tool for the Retrieval of Interacting Genes (STRING) (http://string-db.org) database (32). In the present study, PPI network
analysis of M1/M2 markers and Rb/E2F1 pathway proteins was
conducted to examine their interactions using the STRING database.
An interaction score >0.4 was considered statistically
significant.
THP-1 cell culture and macrophage
differentiation
Human monocyte THP-1 cells (ATCC®
TIB-202TM) were maintained at 37°C in a humidified incubator with
5% CO2. Cells were cultured in Roswell Park Memorial
Institute medium (RPMI-1640; Invitrogen; Thermo Fisher Scientific,
Inc.) supplemented with 10% heat-inactivated foetal bovine serum
(FBS Natocor), 100 U/ml penicillin and 100 µg/ml streptomycin (all
from Invitrogen; Thermo Fisher Scientific, Inc.). Resting
macrophages (M0) were differentiated from THP-1 monocytes with
5–100 ng/ml phorbol 12-myristate-13 acetate (PMA, P8139,
Sigma-Aldrich; Merck KGaA) for 24 h, and 10 ng/ml PMA was
considered optimal. M1 macrophages were polarized with 5–40 ng/ml
IFN-γ (11725-HNAS; Sino Biological Inc.) and 20–100 ng/ml LPS
(L2880; Sigma-Aldrich; Merck KGaA), and 20 ng/ml IFN-γ and 100
ng/ml LPS were considered optimal. M2 macrophages were obtained by
incubation with 5–40 ng/ml IL-4 (#20004; PeproTech, Inc.) at 37°C
for 48 h, and 10 ng/ml IL-4 was considered optimal. M1/M2
macrophages were further induced with BCG (Shanghai Institute of
Biological Products, China) at various concentrations (0.2–40
µg/ml) for 48 h.
Enzyme-linked immunosorbent assay
(ELISA)
Macrophage supernatants were collected from the
THP-1/M0/M1/M2 groups. After 48 h, the levels of IL-10 (ELISA kit,
88-7106; eBioscience) and IL-12 (Elisa Kit, 88-7929; eBioscience)
of serum samples were assayed using ELISA multiplex kits according
to the manufacturer's protocol.
Reverse transcription-quantitative PCR
(RT-qPCR)
RNA was extracted using TRIzol® reagent
(Invitrogen; Thermo Fisher Scientific, Inc.), and RNA purity and
concentration were determined using a UV spectrophotometer. RNA was
reverse transcribed into cDNA using a PrimeScriptTMRT
reagent kit (Takara Bio, Inc.). According to the SYBR®
Premix Ex Taq (Takara Bio, Inc.) manufacturer's protocol,
amplification was performed using a CFX96TM Real-Time PCR Detection
System (Bio-Rad Laboratories, Inc.). The standard reaction
conditions were 95°C for 30 sec; and 95°C for 5 sec and 60°C for 30
sec for 40 cycles. Relative quantification was performed using the
2−ΔΔCq method (33). The
Cq value was automatically analysed based on the amplification
curve. The 2−ΔΔCq value represents the expression level
of a target gene in each group relative to the expression level of
the internal reference gene (34).
GAPDH served as the control gene for normalization. All the
reactions were performed in triplicate. The primer sequences are
listed in Table I.
 | Table I.Primer sequences used for
RT-qPCR. |
Table I.
Primer sequences used for
RT-qPCR.
| IL-10 | Forward primer:
CACTGCTCTGTTGCCTGGTC |
|
| Reverse primer:
GAAGCATGTTAGGCAGGTTGC |
| IL-12 | Forward primer:
GGACCTTGGACCAGAGCAGT |
|
| Reverse primer:
GGCTTAGAACCTCGCCTCCT |
| ARG | Forward primer:
TTGGCTTGAGAGACGTGGAC |
|
| Reverse primer:
CCATCACCTTGCCAATTCCT |
| iNOS | Forward primer:
GAGCCAGGCCACCTCTATGT |
|
| Reverse primer:
GTCCTCGACCTGCTCCTCAT |
| GAPDH | Forward primer:
CGGAGTCAACGGATTTGGTCGTAT |
|
| Reverse primer:
AGCCTTCTCCATGGTGGTGAAGAC |
Western blot analysis
THP-1/macrophages and cervical carcinoma HeLa cells
(Shanghai Institutes for Biological Sciences) were co-cultured in a
separate chamber at 37°C in a humidified incubator with 5%
CO2 for 96 h. Total proteins were extracted from the
HeLa cells using lysis buffer (Beyotime Institute of
Biotechnology). The protein concentration was determined using the
BCA-100 protein quantitation method (Nanjing KeyGen Biotech Co.,
Ltd.). An 8–12% separation gel and a 5% stacking gel were prepared
for electrophoresis. The separated proteins (20 µg) were
transferred to a PVDF membrane (EMD Millipore) with the wet
transfer method. The membranes were blocked with BSA (Beijing
Solarbio Science & Technology Co., Ltd.) for 1 h, and anti-p53
(1:1,000, cat. no. 2524) anti-phosphorylated (p-)Rb (1:1,000, cat.
no. 9313), anti-E2F1(1:1,000, cat. no. 3742), anti-ARG (1:1,000,
cat. no. 93668) anti-GAPDH antibodies (1:1,000, cat. no. D16H11)
(all from Cell Signaling Technology, Inc.) and anti-iNOS (1:500,
MAB9502; R&D Systems, Inc.) were added overnight at 4°C. The
membranes were washed with 1X Tris-buffered saline-Tween (TBST,
Beijing Solarbio Science & Technology Co., Ltd.) for 5 min (3
times). A goat anti-rabbit antibody and goat anti-mouse antibody (H
+ L; Invitrogen; Thermo Fisher Scientific, Inc.) were used as the
secondary antibodies. The membranes were incubated with the
secondary antibodies (anti-mouse IgG, 1:2,000, cat. no. 7076; Cell
Signaling Technology, Inc.; anti-rabbit IgG, 1:2,000, cat. no.
7074; Cell Signaling Technology, Inc.) at room temperature for 1–2
h and washed with 1X TBST for 5 min (3 times). The membranes were
incubated with enhanced chemiluminescence (ECL) substrate (EMD
Millipore), and the blots were developed on an ultrasensitive
chemiluminescence imaging system (Bio-Rad Laboratories, Inc.). All
the reactions were performed in triplicate. GAPDH served as a
control protein to quantify the expression of target proteins using
Image Lab software 4.0 (Bio-Rad Laboratories, Inc.).
Flow cytometric analysis
Cells were separated with trypsin and washed with
PBS Gibco; Thermo Fisher Scientific, Inc.). The cells were cultured
with a PE-conjugated anti-mouse CD80 antibody (cat. no. 561134; BD
Biosciences) for 20 min at room temperature and analysed on a
FACS-Verse flow cytometer (BD Biosciences). The data were analysed
using FlowJo software 7.6 (BD Biosciences).
For the cell cycle assay, the cells were isolated
with trypsin and suspended in PBS. The cells were incubated in
pre-cooled 70% ethanol, and the ethanol was discarded following
centrifugation at room temperature at 800 × g for 6 min. The cells
were suspended in PBS. Propidium iodide (PI, 450 µl)/RNase (50 µl)
staining buffer (BD Pharmingen; BD Biosciences) was added, and the
reaction was performed in the dark at room temperature for 30 min.
A FACS flow cytometer was used (FACS Verse; BD Biosciences).
For the Cell apoptosis assay: Cells were digested
with trypsin without ethylenediaminetetraacetic acid (EDTA). The
cells were suspended in PBS. To each sample, 100 µl of 1X binding
buffer, 5 µl of FITC-Annexin V (eBioscience; Thermo Fisher
Scientific, Inc.) and 10 µl PI (eBioscience; Thermo Fisher
Scientific, Inc.) were added in the dark at room temperature for 15
min and mixed with 400 µl PBS. A FACS flow cytometer was used (FACS
Verse; BD Biosciences).
Statistical analysis
Statistical analyses were performed using SPSS 20.0
statistical software (IBM Corp.). Statistical analyses were
performed using an unpaired Student's t-test (between 2 groups) or
one-way analysis of variance (ANOVA) (between multiple groups)
followed by LSD or Tukey's post hoc multiple comparisons.
Measurement data are expressed as the mean ± standard error of the
mean (SEM) from one representative experiment of three independent
experiments. P<0.05 was considered to indicate a statistically
significant difference. Figures were generated using GraphPad Prism
7 (GraphPad Software, Inc.) and Adobe illustrator CS6 (Adobe
Systems, Inc.).
Results
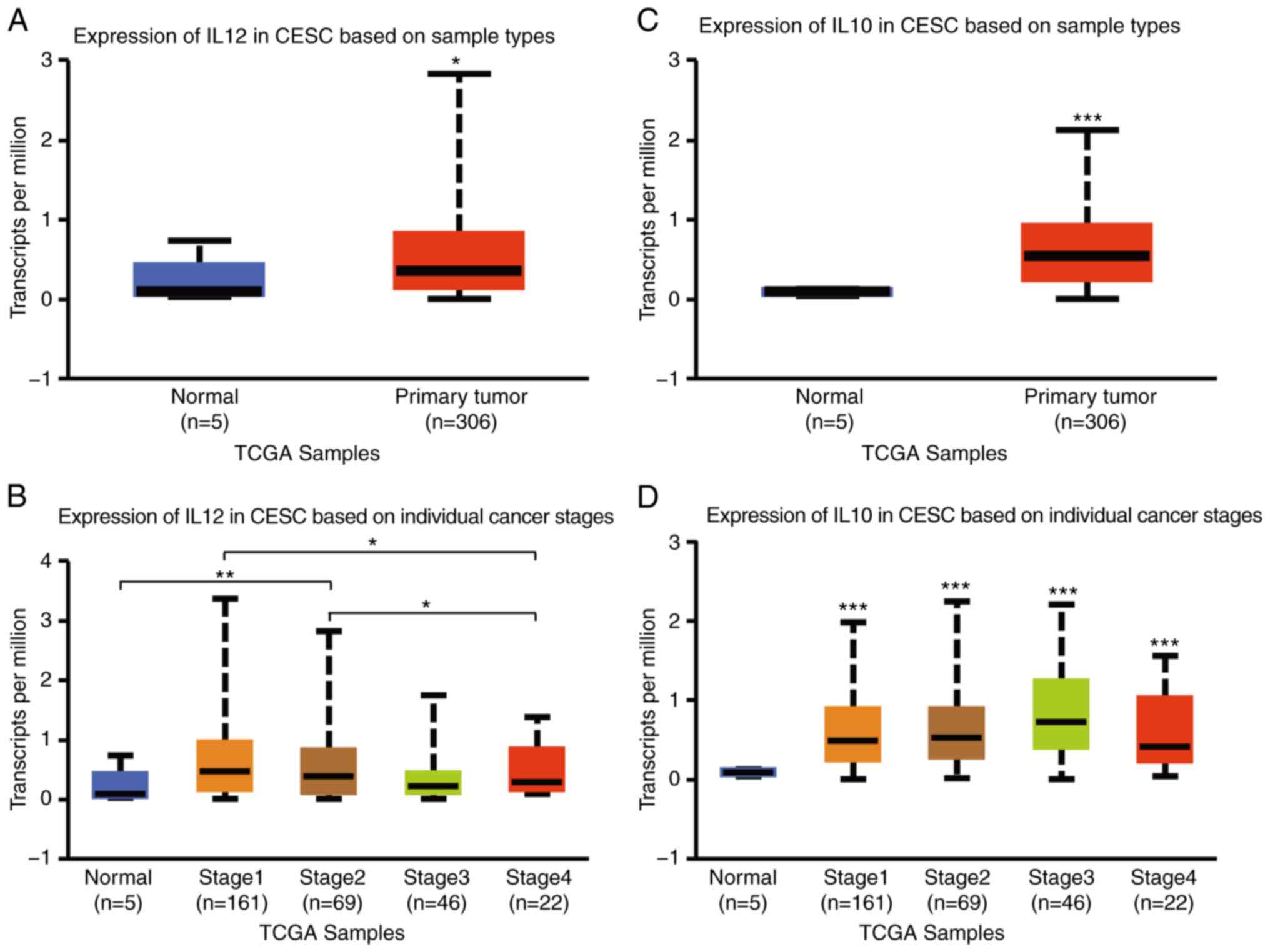
Aberrant expression of IL-12 and IL-10
in patients with CESC
To examine the distinct prognostic and potential
therapeutic value of M1/M2 markers in patients with CESC, the mRNA
expression levels of IL-12 and IL-10 were analysed in primary CESC
tissues, and their association with pathological stages were
assessed with UALCAN and the GEO. IL-12 mRNA expression was
slightly higher in CESC tissues than in normal tissues (P<0.05)
(Fig. 1A) and tended to be weakly
expressed in patients with more advanced stages according to UALCAN
(Fig. 1B). The mRNA expression of
IL-10 was significantly higher in CESC tissues than in normal
tissues according to UALCAN (P<0.001) (Fig. 1C). IL-10 expression was markedly
associated with CESC stages and tended to be more highly expressed
in patients with more advanced stages of the disease (Fig. 1D).
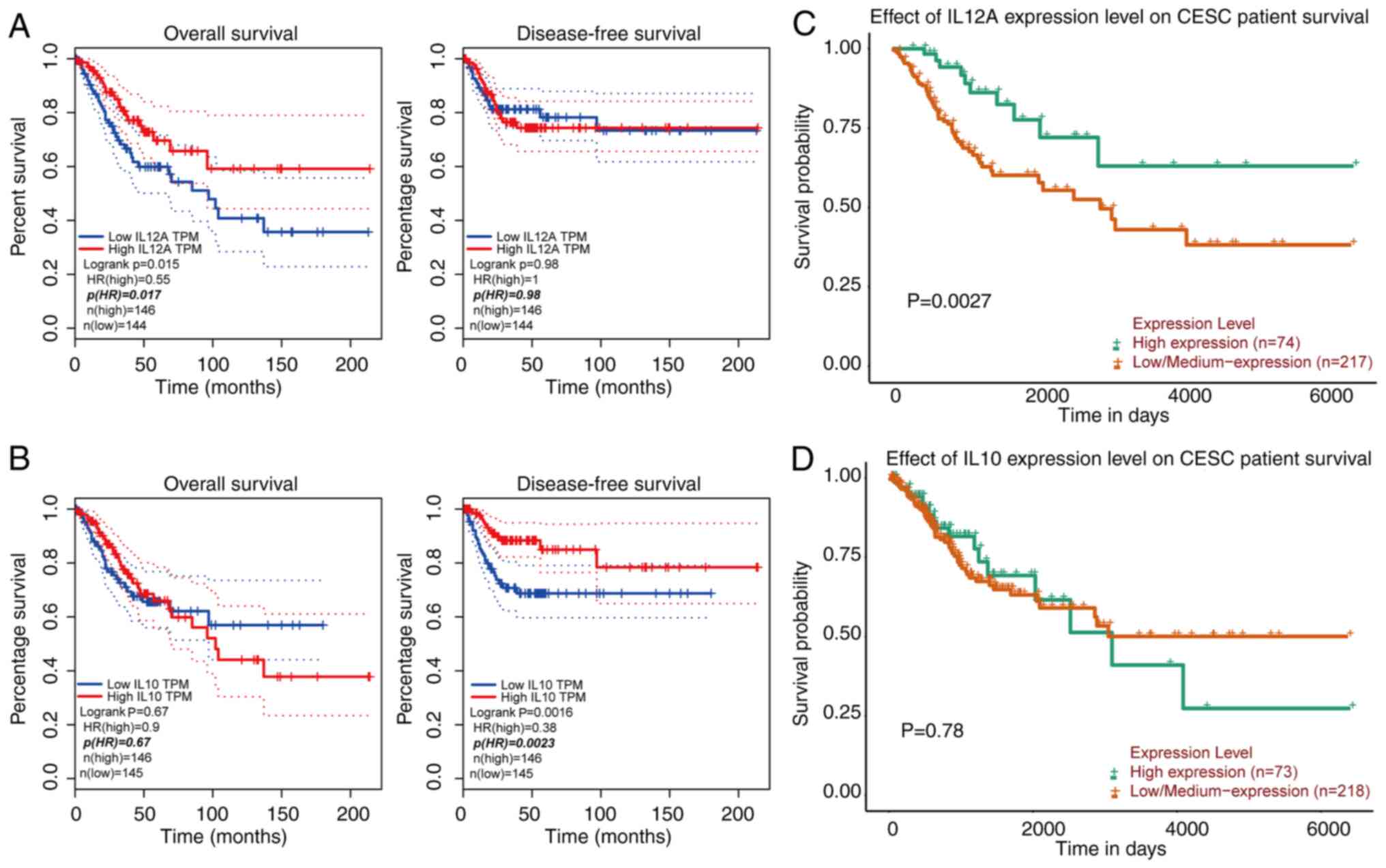
Prognostic value of IL-12 and IL-10 in
patients with CESC
To evaluate the role of IL-12 and IL-10 in the
progression of CESC, the associations of IL-12 and IL-10 expression
and the clinical outcomes of patients were assessed using GEPIA and
UALCAN. The results of overall survival (OS) and disease-free
survival (DFS) are presented in Fig.
2. It was found that high transcriptional levels of IL-12 were
significantly associated with a prolonged OS in patients with CESC
(GEPIA, P<0.05; UALCAN, P<0.001) (Fig. 2A and C). High transcriptional levels
of IL-10 were significantly associated with DFS according to GEPIA
(P<0.001) (Fig. 2B and D).
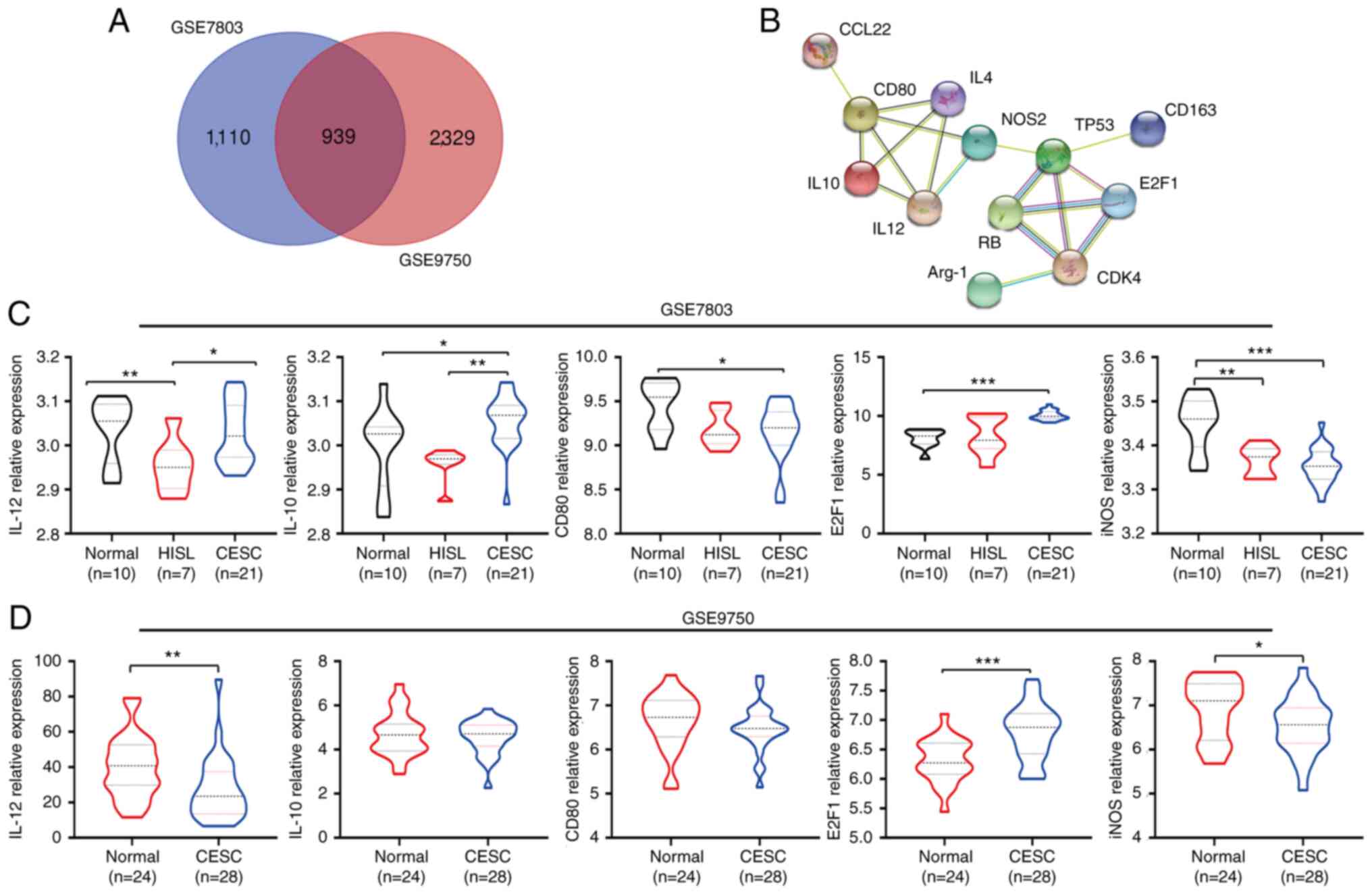
Identification, KEGG and GO enrichment
analyses of DEGs in GSE7803/9750
DEGs (2,049 in GSE7803 and 3,268 in GSE9750) were
identified following the standardization of the microarray results.
Among the two datasets, 939 genes overlapped, as displayed in the
Venn diagram in Fig. 3A. The mRNA
expression of IL-10 was also significantly upregulated in CESC
tissues compared with normal and HSIL tissues from GSE7803
(P<0.05; P<0.001) (Fig. 3B).
The mRNA expression of IL-12 was significantly downregulated in
CESC tissues compared with normal tissues from GSE9750 (P<0.001)
(Fig. 3C). The functional and pathway
enrichment analyses of the DEGs were predicted with GO and KEGG in
DAVID. It was found that GO: 0051301 (cell division), GO: 0000082
(G1/S transition of mitotic cell cycle), GO: 0008283 (cell
proliferation), GO: 0007049 (cell cycle), GO: 1901796 (regulation
of signal transduction by p53) and GO: 0006955 (immune response)
were significantly enriched in DEGs in GSE7803/9750 (Table II). KEGG pathway analysis revealed
that hsa04110 (cell cycle), hsa03030 (DNA replication), hsa03420
(nucleotide excision repair) and hsa04115 (p53 signalling pathway)
were enriched in DEGs in GSE7803/9750 (Table II).
 | Table II.GO and KEGG pathway enrichment
analysis of DEGs in GSE 7803/9750 CESC samples. |
Table II.
GO and KEGG pathway enrichment
analysis of DEGs in GSE 7803/9750 CESC samples.
| Pathway ID | Description | Count in gene
set | P-value |
|---|
| GO:0051301 | Cell division | 66 | <0.001 |
| GO:0006260 | DNA
replication | 43 | <0.001 |
| GO:0000082 | G1/S transition of
mitotic cell cycle | 31 | <0.001 |
| GO:0000086 | G2/M transition of
mitotic cell cycle | 29 | <0.001 |
| GO:0008283 | Cell
proliferation | 47 | <0.001 |
| GO:0007049 | Cell cycle | 26 | <0.001 |
| GO:1901796 | Regulation of
signal transduction by p53 | 18 | <0.001 |
| GO:0075733 | Intracellular
transport of virus | 10 | <0.001 |
| GO:0006955 | Immune
response | 34 | <0.01 |
| GO:0043066 | Negative regulation
of apoptotic process | 36 | <0.01 |
| hsa04110 | Cell cycle | 34 | <0.001 |
| hsa03030 | DNA
replication | 18 | <0.001 |
| hsa03420 | Nucleotide excision
repair | 9 | <0.01 |
| hsa04115 | p53 signalling
pathway | 10 | <0.05 |
PPI network construction
The PPI network analysis of M1/M2 markers and
RB/E2F1 pathway proteins was performed to examine the interactions
between IL-12, IL-10, IL-4, CD80, ARG-1, NOS, p53, Rb and E2F1
using the STRING database. As was expected, the results revealed
several nodes (12) and several edges
(18) in the PPI network (Fig. 3D).
Characterization of macrophage
polarization from THP-1 monocytes
M0 macrophages were differentiated from THP-1
monocytes using 5–100 ng/ml PMA in 6-well plates for 24 h. The
hallmarks of macrophages were observed under a microscope as THP-1
cells became adherent from the suspension and spread. The results
revealed that the optimal condition was 10 ng/ml PMA for M0
macrophage differentiation (Fig. 4A).
M1 macrophages were obtained by incubating M0 macrophages with 5–40
ng/ml IFN-γ and 20–100 ng/ml LPS. M2 macrophages were induced with
5–40 ng/ml IL-4. The mRNA and protein expression of IL-10 revealed
that 10 ng/ml IL-4 was the optimal concentration for M2 macrophage
polarization (Fig. 4B and C). The
mRNA and protein expression of IL-12 indicated that 20 ng/ml IFN-γ
and 100 ng/ml LPS were optimal for M1 macrophage polarization
(Fig. 4D-F).
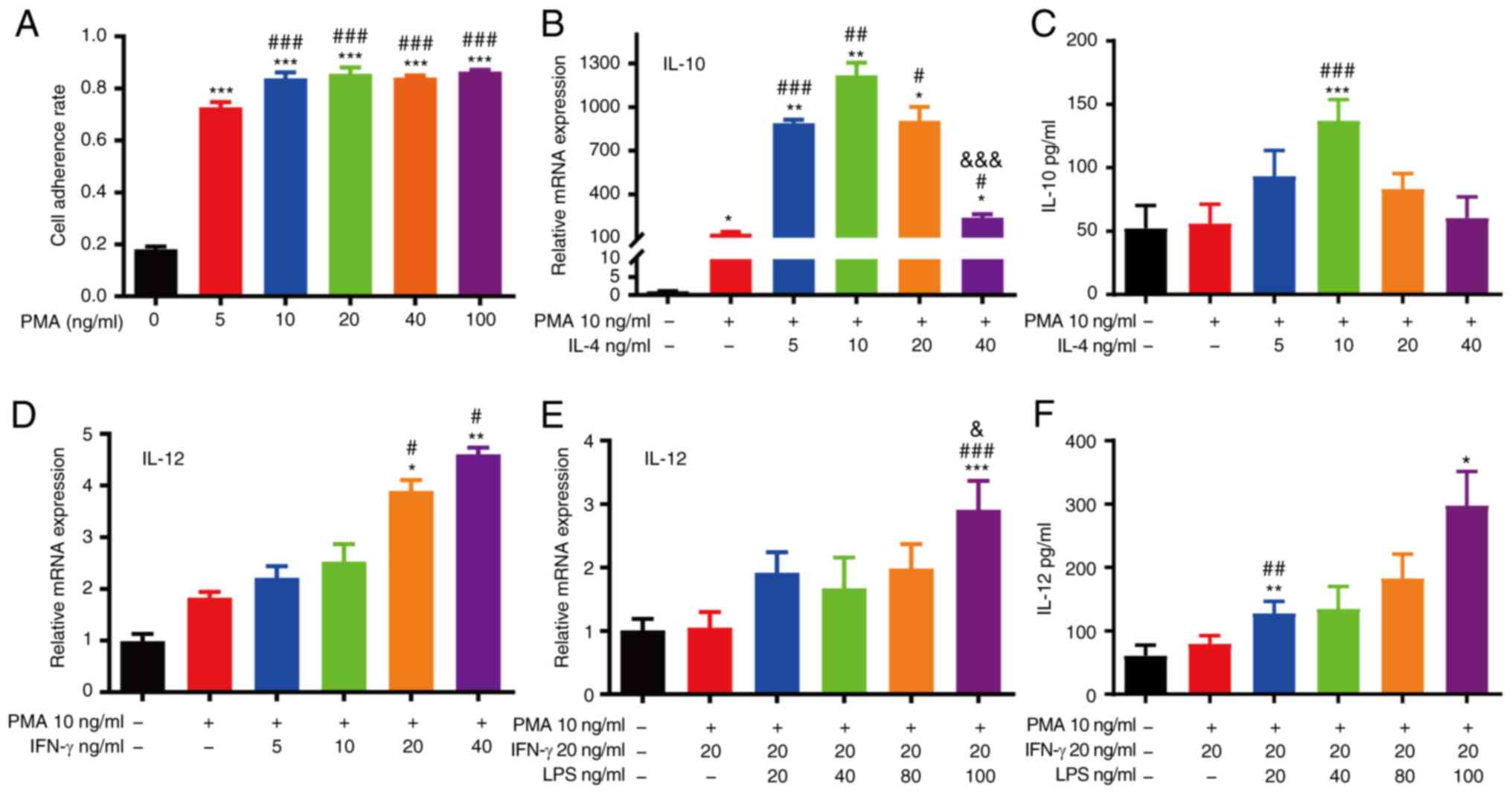 | Figure 4.Differentiation of THP-1 monocytes
into macrophages. (A) M0 macrophages were differentiated from THP-1
cells with various concentrations of PMA (***P<0.001, as
compared with the THP-1 group; ###P<0.001, as
compared with the 5 ng/ml PMA group). (B) RT-qPCR analysis of IL-10
expression in THP-1 cells with PMA and various concentrations of
IL-4 (*P<0.05 and **P<0.01, as compared with the THP-1 group;
#P<0.05, ##P<0.01 and
###P<0.001, as compared with the M0 group;
&&&P<0.001, as compared with the IL-4 5
ng/ml group). (C) ELISA of IL-10 expression in THP-1 cells with PMA
and various concentrations of IL-4 (***P<0.001, as compared with
the THP-1 group; ###P<0.001, as compared with the M0
group). (D) RT-qPCR analysis of IL-12 expression in THP-1 cells
with PMA and various concentrations of IFN-γ (*P<0.05 and
**P<0.01, as compared with the THP-1 group;
#P<0.05, as compared with the M0 group). (E) RT-qPCR
analysis of IL-12 expression in THP-1 cells with PMA, IFN-γ and
various concentrations of LPS (***P<0.001, as compared with the
THP-1 group; ###P<0.001, as compared with the M0 and
20 ng/ml IFN-γ group; &P<0.05, as compared with
the M0 in 20 ng/ml IFN-γ and 20 ng/ml LPS group). (F) ELISA of
IL-12 expression in THP-1 cells with PMA, IFN-γ and various
concentrations of LPS (*P<0.05 and **P<0.01, compared with
the THP-1 group; ##P<0.01, as compared with the M0
and 20 ng/ml IFN-γ group). PMA, phorbol 12-myristate-13 acetate;
LPS, lipopolysaccharide. |
THP-1 cells were differentiated into M0 macrophages
with PMA for 24 h and further polarized toward M1/M2 phenotype
macrophages with IFN-γ and LPS or IL-4 at the appropriate
concentrations for 48 h. The results of RT-qPCR revealed that the
M2 macrophages had a higher IL-10 mRNA expression than the M0/M1
macrophages (Fig. 5A), and IL-12 mRNA
expression in M1 macrophages was significantly higher than that in
M0/M2 macrophages (Fig. 5B). The FACS
results demonstrated that M1 macrophages had higher CD80 protein
expression than M0/M1 macrophages (Fig.
5C and D). The ELISA results demonstrated that the IL-10 levels
in M2 macrophages were higher than those in M0/M1 macrophages
(Fig. 5E); however, the IL-12 levels
in M1 macrophages were higher than those in M0/M2 macrophages
(Fig. 5F).
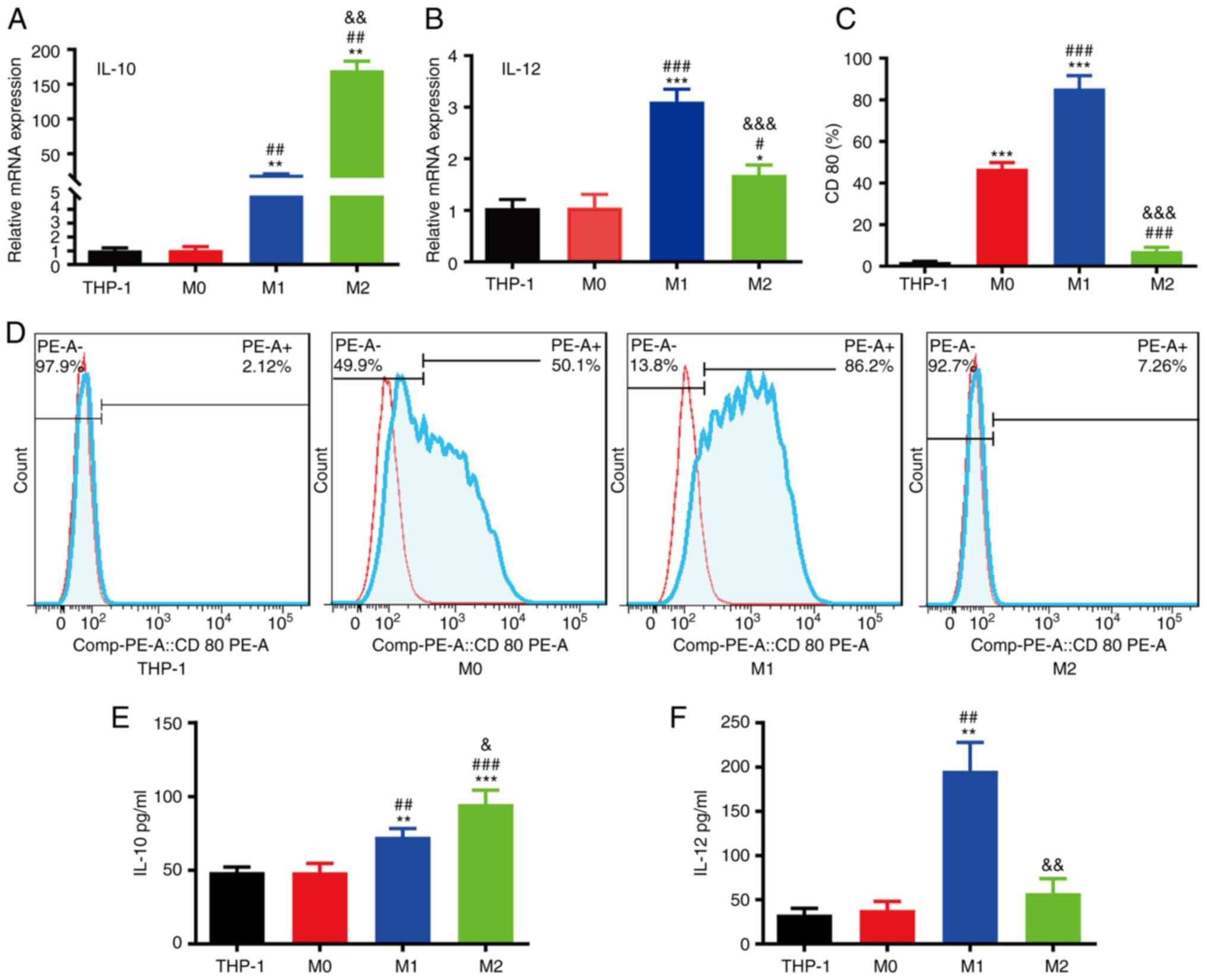 | Figure 5.Characterization of macrophage
polarization. (A) RT-qPCR analysis of IL-10 mRNA expression in the
THP-1, M0, M1 and M2 groups. (B) RT-qPCR analysis of IL-12 mRNA
expression in the THP-1, M0, M1 and M2 groups. (C) Quantification
of CD80 in the THP-1, M0, M1 and M2 groups. (D) Flow cytometric
analysis of CD80 protein expression in the THP-1, M0, M1 and M2
groups. (E) ELISA of IL-10 expression in the THP-1, M0, M1 and M2
groups. (F) ELISA of IL-12 expression in the THP-1, M0, M1 and M2
groups (*P<0.05, **P<0.01 and ***P<0.001, as compared with
the THP-1 group; #P<0.05, ##P<0.01 and
###P<0.001, as compared with the M0 group;
&P<0.05, &&P<0.01 and
&&&P<0.001, as compared with the M1
group). |
BCG promotes macrophage polarization
toward the M1 phenotype and enhances the transition of M2 to M1
macrophages
To observe the effects of BCG on macrophage
polarization, the M1/M2 macrophages were treated with various
concentrations (0.2–40 µg/ml) of BCG for 48 h (35). The mRNA or protein expression of IL-12
and iNOS (M1 markers) steadily increased in the 0.2 to 20 µg/ml
BCG-activated M1/M2 macrophage groups, particularly in the M1
macrophage group, and decreased in the 40 µg/ml BCG-activated M1
macrophage group, likely due to toxicity (Fig. 6A-C). The mRNA or protein expression
levels of IL-10 and ARG (M2 markers) were inhibited in the 0.2–20
µg/ml BCG-activated M1/M2 macrophage groups, and the levels of
these markers were particularly decreased in the M2 macrophage
group. No significant differences in ARG expression were found
between the 0.2–40 µg/ml BCG-activated M1 macrophage groups and the
M1 macrophage group (Fig. 6D-F).
These findings indicated that the 20 µg/ml BCG condition led to
maximum activation.
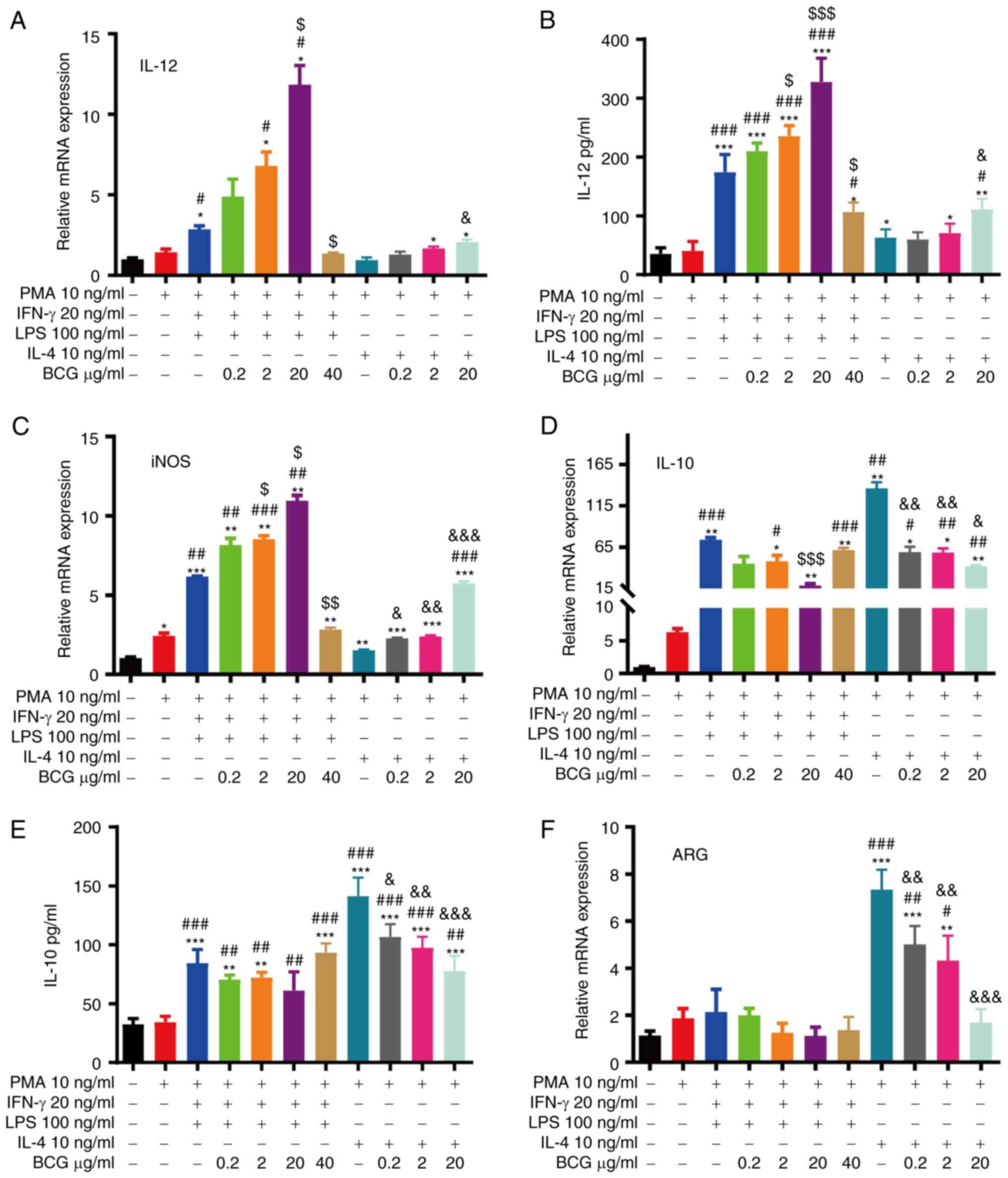 | Figure 6.BCG promotes macrophage polarization
towards the M1 phenotype and enhances the transition of M2 to M1
macrophages. RT-qPCR analysis of (A) IL-12, (C) iNOS, (D) IL-10 and
(F) ARG mRNA expression in each group. ELISA of (B) IL-12 and (E)
IL-10 expression in each group (*P<0.05, **P<0.01 and
***P<0.001, as compared with the THP-1 group;
#P<0.05, ##P<0.01 and
###P<0.001, as compared with the M0 group;
$P<0.05, $$P<0.01 and
$$$P<0.001, as compared with the M1 group;
&P<0.05, &&P<0.01 and
&&&P<0.001, as compared with the M2
group). BCG, Bacillus Calmette-Guérin; iNOS, inducible nitric oxide
synthase; ARG, arginase. |
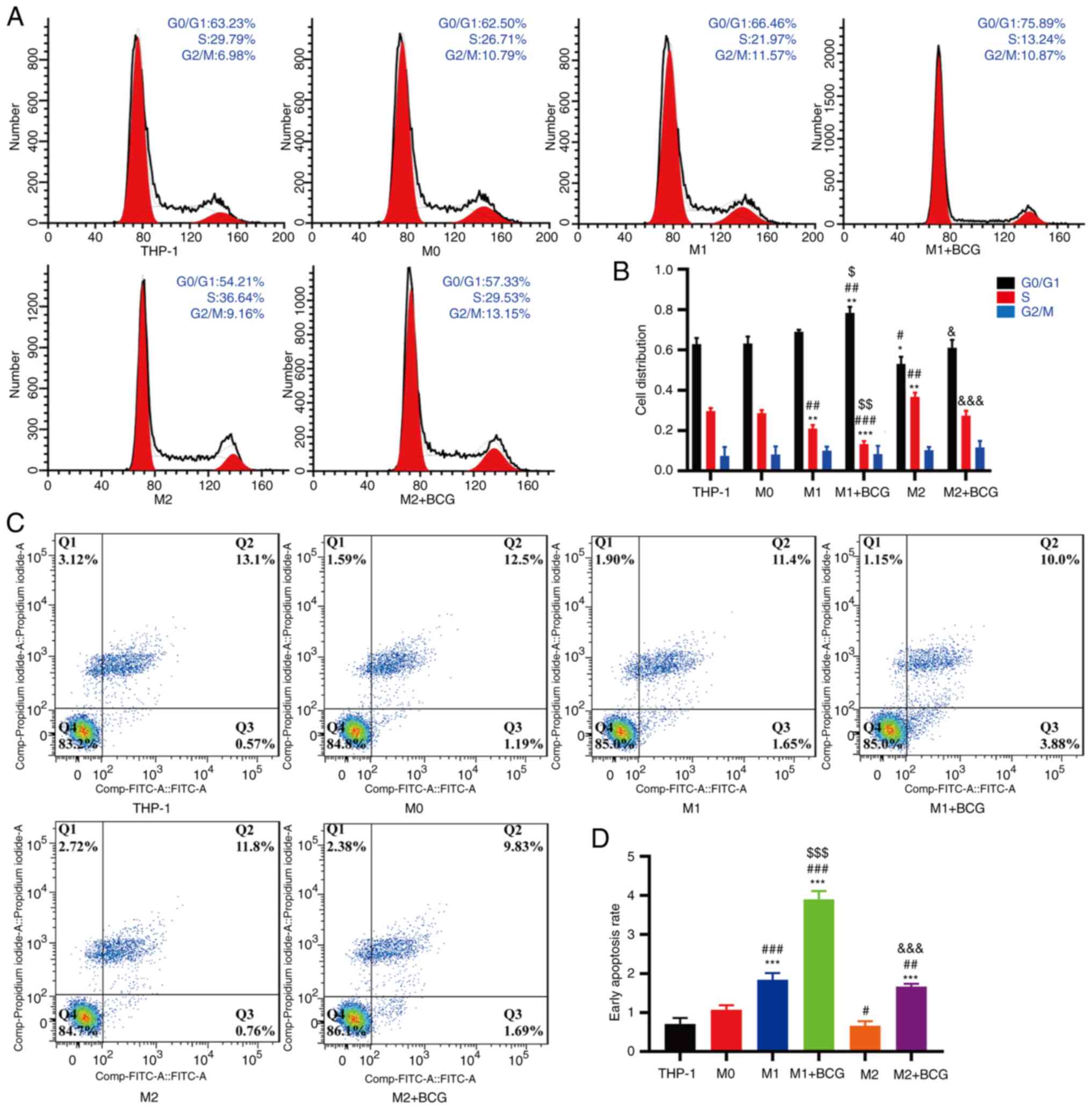
BCG promotes the anti-tumour
progression of M1 macrophages and inhibits the pro-tumour
activation of M2 macrophages in HeLa cells
The effects of polarized macrophages induced by BCG
on HeLa cell proliferation and apoptosis were further assessed.
THP-1/macrophages and HeLa cells were co-cultured in a separate
chamber for 96 h. Cell cycle analysis revealed that the M1
macrophages had an increased the proportion of cells in the G0/G1
phase, and the number of cells in the S phase was decreased in the
HeLa cells compared with the THP-1/M0 macrophages. M2 macrophages
exhibited a decreased number of cells in the G0/G1 phase and an
increased proportion of cells in the S phase in HeLa cells compared
with the THP-1/M0 macrophages. (Fig. 7A
and B). Cell apoptosis analysis revealed that M1 macrophages
increased the percentage of HeLa cells in early apoptosis, and BCG
further increased the proportion of cells in early apoptosis
compared with M2 macrophages without BCG (Fig. 7C and D).
Polarized macrophages induced by BCG
affect HeLa cell proliferation via the Rb/E2F1 signalling
pathway
The present study then investigated whether Rb/E2F1
expression in HeLa cells was influenced by BCG-induced M1/M2
macrophages. The results revealed that the expression of p-Rb/E2F1
proteins was significantly higher in the M2 macrophage group than
in the other groups, although their expression decreased following
treatment with BCG (Fig. 8A, E and
F). iNOS (M1 marker) protein expression increased and ARG-1 (M2
marker) protein expression decreased in the BCG-induced M1
macrophage group compared with the M1 macrophage group (Fig. 8A-C). ARG-1 (M2 marker) protein
expression decreased and iNOS (M1 marker) protein expression
increased in the BCG-induced M2 macrophage group compared with the
M2 macrophage group. The expression of the p53 protein was higher
in M1/M2 macrophages with BCG than in those without BCG treatment
(Fig. 8A and D).
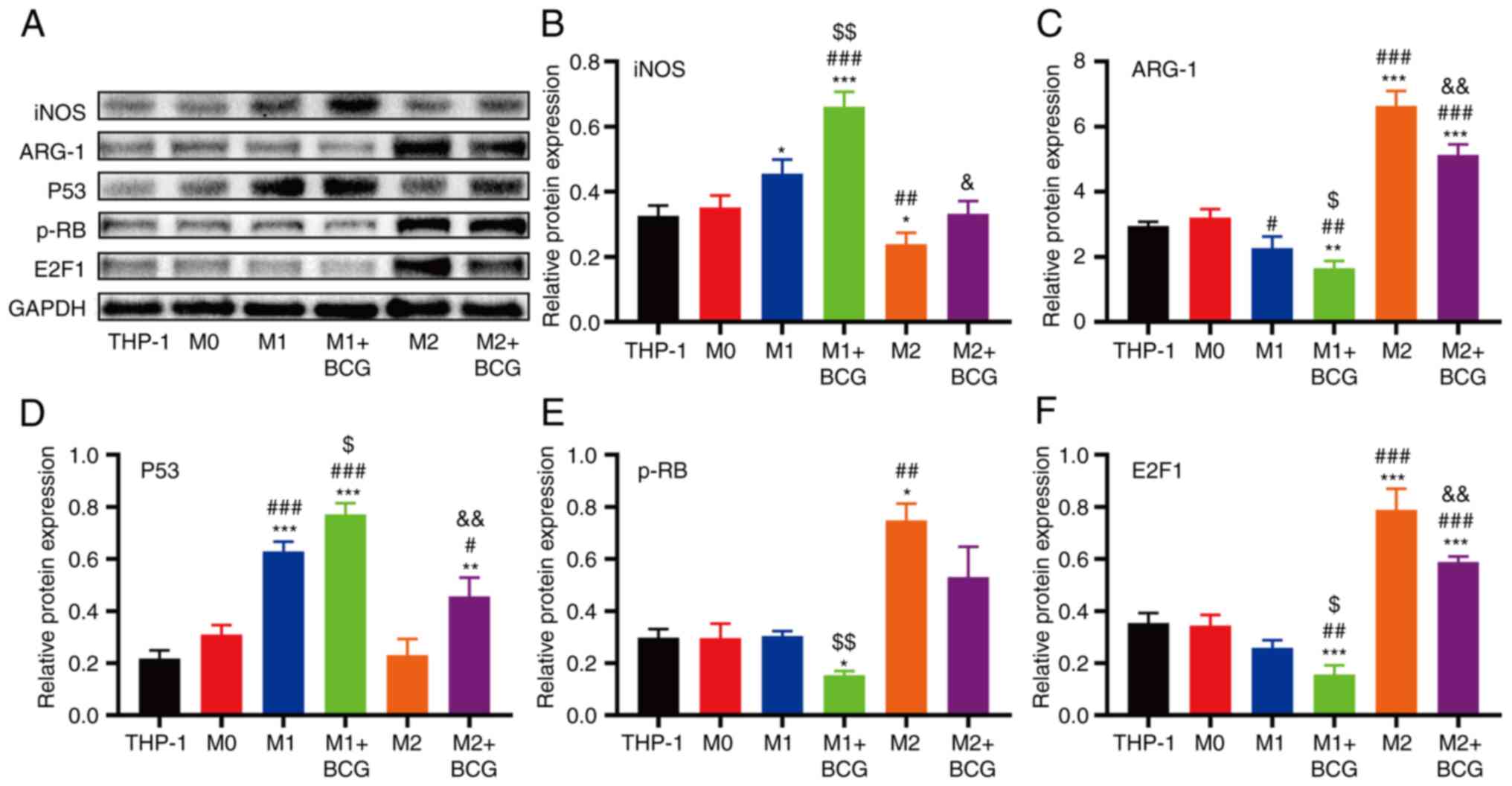 | Figure 8.Effects of the Rb/E2F1 signalling
pathway during BCG-induced M1/M2 macrophage activation. (A) Protein
expression levels of iNOS, ARG-1, p53, p-Rb and E2F1 in each group
were analysed using western blot analysis. Densitometric analysis
results of (B) iNOS, (C) ARG-1, (D) p53, (E) p-Rb and (F) E2F1
expression (*P<0.05, **P<0.01 and ***P<0.001, as compared
with the THP-1 group; #P<0.05, ##P<0.01
and ###P<0.001, as compared with the M0 group;
$P<0.05 and $$P<0.01, as compared with
the M1 group; &P<0.05 and
&&P<0.01, as compared with the M2 group).
BCG, Bacillus Calmette-Guérin; iNOS, inducible nitric oxide
synthase; ARG-1, arginase; p-RB, retinoblastoma protein; E2F1,
transcription factor E2F1. |
Discussion
The tumour microenvironment (TME), which consists of
fibroblasts, lymphatics, blood vessels, cells and inflammatory
immune cells (36), plays a critical
role in the progression of the occurrence, development, and
prognosis of cervical cancer. TAMs are the main contributors to the
TME via the accumulation and polarization of these cells. M1
macrophages, known as the ‘fighting’ macrophages, are tumoricidal
directly via the excretion of pro-inflammatory cytokines, such as
IL-6, IL-12, IL-23 and TNF-α (18),
the depletion of the tumour stoma and their high antigen
presentation capabilities (17).
Therefore, these cells play a critical role in immune surveillance
and are associated with a good prognosis in cancer (21,37). M1
macrophages are associated with the improved survival of patients
with cervical cancer (38,39). The present study found that high
transcriptional levels of IL-12 were significantly associated with
a prolonged OS in patients with cervical cancer using the UALCAN
and GEPIA databases. The results also demonstrated that IL-12, CD80
and iNOS were enriched in M1 macrophages, and M1 macrophages
inhibited HeLa cell proliferation and promoted HeLa cell apoptosis.
M2 macrophages, known as the ‘aggressor’ of tumours, may support
tumour growth directly via the secretion of tumour-promoting
cytokines and indirectly promote angiogenesis, cancer stem cells or
the development of an immune-evasive microenvironment (40,41). The
present study demonstrated that patients with cervical cancer with
low transcriptional levels of IL-10 had a prolonged OS using the
UALCAN, GEPIA and GSE databases. Moreover, it was confirmed that
IL-10 and ARG expression was higher in the M2 macrophage group than
in the M1 macrophage group.
Cervical cancer is closely related to a persistent
infection of high-risk HPV. HPV vaccines are effective in
preventing cervical cancer; however, these vaccines do not
eliminate persistent HPV infections or prevent infected tissues
from developing into malignant tumours (42). BCG has largely been used as a
tuberculosis (TB) vaccine for 100 years (43). The intravesical treatment of BCG is
the primary choice for treatment, and it has become the gold
standard of immunotherapy for superficial bladder cancer (5,44).
Immunostimulation with BCG markedly annihilates existing carcinoma
in situ and suppresses the possibility of tumour progression
or recurrence in treating bladder cancer, melanoma, kidney cancer,
liver cancer, lymphoma, and acute leukaemia (45–47). The
wide range of protection by BCG vaccine against non-associated
pathogens and cancer to promote innate immunity and connect
adaptive immune responses, stresses the urgent need to re-examine
such microbe-based combination therapeutics for cancer treatment,
particularly through local administration (48–50). BCG
immunotherapy affects macrophage polarization in bladder carcinoma
(51). In the present study, it was
found that the levels of M1 markers, such as IL-12 and iNOS, were
significantly increased in BCG-activated macrophages and that the
levels of M2 markers, such as IL-10 and ARG, were decreased in
BCG-activated macrophages. These results indicate that BCG promotes
macrophage polarization toward the M1 phenotype and enhances the
transition of M2 to M1 macrophages.
The molecular mechanism of HPV carcinogenesis is the
integration of viral DNA into the chromosome of the host, and the
E6 and E7 oncoproteins combine with the tumour suppressor genes,
p53 and Rb, respectively, which leads to the inactivation of p53
and Rb, alterations in the cell cycle and DNA repair, and the
progression of invasive cervical cancer (2,52). Rb is a
key player in cell cycle regulation in which the tumour suppression
function is inactivated via phosphorylation, and the tumour
promotion function is inhibited via caspase cleavage (53). Rb inhibits cell proliferation by
directly suppressing the transcription factor E2F1, which promotes
cell growth, cell differentiation and DNA synthesis (54). The present study examined a series of
DEGs associated with survival and identified the survival-based
cell cycle, immune response and p53 signalling pathway in cervical
cancer from the GEO database. The results revealed that the
expression of p-Rb/E2F1 proteins was significantly higher in the M2
macrophage group than in the other groups and decreased after
treatment with BCG.
In the present study, it was also demonstrated that
BCG suppressed the activation of M2 macrophages by enhancing the
proliferation of HeLa cells. It was also identified that M1
macrophages promoted apoptosis and that BCG further promoted the
apoptosis of HeLa cells. These data suggest that BCG promotes the
anti-tumour progression of M1 macrophages and inhibits the
pro-tumour activation of M2 macrophages in HeLa cells. It was also
found that BCG-induced M1/M2 macrophages influenced HeLa cell
proliferation by increasing Rb/E2F1 expression.
In conclusion, the BCG vaccine has immunomodulatory
properties that have recently been revealed (55). The findings of the present study
provide valuable insight into the potential clinical therapeutic
effects of BCG that may benefit patients with cervical cancer. BCG
may prove to be an effective preventive measure for cervical
cancer. Consequently, further experiments are needed to obtain a
better understanding of the molecular mechanisms through which BCG
regulates M1/M2 polarization and activates the cervical cancer
microenvironment, and to confirm the findings presented herein.
Acknowledgements
The authors would like to thank Dr Taoliang Chen and
Dr Weimin Huang for their insightful discussions with the present
study. The authors would also thank Professor Linlang Guo
(Department of Pathology, Zhujiang Hospital, Southern Medical
University, Shenzhen, China) for editing this manuscript.
Funding
The present study was supported by grants from the
Shenzhen Science and Technology Innovation Committee (nos.
JCYJ20160427190929616 and JCYJ20170307091700193) and the Shenzhen
San Ming Project of Medicine (no. SZSM 201612042).
Availability of data and materials
The datasets used and/or analysed during the current
study are available from the corresponding author upon reasonable
request.
Authors' contributions
ZL, LL, HH and KL designed the experiments. LL, WS
and XX performed the experiments. LL, XW and SY performed the
statistical analyses. LL wrote the first draft of the manuscript,
and all authors commented on the subsequent drafts. ZL and LL
reviewed the final draft and confirmed the authenticity of all the
raw data. All authors have read and approved the final
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Cancer Genome Atlas Research Network,
Albert Einstein College of Medicine, Analytical Biological
Services, Barretos Cancer Hospital, Baylor College of Medicine,
Beckman Research Institute of City of Hope; Buck Institute for
Research on Aging; Canada's Michael Smith Genome Sciences Centre;
Harvard Medical School, ; Helen F, et al: Integrated genomic and
molecular characterization of cervical cancer. Nature. 543:378–384.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Mossanen M and Gore JL: The burden of
bladder cancer care: Direct and indirect costs. Curr Opin Urol.
24:487–491. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Gandhi NM, Morales A and Lamm DL: Bacillus
Calmette-Guerin immunotherapy for genitourinary cancer. BJU Int.
112:288–297. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kiselyov A, Bunimovich-Mendrazitsky S and
Startsev V: Treatment of non-muscle invasive bladder cancer with
Bacillus Calmette-Guerin (BCG): Biological markers and simulation
studies. BBA Clin. 4:27–34. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Pichler R, Borena W, Schäfer G, Manzl C,
Culig Z, List S, Neururer S, Von Laer D, Heidegger I, Klocker H, et
al: Low prevalence of HPV detection and genotyping in non-muscle
invasive bladder cancer using single-step PCR followed by reverse
line blot. World J Urol. 33:2145–2151. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Metawea B, El-Nashar AR, Kamel I, Kassem W
and Shamloul R: Application of viable bacille Calmette-Guerin
topically as a potential therapeutic modality in condylomata
acuminata: A placebo-controlled study. Urology. 65:247–250. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Fayed ST, Amer M, Ammar E and Salam MA:
Local BCG injection administered to patients with flat condyloma of
the cervix. Int J Gynaecol Obstet. 107:253–254. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Böhle A, Büttner H and Jocham D: Primary
treatment of condylomataacuminata with viable bacillus
Calmette-Guerin. J Urol. 165:834–836. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Cappello F: Effect of of cervical
antitumoral immunity activation on CIN. Minerva Ginecol.
43:405–408. 1991.(In Italian). PubMed/NCBI
|
|
11
|
Lu X, Wu L, Liu Z, Xie L and Wang S:
Peripheral blood mononuclear cells inhibit proliferation and
promote apoptosis of HeLa cells following stimulation with Bacillus
Calmette-Guerin. Exp Ther Med. 5:561–566. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sonoda T, Sugimura K, Ikemoto SI,
Kawashima H and Nakatani T: Significance of target cell infection
and natural killer cells in the anti-tumor effects of bacillus
Calmette-Guerin in murine bladder cancer. Oncol Rep. 17:1469–1474.
2007.PubMed/NCBI
|
|
13
|
Piaggio F, Kondylis V, Pastorino F, Di
Paolo D, Perri P, Cossu I, Schorn F, Marinaccio C, Murgia D, Daga
A, et al: A novel liposomal Clodronate depletes tumor-associated
macrophages in primary and metastatic melanoma: Anti-angiogenic and
anti-tumor effects. J Control Release. 223:165–177. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Geissmann F, Manz MG, Jung S, Sieweke MH,
Merad M and Ley K: Development of monocytes, macrophages, and
dendritic cells. Science. 327:656–661. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Mantovani A, Sozzani S, Locati M, Allavena
P and Sica A: Macrophage polarization tumor-associated macrophages
as a paradigm for polarized M2 mononuclear phagocytes. Trends
Immunol. 11:232002.PubMed/NCBI
|
|
16
|
Sica A and Mantovani A: Macrophage
plasticity and polarization: In vivo veritas. J Clin Invest.
122:787–795. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
17
|
Biswas SK and Mantovani A: Macrophage
plasticity and interaction with lymphocyte subsets: Cancer as a
paradigm. Nat Immunol. 11:889–896. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zheng X, Turkowski K, Mora J, Brüne B,
Seeger W, Weigert A and Savai R: Redirecting tumor-associated
macrophages to become tumoricidal effectorsas a novel strategy.
Oncotarget. 29:48436–48452. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Liu Q, Tian Y, Zhao X, Jing H, Xie Q, Li
P, Li P, Li D, Yan D and Zhu X: NMAAP1 expressed in BCG-activated
macrophage promotes M1 macrophage polarization. Mol Cells.
38:886–894. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
van Dalen FJ, van Stevendaal M, Fennemann
FL, Verdoes M and Ilina O: Molecular repolarisation of
tumour-associated macrophages. Molecules. 24:92018. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Mills CD: M1 and M2 macrophages: Oracles
of health and disease. Crit Rev Immunol. 32:463–488. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Schiffman M, Doorbar J, Wentzensen N, de
Sanjose S, Fakhry C, Monk BJ, Stanley MA and Franceschi S:
Carcinogenic human papillomavirus infection. Nat Rev Dis Primers.
2:160862016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
de Martel C, Plummer M, Vignat J and
Franceschi S: Worldwide burden of cancer attributable to HPV by
site, country and HPV type. Int J Cancer. 141:664–670. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Stevanovic S, Draper LM, Langhan MM,
Campbell TE, Kwong ML, Wunderlich JR, Dudley ME, Yang JC, Sherry
RM, Kammula US, et al: Complete regression of metastatic cervical
cancer after treatment with human papillomavirus-targeted
tumor-infiltrating T cells. J Clin Oncol. 33:1543–1550. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Seiwert TY, Burtness B, Mehra R, Weiss J,
Berger R, Eder JP, Heath K, McClanahan T, Lunceford J, Gause C, et
al: Safety and clinical activity of pembrolizumab for treatment of
recurrent or metastatic squamous cell carcinoma of the head and
neck (KEYNOTE-012): An open-label, multicentre, phase 1b trial.
Lancet Oncol. 17:956–965. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Morris VK, Salem ME, Nimeiri H, Iqbal S,
Singh P, Ciombor K, Polite B, Deming D, Chan E, Wade JL, et al:
Nivolumab for previously treated unresectable metastatic anal
cancer (NCI9673): A multicentre, single-arm, phase 2 study. Lancet
Oncol. 18:446–453. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
van der Burg SH and Melief CJ: Therapeutic
vaccination against human papilloma virus induced malignancies.
Curr Opin Immunol. 23:252–257. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Stevanovic S, Pasetto A, Helman SR,
Gartner JJ, Prickett TD, Howie B, Robins HS, Robbins PF, Klebanoff
CA, Rosenberg SA and Hinrichs CS: Landscape of immunogenic tumor
antigens in successful immunotherapy of virally induced epithelial
cancer. Science. 356:200–205. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Tang Z, Li C, Kang B, Gao G, Li C and
Zhang Z: GEPIA: A web server for cancer and normal gene expression
profiling and interactive analyses. Nucleic Acids Res. 45:W98–W102.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Chandrashekar DS, Bashel B, Balasubramanya
SAH, Creighton CJ, Ponce-Rodriguez I, Chakravarthi B and Varambally
S: UALCAN: A portal for facilitating tumor subgroup gene expression
and survival analyses. Neoplasia. 19:649–658. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Huang DW, Sherman BT and Lempicki RA:
Systematic and integrative analysis of large gene lists using DAVID
bioinformatics resources. Nat Protoc. 4:44–57. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Franceschini A, Szklarczyk D, Frankild S,
Kuhn M, Simonovic M, Roth A, Lin J, Minguez P, Bork P, von Mering C
and Jensen LJ: STRING v9.1: Protein-protein interaction networks,
with increased coverage and integration. Nucleic Acids Res.
41:D808–D815. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Liu H, Zhang L and Wang P: Complement
factor H-related 3 overexpression affects hepatocellular carcinoma
proliferation and apoptosis. Mol Med Rep. 20:2694–2702.
2019.PubMed/NCBI
|
|
35
|
Sánchez-Rodríguez C, Cruces KP, Ayora JR,
Martín-Sanz E and Sanz-Fernández R: BCG immune activation reduces
growth and angiogenesis in an in vitro model of head and neck
squamous cell carcinoma. Vaccine. 35:6395–6403. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Greten FR and Grivennikov SI: Inflammation
and cancer: Triggers, mechanisms, and consequences. Immunity.
51:27–41. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Honkanen TJ, Tikkanen A, Karihtala P,
Mäkinen M, Väyrynen JP and Koivunen JP: Prognostic and predictive
role of tumour-associated macrophages in HER2 positive breast
cancer. Sci Rep. 9:109612019. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Yang M, McKay D, Pollard JW and Lewis CE:
Diverse functions of macrophages in different tumor
microenvironments. Cancer Res. 78:5492–5503. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
de Vos van Steenwijk PJ, Ramwadhdoebe TH,
Goedemans R, Doorduijn EM, van Ham JJ, Gorter A, van Hall T,
Kuijjer ML, van Poelgeest MIE, van der Burg SH and Jordanova ES:
Tumor-infiltrating CD14-positive myeloid cells and CD8-positive
T-cells prolong survival in patients with cervical carcinoma. Int J
Cancer. 133:2884–2894. 2013.PubMed/NCBI
|
|
40
|
Jayasingam SD, Citartan M, Thang TH, Zin
AA, Ang KC and Ch'ng ES: Evaluating the polarization of
tumor-associated macrophages into M1 and M2 phenotypes in human
cancer tissue: Technicalities and challenges in routine clinical
practice. Front Oncol. 9:15122019. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Mantovani A, Marchesi F, Malesci A, Laghi
L and Allavena P: Tumour-associated macrophages as treatment
targets in oncology. Nat Rev Clin Oncol. 14:399–416. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
English PMB: Eradicating cervical cancer
is unlikely. BMJ. 366:l49532019. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Luca S and Mihaescu T: History of BCG
vaccine. Maedica (Bucur). 8:53–58. 2013.PubMed/NCBI
|
|
44
|
Locht C and Lerm M: Good old BCG-what a
century-old vaccine can contribute to modern medicine. J Intern
Med. 12:611–613. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Mustafa AS: BCG as a vector for novel
recombinant vaccines against infectious diseases and cancers.
Vaccines (Basel). 84:7362020. View Article : Google Scholar
|
|
46
|
Xue QJ, Li YQ, Yang CQ, Chen T, Li XZ,
Cheng B and Wang CM: Anti-tumour research of recombinant BCG using
BZLF1 and hGM-CSF fusion genes. Vaccine. 14:1599–1607. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Kremenovic M, Schenk M and Lee DJ:
Clinical and molecular insights into BCG immunotherapy for
melanoma. J Intern Med. 288:625–640. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Koti M, Morales A, Graham CH and Siemens
DR: BCG vaccine and COVID-19: Implications for infection
prophylaxis and cancer immunotherapy. J Immunother Cancer.
8:e0011192020. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Usher NT, Chang S, Howard RS, Martinez A,
Harrison LH, Santosham M and Aronson NE: Association of BCG
vaccination in childhood with subsequent cancer diagnoses A 60-year
follow-up of a clinical trial. JAMA Netw Open. 2:e19120142019.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Jabbar IA, Fernando GJ, Saunders N,
Aldovini A, Young R, Malcolm K and Frazer IH: Immune responses
induced by BCG recombinant for human papillomavirus L1 and E7
proteins. Vaccine. 18:2444–2453. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Svatek RS, Zhao XR, Morales EE, Jha MK,
Tseng TY, Hugen CM, Hurez V, Hernandez J and Curiel TJ: Sequential
intravesical mitomycin plus Bacillus Calmette-Guerin for
non-muscle-invasive urothelial bladder carcinoma: Translational and
phase I clinical trial. Clin Cancer Res. 21:303–311. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Doorbar J, Egawa N, Griffin H, Kranjec C
and Murakami I: Human papillomavirus molecular biology and disease
association. Rev Med Virol. 25 (Suppl 1):S2–S23. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Borges HL, Bird J, Wasson K, Cardiff RD,
Varki N, Eckmann L and Wang JY: Tumor promotion by
caspase-resistant retinoblastoma protein. Proc Natl Acad Sci.
102:15587–15592. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
van den Heuvel S and Dyson NJ: Conserved
functions of the pRB and E2F families. Nat Rev Mol Cell Biol.
9:713–724. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Yamazaki-Nakashimada MA, Unzueta A,
Gámez-González LB, González-Saldaña N and Sorensen RU: BCG: A
vaccine with multiple faces. Hum Vaccin Immunother. 16:1841–1850.
2020. View Article : Google Scholar : PubMed/NCBI
|