Introduction
Public health officials in Mexico City were
confronted with an outbreak of influenza late in the 2009 influenza
season. The 2009 pandemic H1N1 virus contained a unique combination
of genes from both North American and Eurasian swine lineages that
had not been previously indentified in either swine or human
populations (1,2). The pandemic 2009 H1N1 hemagglutinin
(HA) was found to be antigenically and genetically distinct from
the HA of contemporary human seasonal influenza H1N1 viruses, but
had a greater similarity to the swine H1N1 influenza virus that
caused an influenza outbreak among military recruits in Fort Dix,
New Jersey, in 1976 (1,3). Little is known about the level of
pre-existing immunity to 2009 H1N1 in humans, which is one of the
determining factors for susceptibility to a novel influenza virus.
Vaccination has been a mainstay of influenza prevention, with
annual vaccination recommended for adults and children at high
risk. However, vaccination with recent seasonal influenza vaccines
induced little or no cross-reactive antibody response to the
pandemic A/ H1N1 2009 influenza virus in any age group in human
populations (4). Accordingly, most
people had low immunity against this novel pathogen, thus resulting
in the worldwide spread of the infection to produce a so-called
‘pandemic’.
A convenient method for the mass production of
antibodies has been developed using the female ostrich (Struthio
camelus) (5,6). The avian egg has proven to be an
attractive source for the noninvasive production of antibodies,
with applications in research, diagnosis and immunotherapy
(7–9). In addition, the production of avian
antibodies offers many advantages over mammalian antibodies with
regard to the specificity for antigens, production cost and their
uses (7). The predominant class of
immunoglobulin in birds is immunoglobulin yolk (IgY), which is
transferred from the serum to the yolk to confer passive immunity
to the embryo (10,11). The ostrich grows to be 250 cm in
height and 160 kg in weight, and their life span is appoximately 60
years. Ostrich eggs weigh approximately 1.5 kg and are 30-fold
larger than chicken eggs. Ostrich can lay one hundred eggs every
year. It is possible to purify about 2–4 g of IgY per ostrich egg.
Accordingly, approximately 400 g of IgY can be obtained from only
one ostrich in the course of a year. Therefore, the ostrich egg
might provide an excellent source of antibodies for industrial
purposes (5).
The present study demonstrated that a large amount
of cross-reactive and neutralizing antibodies to the pandemic
influenza virus A/H1N1 was generated by the ostrich using a simple
and economical method involving immunization with a seasonal
influenza viral vaccine.
Materials and methods
Generation of antibodies against seasonal
influenza virus HA antigens
A mixture of HA antigens of vaccine strains of
seasonal influenza virus, A/NewCaledonia/20/99 (H1N1), A/
Hiroshima/52/2005 (H3N2) and B/Malaysia (The Kitasato Institute
Research Center for Biologicals, Japan) was used as antigens for
the immunization of the ostrich. The female ostrich were immunized
intramuscularly in the lumber region at multiple sites with 30
μg of the mixture of HA. Boosters were administered every
other week with each antigen. The eggs were then collected 4 weeks
after the initial immunization. The yolk was separated from the
albumin of the eggs and diluted 5-fold with TBS buffer [0.02 M
Tris/HCl (pH 7.5), 0.15 M NaCl], and an initial 1/10-fold with 30%
dextran sulfate in TBS and 2/3-fold with 2.5 M CaCl2 in
TBS, and then stored at 4°C for at least 4 h. The supernatant
containing the IgY was collected by centrifugation (10,000 × g at
4°C for 15 min) and precipitated with 45% saturated ammonium
sulfate. The solution was centrifuged again at 10,000 × g at 4°C
for 15 min. The precipitate was then redissolved in TBS and
dialyzed against PBS. Finally, the purified antibody solutions were
verified by 10% SDS-PAGE under non-reducing conditions and stained
with Coomassie Brilliant Blue (CBB).
Enzyme-linked immunosorbent assay
(ELISA)
Each well of a polystyrene ELISA plate (Sumitomo
Bakelite, Japan) was coated with 0.2 μg of HA antigens from
each vaccine strain and pandemic A/H1N1 (Protein Science, USA), and
the plate was incubated overnight at 4°C. Each of the following
incubation steps was preceded by washing the wells twice with PBS
containing 0.05% Tween-20. The wells were blocked for nonspecific
binding by the addition of a commercial blocking buffer (DS Pharma
Biomedical, Japan) and incubated at 37°C for 2 h. Serial dilutions
of purified ostrich IgY generated by the seasonal influenza vaccine
immunization were added vertically to the wells and kept for
incubation at 37°C for 1 h. The HRP-conjugated rabbit IgG against
ostrich IgY (5) diluted 1:5,000 in
PBS was dispensed into each well. The plate was incubated for 1 h
at 37°C and washed. A substrate buffer containing TMB (Sumitomo
Bakelite, Japan) was added to each well and kept for incubation at
37°C for 15 min. The reaction was terminated by the addition of a
stopping reagent (1.25 M sulfuric acid). The absorbance was
recorded at 450 nm using an ELISA plate reader (DS Pharma
Biomedical).
Influenza viruses
Seasonal influenza viruses [A/Osaka/309/2007 (H1N1),
A/Osaka/2587/2005 (H3N2) and B/Osaka 21/2005] and a pandemic virus
[A/Osaka/2040/2009 (H1N1) pdm] cloned from patients in Osaka
Prefecture, Japan, were used throughout this study. The viral
solutions were titered as TCID50 using a cell culture
system (MDCK cells) onto 96-well microtiter plates by serial 4-fold
dilutions of the samples in the routine manner.
Immunocytochemistry
MDCK cells were independently infected with each
influenza virus (102 TCID50) for 2–5 days at
35°C. The infected cells were fixed with 10% buffered formalin for
immunocytochemistry. The cells were washed in PBS, incubated with
the ostrich IgY generated by seasonal influenza vaccine
immunization (1:4000) for 1 h at 37°C and incubated with
FITC-conjugated rabbit IgG (1:4000) against ostrich IgY (5) following a sufficient number of washes
in PBS. Finally, the specific signal was observed using
fluorescence microscopy.
Hemagglutination inhibition (HI)
test
Serial dilutions of ostrich IgY were mixed with 8-HA
units of each influenza virus in clear 96-well micro-test
polystyrene assay plates (Becton Dickinson, USA). The plates were
incubated for 30 min at room temperature. Guinea pig erythrocytes
were added, pipetted gently, and incubation was carried out for
another 45 min at room temperature. Each well was observed, and the
HI titers were scored based on the HA titer with immune IgY versus
the HA titer with preimmune IgY (a higher ratio indicates a
stronger inhibitory activity of the antibody against the pandemic
influenza virus).
Neutralization assays for influenza virus
infection
Serial dilutions of ostrich IgY were mixed at a
ratio of 1:1 with influenza viruses at 102
TCID50, incubated for 1 h at 37°C, and transferred to a
microtiter plate with an MDCK cell monolayer. The cultures were
incubated for 2–3 days at 35°C and inspected to determine the
cytopathic effect (CPE). The neutralizing titer, expressed as the
reciprocal of the IgY dilution at which virus growth is inhibited
by 50%, was calculated by the number of virus negative wells and
the IgY dilution (12).
Results
Cross-reactive antibody responses of
ostrich IgY to the pandemic influenza virus A/H1N1
IgY was purified from eggs produced by female
ostrich immunized with a seasonal influenza vaccine. The molecular
weight of purified IgY was ∼200 kDa (SDS-PAGE; data not shown).
Each immunized ostrich egg yielded ∼4 g of IgY. The reactivity of
the IgY to the seasonal and pandemic influenza viruses was
estimated by ELISA. The antibody titers for each antigen of the
seasonal A/H1N1, A/H3N2 and B viruses were increased dramatically
in the ostrich yolk after immunization (Table I). In addition, it appeared that
the antibody had a high cross-reactivity to the pandemic influenza
viral antigens.
 | Table I.Cross-reactivities of ostrich
antibodies (IgY) generated by seasonal influenza vaccine
immunization. |
Table I.
Cross-reactivities of ostrich
antibodies (IgY) generated by seasonal influenza vaccine
immunization.
| Antibody | Antibody titer
against the indicated influenza virus HA antigens
|
|---|
Seasonal influenza
viruses
| Pandemic influenza
virus
|
|---|
| A/H1N1 | A/H3N2 | B | 2009 A/H1N1 |
|---|
| Ostrich IgY | 102,400 | 204,800 | 102,400 | 51,200 |
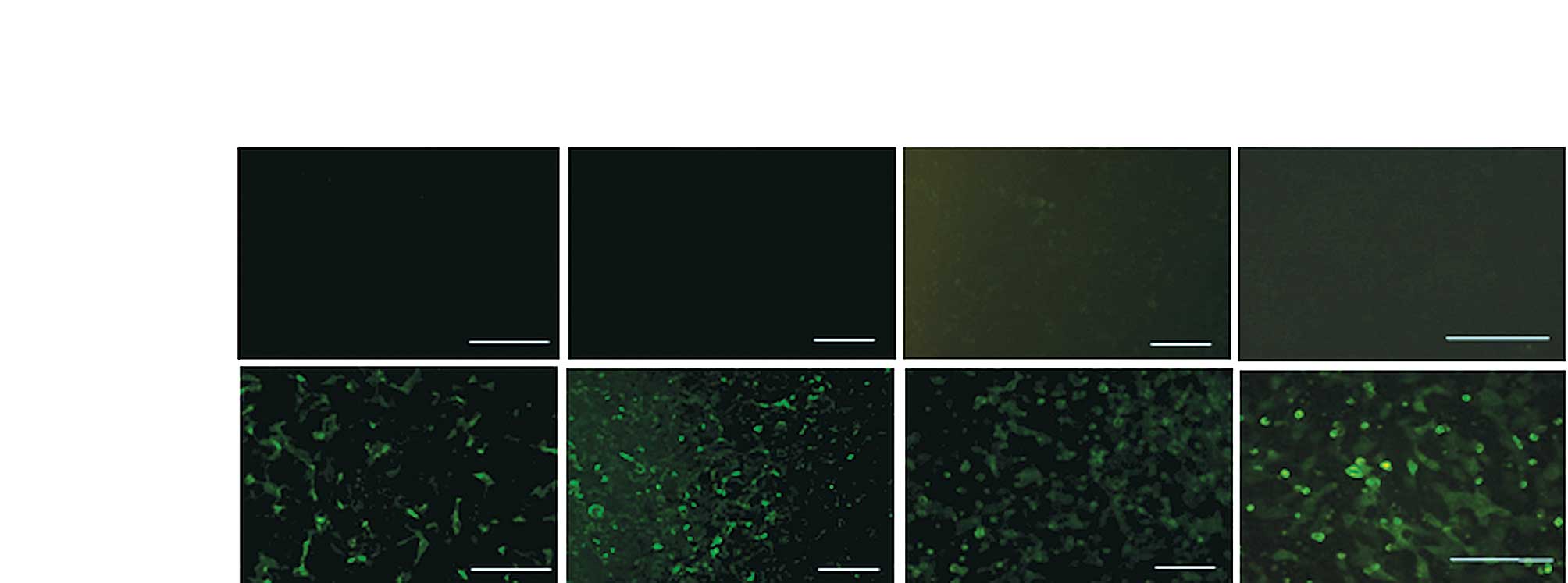
The reactivity of the ostrich IgY to the infectious
influenza viruses was examined by immunocytochemistry. MDCK cells
were infected with each seasonal influenza virus and pandemic
influenza virus A/H1N1. The infected cells were fixed and reacted
with IgY generated by the seasonal influenza vaccine immunization.
The cytoplasm of the cells infected with the seasonal A/H1N1,
A/H3N2 and B viruses was strongly labeled with the IgY (Fig. 1). In contrast, preimmune IgY did
not react with the infected cells. Notably, the cells infected with
the pandemic influenza virus also reacted with the IgY. This
demonstrated the cross-reactivity of the ostrich antibodies to the
pandemic influenza virus A/H1N1, thereby supporting the ELISA
findings.
Inhibition of hemaggregation activities
of pandemic influenza virus A/H1N1 by ostrich IgY
The HA activities of the influenza viruses were
estimated using erythrocytes, since the viral strains in this study
originated from sporadic cases of infection and their
characteristics had not yet been clarified. The highest dilutions
of viral fluids showing hemaggregation were scored as a single HA
unit. Each strain of the influenza virus was used for HI testing at
8-HA units.
Hemaggregation by the seasonal A/H1N1, A/H3N2 and B
viruses was dramatically inhibited by the ostrich IgY. Importantly,
the hemaggregation activities of the pandemic influenza virus
A/H1N1 were also impeded by the IgY (Table II). Accordingly, this antibody
blocked the HA on the surface of the pandemic A/H1N1 virus as well
as on the seasonal influenza viruses, and also inhibited the
interaction of HA and erythrocytes, thereby leading to the
inhibition of hemaggregation.
 | Table II.Inhibitory activity of ostrich IgY on
hemaggregations by the pandemic influenza virus A/H1N1. |
Table II.
Inhibitory activity of ostrich IgY on
hemaggregations by the pandemic influenza virus A/H1N1.
| Seasonal influenza
viruses
| Pandemic influenza
virus
|
|---|
| A/H1N1 | A/H3N2 | B | 2009 A/H1N1 |
|---|
| HI titer | 512 | 512 | 128 | 256 |
Neutralization assays for pandemic
influenza virus A/H1N1 infection
The seasonal and pandemic influenza viruses were
reacted with the ostrich IgY followed by inoculation into MDCK
cells. The degree of neutralization was determined by the
observation of CPE after 4 days of inoculation. As shown in
Table III, the IgY strongly
inhibited the infectivity of all seasonal influenza viruses,
A/H1N1, A/H3N2 and B; even a small volume of IgY obstructed the
infections of the viruses in the MDCK cells. Notably, the infection
of pandemic influenza virus A/H1N1 to MDCK cells was also strongly
inhibited by the ostrich IgY. The IgY appeared to bind to the HA of
the pandemic influenza virus as well as of the seasonal viruses,
and blocked the interaction between viral particles and the
receptors on cells, thus leading to the inhibition of viral
infectivity.
 | Table III.Neutralizing activities of ostrich IgY
against the infectivities of seasonal and pandemic influenza
viruses. |
Table III.
Neutralizing activities of ostrich IgY
against the infectivities of seasonal and pandemic influenza
viruses.
| Antibody (IgY) | Neutralizing titers
(μg/ml)
|
|---|
Seasonal influenza
viruses
| Pandemic influenza
virus
|
|---|
| A/H1N1 | A/H3N2 | B | 2009 A/H1N1 |
|---|
| Preimmune IgY | >384.0 | >384.0 | >384.0 | >384.0 |
| Immunized IgY | 2.6 | 8.9 | 22.3 | 11.2 |
Discussion
Hemagglutinin is essential for viral binding to
cells and entrance into host cells. Therefore, these antigens are
widely used in the vaccination against influenza in humans
(13). The inhibition of HA
antigens by antibodies is useful for protecting against these viral
infections.
The pandemic influenza virus belongs to strain
A/H1N1, but its HA is genetically distinct from the HA of seasonal
A/H1N1 (2). Vaccination with
seasonal influenza vaccines, even when formulated with oil-in-water
adjuvants, was found to provide little or no benefit to any age
group in human populations with respect to an increase in
cross-reactive neutralizing antibodies against pandemic A/H1N1
(4). Importantly, the present
study demonstrated that vaccination of a seasonal trivalent
influenza HA vaccine into the ostrich resulted in a marked increase
in the level of cross-reactive antibody to pandemic influenza A/
H1N1. One important finding of the present study was the
observation that ostrich IgY obstructed the hemaggregation
activities of the pandemic influenza virus A/H1N1. The HA antigens
on the pandemic influenza virus A/H1N1 might be masked by ostrich
antibodies, thus resulting in the effective blocking of viral
adsorption into the cells. The neutralization activities of ostrich
antibodies were assessed using living cells to confirm this
paradigm. The infection of pandemic influenza viruses from patients
was prevented by the IgY, and the CPE of MDCK cells was
dramatically inhibited by the antibodies. Therefore, ostrich
antibodies might inhibit the entrance of the pandemic influenza
virus into cells by blocking HA activities, thus resulting in the
escape of cells from viral infection. The ostrich IgY against
seasonal A/H1N1 in the trivalent vaccine had cross-reactivity to
pandemic A/H1N1, because of the antigenic similarity among strains
of A/H1N1. Ongoing studies are underway to determine the mechanism
by which ostrich produce cross-reactive neutralizing antibodies to
pandemic influenza virus A/H1N1 by immunization with seasonal
influenza vaccine.
There is an increasing need for the development of
antibodies for research, diagnostic and therapeutic purposes.
However, antibodies from experimental mammals, including the mouse
and rabbit, are not suited for industrial use because of their high
production cost. The avian egg has proven to be an attractive
source for the non-invasive production of antibodies, with
applications in research, diagnosis and immunotherapy (8,9). A
simple and economical method has been developed for the mass
production of antibodies, and 4 g of IgY can be purified from one
yolk; thus, 400 g of antibodies can be obtained from one female
ostrich in one year (5). This
suggests that anti-pandemic influenza virus antibodies can be
provided in large quantities at a relatively low price using
ostrich. Accordingly, the ostrich egg might provide an excellent
source of antibodies for industrial purposes.
Recently, various types of facial masks and
air-conditioner filters have been used for the prevention of
airborne infections. However, the small influenza virus can pass
through currently used filters, thus resulting in human infection,
as the virus is still alive even after drying (14–16).
Therefore, a high grade filter employing new prevention mechanisms
must be developed. Ostrich IgY is being applied to filters, and can
protect against influenza viruses by antigen-antibody reactions. In
the present study, a large amount of cross-reactive neutralization
antibodies against the various influenza viruses, including
pandemic A/ H1N1, was produced in a cost-effective manner,
indicating the potential of ostrich antibodies for industrial
purposes. Filters impregnated with ostrich antibodies may therefore
become a powerful tool for protecting humans against pandemic
influenza viruses.
Acknowledgements
We thank Dr Tetsuo Kase at the Osaka
Prefectural Institute of Public Health for providing the clinical
strains of the influenza viruses. We also thank Dr Yoji Goto at The
Kitasato Institute Research Center for Biologicals, Japan, for
providing the HA antigens of the influenza virus vaccine strains.
This study was supported in part by a Grant-in-Aid for Scientific
Research (no. 21380182) from the Ministry of Education, Science,
Sports and Culture, Japan.
References
|
1.
|
Garten RJ, Davis CT, Russell CA, Shu B,
Lindstrom S, Balish A, Sessions WM, Xu X, Skepner E, Deyde V,
Okomo-Adhiambo M, Gubareva L, Barnes J, Smith CB, Emery SL, Hillman
MJ, Rivailler P, Smagala J, De Graaf M, Burke DF, Fouchier RA,
Pappas C, Alpuche-Aranda CM, López-Gatell H, Olivera H, López I,
Myers CA, Faix D, Blair PJ, Yu C, Keene KM, Dotson PD Jr, Boxrud D,
Sambol AR, Abid SH, St George K, Bannerman T, Moore AL, Stringer
DJ, Blevins P, Demmler-Harrison GJ, Ginsberg M, Kriner P, Waterman
S, Smole S, Guevara HF, Belongia EA, Clark PA, Beatrice ST, Donis
R, Katz J, Finelli L, Bridges CB, Shaw M, Jernigan DB, Uyeki TM,
Smith DJ, Klimov AI and Cox NJ: Antigenic and genetic
characteristics of swine-origin 2009 A(H1N1) influenza viruses
circulating in humans. Science. 325:197–201. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Novel Swine-Origin Influenza A (H1N1)
Virus Investigation Team: Emergence of a novel swine-origin
influenza A (H1N1) virus in humans. N Engl J Med. 360:2605–2615.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Nelson MI, Viboud C, Simonsen L, Bennett
RT, Griesemer SB, St-George K, Taylor J, Spiro DJ, Sengamalay NA,
Ghedin E, Taubenberger JK and Holmes EC: Multiple reassortment
events in the evolutionary history of H1N1 influenza A virus since
1918. PLoS Pathog. 4:e10000122008. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Hancock K, Veguilla V, Lu X, Zhong W,
Butler EN, Sun H, Liu F, Dong L, DeVos J, Gargiullo PM, Brammer TL,
Cox NJ, Tumpey TM and Katz JM: Cross-reactive antibody responses to
the 2009 pandemic H1N1 influenza virus. N Engl J Med.
361:1945–1952. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Adachi K, Handharyani E, Sari DK, Takama
K, Fukuda K, Endo I, Yamamoto R, Sawa M, Tanaka M, Konisi I and
Tsukamoto Y: Development of neutralization antibodies against
highly pathogenic H5N1 avian influenza virus using ostrich
(Struthio camelus) yolk. Mol Med Rep. 1:203–209.
2008.PubMed/NCBI
|
|
6.
|
Adachi K, Hagimori K, Kato H, Fukuda K,
Kikuta M and Tsukamoto Y: Potential role of SC1, a cell adhesion
molecule, in mammary gland tumors. Mol Med Rep. 1:219–224.
2008.PubMed/NCBI
|
|
7.
|
Schade R, Pfister C, Halatsch R and
Henklein P: Polyclonal IgY antibodies from chicken egg yolk – an
alternative to the production of mammalian IgG type antibodies in
rabbits. ATLA. 19:403–419. 1991.
|
|
8.
|
Schade R, Schniering A and Hlinak A:
Polyclonal avian antibodies extracted from egg yolk as an
alternative to the production of antibodies in mammals – a review.
ALTEX. 9:43–56. 1992.PubMed/NCBI
|
|
9.
|
Gross M and Speck J: Avian yolk antibodies
in diagnosis and research. Dtsch Tierarztl Wochenschr. 103:417–422.
1996.PubMed/NCBI
|
|
10.
|
Larsson A, Balow RM, Lindahl TL and
Forsberg PO: Chicken antibodies: taking advantage of evolution – a
review. Poult Sci. 72:1807–1812. 1993.PubMed/NCBI
|
|
11.
|
Leslie GA and Clem LW: Phylogeny of
immunoglobulin structure and function. III. Immunoglobulins of the
chicken. J Exp Med. 130:1337–1352. 1969. View Article : Google Scholar
|
|
12.
|
Reed LJ and Muench H: A simple method of
estimating fifty percent endo-points. Am J Hyg. 27:493–797.
1938.
|
|
13.
|
Bosch FX, Orlinch M, Klenk HD and Rott R:
The structure of the hemagglutinin, a determinant for the
pathogenicity of influenza viruses. Virology. 95:197–207. 1979.
View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Wiwanitkit V: N-95 face mask for
prevention of bird-flu virus: An appraisal of nanostructure and
implication for infectious control. Lung. 184:373–374. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Gralton J and McLaws ML: Protecting
healthcare workers from pandemic influenza: N95 or surgical masks?
Crit Care Med. 38:657–667. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Aiello AE, Murray GF, Perez V, Coulborn
RM, Davis BM, Uddin M, Shay DK, Waterman SH and Monto AS: Mask use,
hand hygiene, and seasonal influenza-like illness among young
adults: a randomized intervention trial. J Infect Dis. 201:491–498.
2010. View
Article : Google Scholar : PubMed/NCBI
|