Contents
Molecular genetics in endometriosis-associated
ovarian cancer
Materials and methods
Article selection, data extraction and
assessment
Estrogen receptor expression and prognosis in clear
cell carcinoma
Factors contributing to the expression of estrogen
receptor
Conclusion
Molecular genetics in
endometriosis-associated ovarian cancer
Epithelial ovarian cancer (EOC) remains the number
one cause of death from gynecological malignancies. More than 50%
of the patients relapse within a few years. Among the EOC, the most
common morphological subtype is serous adenocarcinoma (SAC), with
less common subtypes including clear cell (CCC), mucinous (MAC) and
endometrioid (EAC), generally believed to originate from the
ovarian surface epithelium, distal fallopian tube epithelium or
peritoneal mesothelial cells. In Japan, however, CCC is not
uncommon and is the second most frequent (22%) type of EOC
(1). This tumor demonstrates a
clinical behavior different from that of the most common histotype
SAC. Patients with CCC have early stage with a large pelvic mass,
less ascites, chemoresistance and higher incidence of endometriosis
and thromboembolism (2). The
survival rates of patients with advanced CCC are lower than those
of patients with advanced SAC.
Although two histologic types, CCC and EAC, are the
common histology in ovarian cancer patients who have associated
endometriosis (also known as endometriosis-associated ovarian
cancer; EAOC) (3), both tumor
types have also distinct clinicopathological characteristics and
molecular phenotypes (4,5). The exact mechanisms that turn a
process of endometriosis into a cancer precursor are recent topics
of intense research. Animal models that resemble the EAOC are of
paramount importance in studying the carcinogenesis of these
diseases. Several methods for modeling EAC in animals are
available. A variety of molecular events, such as PTEN (phosphatase
and tensin homolog) silencing and KRAS (v-Ki-ras2 Kirsten rat
sarcoma viral oncogene homolog) mutations/activation, have been
frequently identified in EAC (6).
Despite advancements in the molecular analyses of EAOC and the
development of animal models, the mechanisms that underlie the
pathogenesis of CCC remain largely unknown. Representative CCC
animal models have not been available to date. The current
knowledge on the mechanisms involved in CCC carcinogenesis shows
that ovarian hemorrhage or retrograde menstruation carries highly
pro-oxidant factors, such as heme and iron, into the ovarian
endometrioma or peritoneal cavity (7). The persistence of a redox active iron
in the blood at the site of endometriosis development is important
to understand both induction and promotion of the EAOC. Taken
together, DNA damage or loss of heterozygosity (LOH) in an ectopic
endometrium caused by iron-induced oxidative stress may be a
critical factor in the carcinogenic process (8–11).
CCC carries worse prognoses than EAC tumors. EAC was
predominantly positive for estrogen receptor (ER), but CCC
specifically exhibits lower ER expression. Aberrant DNA methylation
of CpG islands has been recognized in carcinogenesis as a common
alteration associated with the loss of expression of a number of
key regulatory genes. Many studies have found that the loss of ER
expression is a result of the hypermethylation of the ER-α
promoter. Although hypermethylation is one of the best known
epigenetic events, other epigenetic events, such as histone
modification (deacetylation and methylation of histones), are
involved in the complex mechanism that regulates promoter
transcription, and drive stable, clonally propagated changes in
gene expression. The exact interplay of these factors in
transcriptional repression activity in CCC is not yet well
understood. This study reviews the current understanding of the
role of the ER information in the pathogenesis of CCC.
Materials and methods
The present article reviews the English language
literature for biological, pathogenetic and pathophysiological
studies on endometriosis and EAOC. We searched PubMed electronic
database for a 20-year period (1990–2010), combining the keywords
‘genome-wide’, ‘microarray’, ‘proteomics’, ‘estrogen receptor’,
‘oxidative stress’, ‘ROS’, ‘carcinogenesis’, ‘iron’, ‘HNF-1β’,
‘mutation’ and ‘chromatin remodeling’ with ‘endometriosis’,
‘ovarian cancer’, ‘clear cell carcinoma’ or
‘endometriosis-associated ovarian cancer’. Several recent studies
are discussed in the context of pathogenesis of CCC and DNA
mutations. Additionally, references in each article were searched
to identify potentially missed studies for a 20-year period.
Article selection, data extraction and
assessment
Although the main interest is the regulation of ER
expression obtained from human ovarian cancer samples, we have
included the in vitro studies in the knowledgebase. We also
included the animal models performed to support human data.
Initially, 58 potentially relevant studies were identified by
screening electronic databases. A total of 21 peer-reviewed journal
articles were additionally identified from references in each
article.
Estrogen receptor expression and prognosis
in clear cell carcinoma
Estrogen plays a role in ovarian tumorigenesis.
In vitro studies indicate that ovarian cancer cell growth is
dependent on estrogen stimulation. In a case of breast cancer,
ER-negative tumors fail to respond to endocrine therapy and have a
poor overall prognosis when compared to ER-positive tumors. The
ER-α mRNA level was a significantly positive prognostic factor for
patient survival. The prognostic significance of ER expression by
ovarian cancers has received little attention to date. One study
reported a correlation between levels of ER expression and cancer
disease stage, with levels declining with increased severity of
disease, suggesting that loss of ER expression in ovarian cancer is
a feature of malignant transformation and aggressiveness (12). Despite the poor prognosis of
patients with ER-negative disease, there remains considerable
heterogeneity in individual outcomes. Indeed, other studies
demonstrated that since ER has emerged as a mitogenic factor, ER
status is a prognostic factor for ovarian cancer with better
survival for ER-negative tumors (12). Furthermore, a higher ER expression
at the mRNA and protein levels was found to be associated with a
longer progression-free survival and overall survival (13). The remaining investigators
reported, however, that neither ER nor progesterone receptor (PR)
independently correlated with survival in the overall study
population. Therefore, there is a controversy as to whether ER
expression is a prognostic factor for outcomes in ovarian
cancer.
Among EAOC, EAC were predominantly positive for ER
and PR (14), but CCC specifically
exhibited lower ER and PR expression (14). The investigators put forward a
model postulating that additional events, particularly deletion of
ER expression, are required for CCC lesion progression. CCC
pathogenesis may be a model to study the disease progression from
estrogen-dependent to estrogen-independent, allowing design of new
strategies targeting the hormone response, thereby modifying
disease outcome. Therefore, loss of estrogen function may be a
turning point in CCC development. There are basically the following
hypotheses regarding the carcinogenesis or pathogenesis of CCC:
initially, the heme and iron-mediated oxidative stress and
persistent inflammation processes occur due to repeated hemorrhage
in endometriosis. These compounds oxidatively modify DNA, proteins
and lipids, and subsequently hypermethylation or ER depletion may
be observed (Fig. 1). Suzuki et
al reported that ER is inactivated mainly through aberrant DNA
methylation (15). A dualistic
model, which has been established on morphological and genomic
basis, differentiates EAOC into two broad categories:
estrogen-dependent ovarian cancers with an EAC morphology, and
estrogen-independent carcinoma with the CCC morphology (4). The genetic pathways utilized by
ER-negative tumors to proliferate in the absence of a mitogenic
estrogen signal are poorly understood. Elucidation of these
pathways is required for the development of improved therapies for
ER-negative CCC patients.
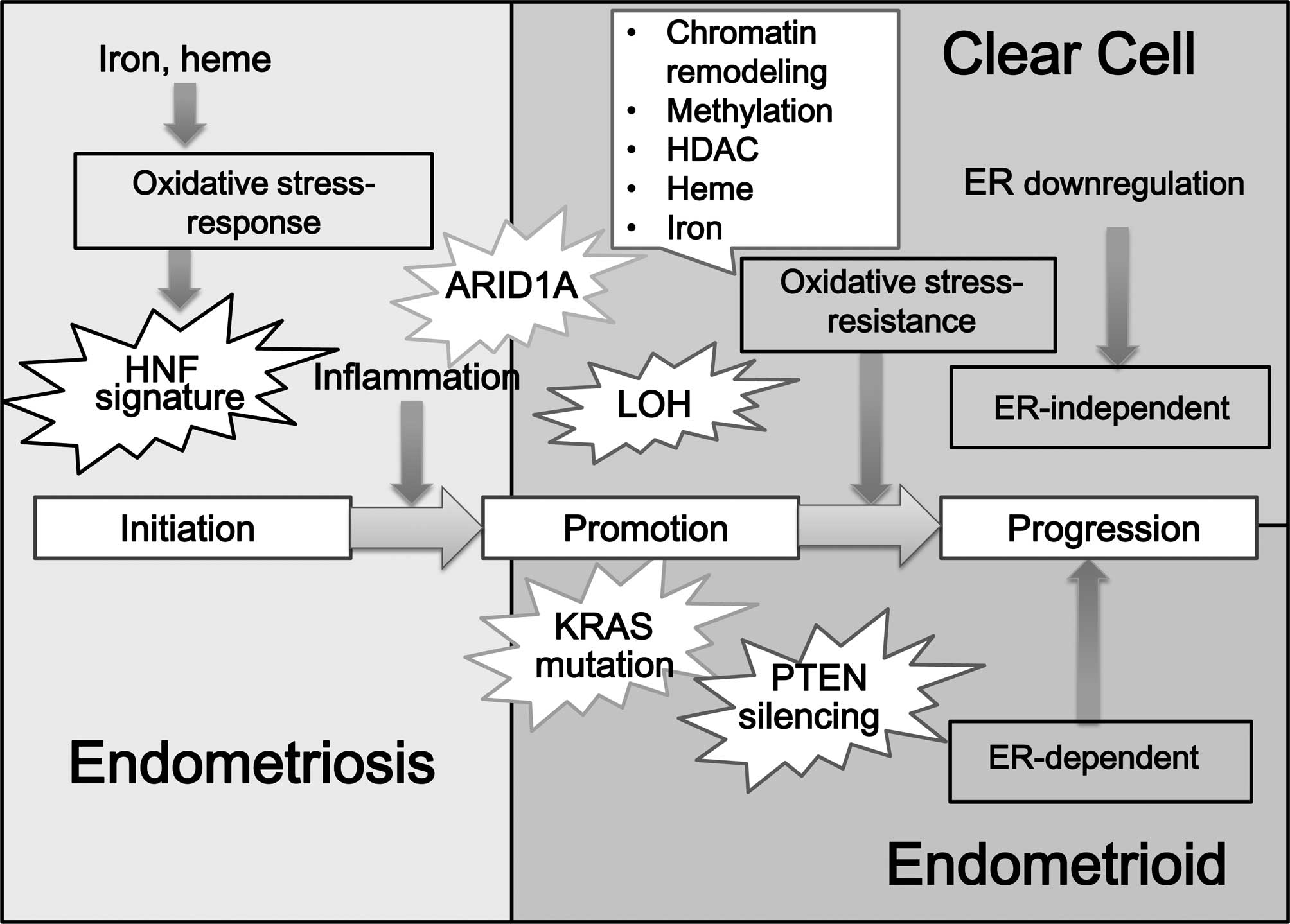 | Figure 1.Hypotheses regarding the
carcinogenesis of CCC: factors contributing to the expression of
ER. Estrogen plays a role in ovarian tumorigenesis. Among EAOC, EAC
was predominantly positive for ER and PR, but CCC specifically
exhibited lower ER and PR expression. A dualistic model, which has
been established on morphological and genomic basis, differentiates
EAOC into two broad categories: estrogen-dependent ovarian cancers
with an EAC morphology, and estrogen-independent carcinoma with a
CCC morphology. There are basically the following hypotheses
regarding the carcinogenesis or pathogenesis of CCC: initially, the
heme and iron-mediated oxidative stress and persistent inflammation
processes occur due to repeated hemorrhage in endometriosis. These
compounds oxidatively modify DNA, proteins and lipids and,
subsequently, ER depletion or hypermethylation may be observed.
Loss of estrogen function may be a turning point in CCC
development. Many factors are implicated as significant regulators
of distinct aspects of ER action and expression, including gene
expression, DNA methylation of the promoter region, histone
deacetylation and heme and iron binding, PPARγ and ubiquitin
protein ligase. The switch from a normal-stress-response phenotype
to a stress-resistant phenotype may involve the mutation of the
specific genes in CCC. Additional candidate gene (e.g., ARID1A)
abnormalities or molecular alterations may cause the progression
towards the CCC. |
Factors contributing to the expression of
estrogen receptor
Estrogen is supposed to actively participate in the
early stages of endometrial tumorigenesis. ER also plays a role in
the onset and development of tumors arising from or outside the
reproductive system. Genomic aberrations of ER-dependent downstream
targets provide cellular growth advantages when the cells are
exposed to estrogen. There are a number of factors that interfere
with ER expression and estrogen activity. Many factors are
implicated as an important regulator of distinct aspects of ER
action and expression, including gene expression, DNA methylation
of the promoter region, histone deacetylation and heme and iron
binding (16) (Fig. 1).
Methylation of the ER gene
DNA damage by iron-mediated oxidative stress could
be one factor leading to the development of EAOC (9,10).
Levels of the oxidatively modified guanosine base 8-oxoguanine
(8-oxoG) were increased in the endometriotic cells (17) and CCC cells (8). Oxidative stress activates signaling
cascades that lead to induction of stress-responsive genes (genetic
alteration). In addition to the genetic alterations, evidence
supports a role of oxidative stress in the epigenetic process. DNA
methylation is among the most studied epigenetic mechanisms
(18). Beside the standard picture
of DNA methylation, factors contributing to the outcome of
oxidative damage to nucleic acids may be chromosomal aberration,
microsatellite instability and telomere shortening (19). Several investigators focus on DNA
methyltransferases (DNMT) and histone deacetylases (HDAC) as
epigenetic targets for cancer treatment. Such lesions of genome DNA
damaged by oxidative stress interfere with the ability of DNA to
function as a substrate for DNMTs, resulting in hypomethylation
(19). Loss of methylation in
normally methylated sequences (hypomethylation) may lead to
activation of HNF-1β and genomic instability, which is evident in
CCC.
In addition, a growing body of evidence has
accumulated that DNA methylation and various histone modifications
involved in chromatin remodeling have been linked to the lack of ER
expression (15,20). This change may lead to
inappropriate methylation in the promoter region of ER gene.
Methylation of the ER gene has been linked to breast, colon,
prostate and hematopoietic malignancies (20). The ER promoter is also methylated
in ovarian cancer cell lines and tissue samples (15). These results indicate that aberrant
hypermethylation is responsible for a significant proportion of
EAOC in which ER expression is lost. This process may play an
important role in the pathogenesis of CCC.
Histone deacetylase (HDAC)
There are several genes that regulate ER
transcription. Histone acetyltransferases (HAT) and HDACs cause
post-transcriptional modification of histone proteins that
participate in ER signaling. HDAC exerts specific roles in breast
cancer progression and estrogen dependence, because this family
suppresses ER transcriptional activity (21). HDAC inhibitor induces stabilization
and expression of ER gene and restores sensitivity of the
ER-negative cancer to chemotherapy. Therefore, several
investigators focus on HDAC as epigenetic targets for cancer
treatment. HDAC may be a potential target for therapeutic approach
or intervention in the treatment of a subset of ER-negative
cancers, including CCC.
Furthermore, of interest is the observation that
metastasis-associated protein 1 (MTA1) acts as a potent corepressor
of ER in breast cancer cells (22). MTA1 is the component of a
nucleo-some remodeling deacetylase complex. This gene represses ER
transcription by recruiting HDAC to the ER response
element-containing target genes, which is accomplished by chromatin
remodeling. MTA1 overexpression also results in down-regulation of
E-cadherin and overexpression of Snail and Slug, leading to the
epithelial mesenchymal transition and enhancing invasion and
metastasis (22). Thus, MTA1 may
be a predictor of aggressive phenotypes. It remains unclear,
however, whether CCC retains a similar cellular activity on the
HDAC-regulated ER expression to that found within breast
cancer.
Iron
In an excess of a redox active iron, this metal is
easily incorporated into the zinc fingers of ER molecule by means
of a zinc/iron exchange, because zinc appears to be kinetically
labile and is exchangeable. Iron fingers of ER molecule will
augment the rate of hydroxyl radical generation (e.g., hydroxyl
radicals via Fenton reaction) and enhance oxidative stress
(23). Iron-induced free radical
formation and subsequent oxidative stress lead to extensive damage
to the proximate DNA, e.g., the estrogen responsive element of ER,
which results in suppression of ER expression. Iron-induced
hydroxyl radical generation also causes adverse consequences, such
as carcinogenesis (23).
Heme
Similar to iron, heme appears to be rich in
endometriotic cyst fluid and peritoneal fluid. Heme is a
physiological ligand of NR1D2 (nuclear receptor subfamily 1, group
D, member 2), belonging to the steroid receptor superfamily of
transcription factors (24). Heme
binding to NR1D2 causes the recruitment of the co-repressor NCOR1
(nuclear receptor co-repressor 1) and SMRT (silencing mediator for
retinoid and thyroid-hormone receptors). SMRT contribute to the
corepressor recruitment and modulation of ER transcriptional
activity. These co-repressors are modulated by the ER's recognition
of cognate DNA binding sites. This complex also leads to repression
of target genes, including ER, by promoting chromatin condensation
and recruitment of HDAC complexes (24). The differential expression pattern
of ER and its co-activator/co-repressor proteins may fine-tune the
modulation of estrogenic action. The up-regulation of NCOR
suppresses estrogen-induced growth in the ER-positive cancer cells.
High NCOR mRNA levels were associated with a better prognosis,
demonstrating that the NCOR level may affect prognosis among women
with breast cancer. In addition, recent data suggest that some
co-repressors, other than NCOR/SMRT, may be involved in ER
signaling. The precise role in heme-dependent regulation of ER
expression remains unclear in CCC.
Peroxisome proliferator-activated
receptor (PPAR) γ
Nuclear receptors are transcription factors that can
be activated by specific ligands (25). On activation by a ligand, nuclear
receptors recruit the retinoid X receptor (RXR) for heterodimer
formation (25). Such nuclear
receptors include the peroxisome proliferator-activated receptor
(PPAR) γ. The PPARγ/RXR heterodimer binds SWI/SNF (mating type
switching/sucrose non-fermenting) chromatin complex (26). The SWI/SNF complex facilitates gene
transcription by remodeling chromatin using the energy of ATP
hydrolysis. This complex always contains either BRG-1
(brahma-related gene 1; hSNF2β) or BRM (brahma; hSNF2) as the
catalytic subunit, together with associated factors (BAFs,
BRG1-associated factors) (26).
BRG-1 and BRM enhance the transcription of genes regulated by
several transcription factors, including ER (26). The SWI/SNF complex reportedly
regulates many important genes involved in energy homeostasis
(diabetes and atherosclerosis) (25). Major rearrangement of the
nucleosomal chromatin facilitates recruitment of BAFs, which have
ten isoforms, including BAF250a (26). BAFs regulate transcription of
certain genes by altering the chromatin structure around those
genes (26). For example, BAF250a
has a DNA-binding domain that specifically binds an AT-rich DNA
sequence and stimulates glucocorticoid receptor-dependent
transcriptional activation. BAF250a is also known as ARID1A (AT
rich interactive domain 1A) (27)
(Fig. 1). BAF250 forms an E3
ubiquitin ligase that targets histone H2B (27). H2B ubiquitination plays critical
roles in regulating many processes within the nucleus, including
transcription initiation and elongation, silencing and DNA repair.
The loss of function of BAF250 due to somatic or genetic mutation
may fail to ubiquitinate the H2B molecule, which results in
aberrant chromatin remodeling.
More recent data for the first time showed that the
specific genes that were somatically mutated in CCC are ARID1A,
PIK3CA (phosphoinositide-3-kinase, catalytic, α polypeptide),
PPP2R1A (protein phosphatase 2, regulatory subunit A, α) and KRAS
(v-Ki-ras2 Kirsten rat sarcoma viral oncogene homolog) (28,29).
Nearly half the CCC carried truncating mutations in ARID1A
(BAF250a) (28,29). These investigators propose that
aberrant chromatin remodeling contributes to the pathogenesis of
CCC (28,29). The mechanism of action of ARID1A
and the SWI/SNF family complex is interesting to consider. ARID1A
may contribute to the development of CCC, possibly through a lack
of tumor suppressor gene and suppression of estrogenic action.
High expression level of PPARγ correlates with
long-term survival in patients with breast cancer. PPARγ expression
is also positively correlated with ER status. These observations
correspond to estimations that up-regulation of HNF-1β expression
in CCC down-regulates ER expression via suppression of PPARγ. The
PPARγ agonist troglitazone (TRG; anti-diabetics) may suppress PI3K
signaling through HDAC inhibition, leading to increased histone
modifications. Estrogen-related receptor α and γ (ERRα and ERRγ)
are alternative targets of TRG important for mediating its growth
suppressive effect. Therefore, it is certainly possible that ARID1A
and its downstream targets may be a therapeutic target for CCC.
Ubiquitin protein ligase E3A (UBE3A,
E6-AP)
Other potential mechanisms underlying ER generation
involve E6-AP (UBE3A, ubiquitin protein ligase E3A) (30). This gene encodes an E3
ubiquitin-protein ligase, part of the ubiquitin protein degradation
system. The E6-AP expression is inversely associated with that of
ER, suggesting that E6-AP suppresses the ER expression. This gene
has been shown to be elevated in ER-negative breast cancer and
drives the proteasomal degradation of ER. In addition, E6-AP
regulates cell proliferation by promoting proteasome-mediated
degradation of cyclin-dependent kinase inhibitor p27.
Conclusion
Epidemiologically and clinicopathologically, EAC and
CCC are believed to develop from endometriosis (EAOC). Under
certain conditions in endometriosis, the biological behavior change
of the local microenvironment may promote the development of EAOC.
Recent studies have demonstrated that a redox active iron overload
and the accompanying chronic inflammatory processes will supply
critical initiators inducing cell growth, survival, anti-apoptosis
and finally the oxidative stress-response, leading to
carcinogenesis. Among EAOC, CCC differ from EAC with respect to
their clinical characteristics and carcinogenesis (10). A growing body of evidence has
accumulated that, compared to EAC, CCC is relatively resistant to
conventional taxane plus platinum-based chemotherapy (31). Therefore, EAC shows favorable
prognosis, whereas CCC contributes to poor prognosis. There is a
review focusing on the specific gene expression that may predict
the response to chemotherapy and/or prognosis (7).
The genome-wide expression analyses identified
differences in the expression of several genes and proteins between
CCC and EAC (4,5,7–11,32).
The literature data on the expression of ER in CCC differ from that
in EAC (14). Compared to EAC, CCC
exhibits very low ER expression. CCC characterized by ER negativity
may be an important reason for an aggressive tumor with poor
prognosis. Several studies have defined sets of genes with
differential expression levels between ER-positive and ER-negative
tumor types. For example, ER-negative breast cancer exhibits a
greater proliferation signal, failure of chemotherapy and an
aggressive tumor with poor prognosis. We put forward a model
postulating that deletion of ER expression is required for CCC
lesion progression and aggressiveness.
The present review demonstrated that many factors
are implicated as critical regulators of distinct aspects of
estrogenic action and ER expression, including gene overexpression
and silencing, DNA methylation of the promoter region, histone
deacetylation, heme and iron binding, PPARγ action and ubiquitin
ligase activity. Consequently, loss of ER expression may be
associated with a specific additional risk for developing CCC, but
not EAC. Our preliminary microarray data on the HNF-1β siRNA
experiments support the notion that the estrogenic action and ER
expression are down-regulated in response to HNF-1β (Shigetomi
et al, 2011, unpublished data). The up-regulation of HNF-1β
expression led to subsequent modulation of its downstream targets
that directly or indirectly suppress the estrogenic action. We have
defined the gene set in CCC, with a view to identify the
HNF-1β-dependent estrogenic action. Some of the genes predominantly
identified in CCC were related to ER modulation, which may be
involved in downstream targets of HNF-1β gene. This result is
particularly interesting because CCC cells that show overexpression
of HNF-1β gene may down-regulate the estrogenic action and ER
expression.
More recent data showed that the specific genes that
were somatically mutated in CCC are ARID1A, PIK3CA, PPP2R1A and
KRAS (28,29). ARID1A mutation results in aberrant
chromatin remodeling and may contribute to the development of CCC,
possibly through a lack of tumor suppressor gene (28,29)
and also suppression of estrogenic action. Additionally, PPP2R1A is
another gene that exhibits acquired somatic mutations in the genome
in CCC (29). PPP2R1A functions,
when mutated, as an oncogene. PPP2R1A is a regulatory subunit A-α
of PP2A (serine/threonine protein phosphatase type 2A). PP2A plays
an essential role in cell cycle regulation and induction of G2
arrest by a mechanism of phosphorylation/dephosphorylation with a
variety of protein kinases. Our preliminary data showed that
PPP2R1A (29) and PP2A can be
deregulated in CCC either by somatic mutation and transcriptional
repression by HNF-1β, respectively (Shigetomi et al, 2010,
unpublished data). Furthermore, the PP2A complex is also associated
with ER and leads to its dephosphorylation and inhibiting
transcription.
HNF-1β is differentially expressed among the EAOC.
The nuclear immunostaining of HNF-1β protein is observed in the
majority of CCC, but not in EAC, demonstrating that this aberration
is histotype-specific. Recent biochemical studies based on
genome-wide microarray analysis technology have noted specific
expression of HNF-1β in CCC (33).
Kato et al reported that hypomethylation of the HNF-1β CpG
island participates in the HNF-1β up-regulation in CCC (34). Among the genes highly up-regulated
in CCC, more than 80% genes were associated with the redox-related
gene (32). Several important
CCC-related genes overlap with those known to be regulated by
HNF-1β (32).
Several investigators have described the
HNF-1β-dependent pathophysiology of CCC and discussed its role in
oxidative stress-induced carcinogenesis (7–11). A
majority of CCC likely develops as the result of a stepwise
accumulation of alterations in cellular regulatory pathways, such
as DNA methylation, genomic DNA mutation, LOH, oncogene activation,
tumor suppressor gene inactivation and aberrant chromatin
remodeling. The gene expression profiling and signaling pathways of
HNF-1β, so called ‘HNF signature’, has been assessed in CCC: the
catalog of CCC-specificity may be a manifestation of several
essential alterations in cell physiology, including oxidative
stress, detoxification and metabolism (7,8). The
‘HNF signature’ shows evidence of biological functions, including
glycogen storage (energy accumulation), anti-apoptosis (survival)
and detoxification (stress-resistance).
Up-regulation of HNF-1β expression was evident in
the CCC and contiguous atypical endometriosis and even in distant
endometriotic lesions (35). A
candidate early precursor to CCC, the ‘HNF signature’, is commonly
found even in endometriosis in the absence of any morphological
abnormality (35–38). The ‘HNF signature’ may be an early
event of the clear cell directionality (4,7–10,32).
Therefore, up-regulation of HNF-1β expression is not sufficient for
the development of CCC.
Birrer proposed that the switch from a
normal-stress-response phenotype to a stress-resistant phenotype
may involve the mutation of the specific genes in CCC (39). Additional candidate gene
abnormalities or molecular alterations may cause the progression
towards the CCC. ARID1A mutations are evident in the CCC and
contiguous atypical endometriosis, but not in distant endometriotic
lesions (28). Therefore, ARID1A
is considered to be an early event in the transformation of
endometriosis into ovarian cancer (28).
In conclusion, this review examines the suppression
of estrogenic action and ER expression in CCC. The basic findings
of this comprehensive review suggest that the interactions between
the iron-mediated oxidative stress and down-regulation of ER
expression may have clear implications for developing CCC via the
up-regulation of HNF-1β expression. These genes and proteins
significantly elevated and repressed in CCC subjects may provide a
foundation for further validation in larger patient cohorts. Future
studies will address the mechanism of synergistic effects of the
specific gene mutations and up-regulation of HNF-1β gene and its
downstream target genes on the estrogenic action. Thus, targeting
HNF-1β-mediated signaling may serve as a novel therapeutic strategy
for the treatment of CCC. Certain inhibitors are currently being
studied as new drugs able to restore ER-α protein expression in
ER-α-negative cancer cells and to promote apoptosis and
differentiation. Demethylating agents and HDAC inhibitors are
candidates for becoming potent new drugs in cancer therapy.
Acknowledgements
This study was supported by KAKENHI
[Japan Society for the Promotion of Science (JSPS) Grant-in-Aid].
The authors thank all the study participants for their time and
efforts. They thank Mikiko Kita for the editorial assistance.
References
|
1.
|
M TakanoY KikuchiN YaegashiK KuzuyaM UekiH
TsudaM SuzukiJ KigawaS TakeuchiH TsudaT MoriyaT SugiyamaClear cell
carcinoma of the ovary: a retrospective multicentre experience of
254 patients with complete surgical stagingBr J
Cancer9413691374200610.1038/sj.bjc.660311616641903
|
|
2.
|
T KitaY KikuchiK KudohM TakanoT GotoJ
HirataT TodeI NagataExploratory study of effective chemotherapy to
clear cell carcinoma of the ovaryOncol Rep7327331200010671681
|
|
3.
|
DA BellOrigins and molecular pathology of
ovarian cancerMod
Pathol18S19S32200510.1038/modpathol.380030615761464
|
|
4.
|
M MandaiK YamaguchiN MatsumuraT BabaI
KonishiOvarian cancer in endometriosis: molecular biology,
pathology, and clinical managementInt J Clin
Oncol14383391200910.1007/s10147-009-0935-y19856044
|
|
5.
|
H KobayashiOvarian cancer in
endometriosis: epidemiology, natural history, and clinical
diagnosisInt J Clin
Oncol14378382200910.1007/s10147-009-0931-219856043
|
|
6.
|
DM DinulescuTA InceBJ QuadeSA ShaferD
CrowleyT JacksRole of K-ras and Pten in the development of mouse
models of endometriosis and endometrioid ovarian cancerNat
Med116370200510.1038/nm117315619626
|
|
7.
|
H KobayashiY YamadaS KanayamaN FurukawaT
NoguchiS HarutaS YoshidaM SakataT SadoH OiThe role of hepatocyte
nuclear factor-1beta in the pathogenesis of clear cell carcinoma of
the ovaryInt J Gynecol
Cancer19471479200910.1111/IGC.0b013e3181a19eca19407577
|
|
8.
|
K YamaguchiM MandaiS ToyokuniJ HamanishiT
HiguchiK TakakuraS FujiiContents of endometriotic cysts, especially
the high concentration of free iron, are a possible cause of
carcinogenesis in the cysts through the iron-induced persistent
oxidative stressClin Cancer
Res143240200810.1158/1078-0432.CCR-07-161418172249
|
|
9.
|
K YamaguchiM MandaiT OuraN MatsumuraJ
HamanishiT BabaS MatsuiSK MurphyI KonishiIdentification of an
ovarian clear cell carcinoma gene signature that reflects inherent
disease biology and the carcinogenic
processesOncogene2917411752201010.1038/onc.2009.47020062075
|
|
10.
|
H KobayashiH KajiwaraS KanayamaY YamadaN
FurukawaT NoguchiS HarutaS YoshidaM SakataT SadoH OiMolecular
pathogenesis of endometriosis-associated clear cell carcinoma of
the ovary (Review)Oncol Rep22233240200919578761
|
|
11.
|
S YoshidaN FurukawaS HarutaY TanaseS
KanayamaT NoguchiM SakataY YamadaH OiH KobayashiTheoretical model
of treatment strategies for clear cell carcinoma of the ovary:
focus on perspectivesCancer Treat
Rev35608615200910.1016/j.ctrv.2009.07.00219665848
|
|
12.
|
KK ChanN WeiSS LiuL Xiao-YunAN CheungHY
NganEstrogen receptor subtypes in ovarian cancer: a clinical
correlationObstet
Gynecol111144151200810.1097/01.AOG.0000296715.07705.e918165403
|
|
13.
|
C ZamagniRM WirtzP de IacoM RosatiE
VeltrupF RosatiE CapizziN CacciariC AlboniA BernardiF MassariS
QuerciaA D'Errico GrigioniM DietelJ SehouliC DenkertAA
MartoniOestrogen receptor 1 mRNA is a prognostic factor in ovarian
cancer patients treated with neo-adjuvant chemotherapy:
determination by array and kinetic PCR in fresh tissue
biopsiesEndocr Relat Cancer1612411249200910.1677/ERC-08-0342
|
|
14.
|
RA SoslowHistologic subtypes of ovarian
carcinoma: an overviewInt J Gynecol Pathol27161174200818317227
|
|
15.
|
F SuzukiJ AkahiraI MiuraT SuzukiK ItoS
HayashiH SasanoN YaegashiLoss of estrogen receptor beta isoform
expression and its correlation with aberrant DNA methylation of the
5′-untranslated region in human epithelial ovarian carcinomaCancer
Sci9923652372200819032364
|
|
16.
|
E SwedenborgKA PowerW CaiI PongratzJ
RüeggRegulation of estrogen receptor beta activity and implications
in health and diseaseCell Mol Life
Sci6638733894200910.1007/s00018-009-0118-z19669093
|
|
17.
|
SH KaoHC HuangRH HsiehSC ChenMC TsaiCR
TzengOxidative damage and mitochondrial DNA mutations with
endometriosisAnn NY Acad
Sci1042186194200510.1196/annals.1338.02115965062
|
|
18.
|
LS KristensenHM NielsenLL
HansenEpigenetics and cancer treatmentEur J
Pharmacol625131142200910.1016/j.ejphar.2009.10.01119836388
|
|
19.
|
KV DonkenaCY YoungDJ TindallOxidative
stress and DNA methylation in prostate cancerObstet Gynecol
IntJune2010(E-pub ahead of print).
|
|
20.
|
YL OttavianoJP IssaFF ParlHS SmithSB
BaylinNE DavidsonMethylation of the estrogen receptor gene CpG
island marks loss of estrogen receptor expression in human breast
cancer cellsCancer Res542552255519948168078
|
|
21.
|
L GiacintiPP ClaudioM LopezA
GiordanoEpigenetic information and estrogen receptor alpha
expression in breast
cancerOncologist1118200610.1634/theoncologist.11-1-116401708
|
|
22.
|
C DannenmannN ShabaniK FrieseU JeschkeI
MylonasA BrüningThe metastasis-associated gene MTA1 is upregulated
in advanced ovarian cancer, represses ERbeta, and enhances
expression of oncogenic cytokine GROCancer Biol
Ther714601467200810.4161/cbt.7.9.642718719363
|
|
23.
|
D ConteS NarindrasorasakB SarkarIn vivo
and in vitro iron-replaced zinc finger generates free radicals and
causes DNA damageJ Biol
Chem27151255130199610.1074/jbc.271.9.51258617792
|
|
24.
|
S RaghuramKR StayrookP HuangPM RogersAK
NosieDB McClureLL BurrisS KhorasanizadehTP BurrisF
RastinejadIdentification of heme as the ligand for the orphan
nuclear receptors REV-ERBalpha and REV-ERBbetaNat Struct Mol
Biol1412071213200710.1038/nsmb134418037887
|
|
25.
|
MC SugdenMJ HolnessRole of nuclear
receptors in the modulation of insulin secretion in lipid-induced
insulin resistanceBiochem Soc
Trans36891900200810.1042/BST036089118793157
|
|
26.
|
J HuuskonenM VishnuPE FieldingCJ
FieldingActivation of ATP-binding cassette transporter A1
transcription by chromatin remodeling complexArterioscler Thromb
Vasc
Biol2511801185200510.1161/01.ATV.0000163186.58462.c515774904
|
|
27.
|
XS LiP TrojerT MatsumuraJE TreismanN
TaneseMammalian SWI/SNF – a subunit BAF250/ARID1 is an E3 ubiquitin
ligase that targets histone H2BMol Cell Biol30167316882010
|
|
28.
|
KC WiegandSP ShahOM Al-AghaARID1A
mutations in endometriosis-associated ovarian carcinomasN Engl J
Med36315321543201010.1056/NEJMoa100843320942669
|
|
29.
|
S JonesTL WangIeM ShihTL MaoK NakayamaR
RodenR GlasD SlamonLA Diaz JrB VogelsteinKW KinzlerVE VelculescuN
PapadopoulosFrequent mutations of chromatin remodeling gene ARID1A
in ovarian clear cell
carcinomaScience330228231201010.1126/science.119633320826764
|
|
30.
|
T OhtaM FukudaUbiquitin and breast
cancerOncogene2320792088200410.1038/sj.onc.120737115021895
|
|
31.
|
H ItamochiJ KigawaN TerakawaMechanisms of
chemoresistance and poor prognosis in ovarian clear cell
carcinomaCancer
Sci99653658200810.1111/j.1349-7006.2008.00747.x18377417
|
|
32.
|
H KajiharaY YamadaS KanayamaN FurukawaT
NoguchiS HarutaS YoshidaT SadoH OiH KobayashiClear cell carcinoma
of the ovary: potential pathogenic mechanisms (Review)Oncol
Rep2311931203201020372830
|
|
33.
|
A TsuchiyaM SakamotoJ YasudaM ChumaT OhtaM
OhkiT YasugiY TaketaniS HirohashiExpression profiling in ovarian
clear cell carcinoma: identification of hepatocyte nuclear factor-1
beta as a molecular marker and a possible molecular target for
therapy of ovarian clear cell carcinomaAm J
Pathol16325032512200310.1016/S0002-9440(10)63605-X
|
|
34.
|
N KatoG TamuraT MotoyamaHypomethylation of
hepatocyte nuclear factor-1beta (HNF-1beta) CpG island in clear
cell carcinoma of the ovaryVirchows
Arch452175180200810.1007/s00428-007-0543-z18066692
|
|
35.
|
N KatoS SasouT MotoyamaExpression of
hepatocyte nuclear factor-1beta (HNF-1beta) in clear cell tumors
and endometriosis of the ovaryMod
Pathol198389200610.1038/modpathol.380049216258507
|
|
36.
|
N KatoM ToukairinI AsanumaT
MotoyamaImmunocytochemistry for hepatocyte nuclear factor-1beta
(HNF-1beta): a marker for ovarian clear cell carcinomaDiagn
Cytopathol35193197200710.1002/dc.2062317351940
|
|
37.
|
N KatoT MotoyamaHepatocyte nuclear
factor-1beta (HNF-1beta) in human urogenital organs: its expression
and role in embryogenesis and tumorigenesisHistol
Histopathol2414791486200919760597
|
|
38.
|
S YamamotoH TsudaS AidaH ShimazakiS TamaiO
MatsubaraImmunohistochemical detection of hepatocyte nuclear factor
1beta in ovarian and endometrial clear-cell adenocarcinomas and
nonneoplastic endometriumHum
Pathol3810741080200710.1016/j.humpath.2006.12.01817442376
|
|
39.
|
MJ BirrerThe origin of ovarian cancer – is
it getting clearer?N Engl J Med363157415752010
|















