Introduction
Cardiovascular disease is the leading cause of death
worldwide, accounting for more than 900,000 deaths annually in the
United States alone (1). Clinical
study shows that its main cause of death is acute myocardial
infarction (AMI) (2). The most
common cause of MI is reduced myocardial perfusion due to coronary
artery narrowing caused by a thrombus, that develops on a disrupted
atherosclerotic plaque. Pathological studies have shown that the
most common underlying molecular and cellular pathophysiology of
disrupted atherosclerotic plaque is arterial inflammation in MI
(3), and also oxidative stress
plays a critical role in AMI (4,5). In
recent years, pharmacological studies have indicated that many
traditional plants and their extracts have anti-inflammatory
(6–9) and anti-oxidant (10,11)
effects on cardiovascular disease.
Dan-Shen-Yin (DSY) is a well-known traditional
Chinese formula comprising Salvia Miltiorrhiza, Sandalwood
and Fructus Amomi. It is widely used in clinical practice
for the treatment of diabetes (12,13)
and coronary heart disease (CHD) (14,15)
and has produced a favorable effect (12–15).
However, the anti-inflammatory and anti-oxidant
mechanisms of DSY are unclear. The object of the present study was
to evaluate whether or not acute oral DSY can protect the heart
against AMI rats. If so, we would then investigate the
anti-inflammatory and anti-oxidant mechanisms involved.
Materials and methods
Preparation of DSY decoction
The crude drugs of DSY, including 120 g of Salvia
Miltiorrhiza, 18 g of Sandalwood and 18 g of Fructus
Amomi, were purchased from LBX Pharmacy (Zhengzhou, China). To
keep the consistency of the herbal chemical ingredients, all the
herbal components were originally obtained from the standard native
sources, as stated above, with GAP grade, and the drugs were
extracted with standard methods according to the Chinese
Pharmacopeoia (China Pharmacopoeia and Committee, 2000). Voucher
specimens (No. 100322) had been kept. The mixture of DSY was
produced by boiling with distilled water at 100°C for 30 min twice,
and the drug solution was vacuum cool-dried, rendered into drug
powder and dissolved with distilled water, with a final
concentration of 2.5 g/ ml (equivalent to dry weight of raw
materials).
Experimental protocol
Male Sprague-Dawley rats (n=40) weighing 180–220 g
were purchased from the Henan Laboratory Animal Center (Henan,
Zhengzhou, China). Rats were housed in temperature-controlled
(22±2°C) and humidity-controlled (55±5%) rooms under 12-h
light-dark cycles. Rats had free access to solid rodent chow and
tap water. Animals were allowed a 1-week acclimatization period
prior to entry into any experimental protocol. The animal
experiments were conducted in accordance with the Use of Laboratory
Animals by the US National Institutes of Health (1996). The rat
dose of DSY in the present study was converted according to the
principles described below. The low dosage of DSY used in rats was
5 g/kg, that is, the dose used was 6.7 times the dose used in
humans. This is consistent with a previous study (16). The high dose used in rats was 10
g/kg, which is equivalent to the dose administered to clinical
patients without causing significant side-effects. The experiments
were performed 30 min after the administration of DSY (5 or 10
g/kg) or 0.9% NaCl 20 ml/kg.
Surgical preparation of animals
We performed the surgical procedure according to a
previous study (17), with some
modifications. In brief, the rats were anesthetized
intraperitoneally with pentobarbitone at a dose of 36 mg/kg and
subcutaneous peripheral limb electrodes were inserted and the ECG
was recorded for the entire duration of the experiment (AD
Instruments, Australia). After performing a left thoracotomy, the
incised area was extended using forceps and the pericardium was
opened. The heart was then pushed out of the chest and the left
anterior descending coronary artery was ligated using a 3/0 silk
thread. Successful ligation was verified by the occurrence of
arrhythmias and, visually, by the color change of the ischemic
area. The heart was immediately returned to its anatomical position
and the chest was closed, while slight pressure was applied from
the outside, so that air did not remain in the chest. The skin was
then sutured. Forty male Sprague-Dawley rats (180–220 g) were
randomly assigned to four groups: i) sham-operated control group
(sham MI), rats underwent the same surgical procedures except that
the suture passing under the coronary artery was not tied; ii)
vehicle control group (MI + vehicle), rats were orally administered
the vehicle (0.9% NaCl, 20 ml/kg); iii) low-dose DSY group (MI +
DSY), rats were orally administered DSY extract (5 g/kg) 30 min
prior to ischemia induction; iv) high-dose DSY group (MI + DSY),
rats were orally administered DSY extract (10 g/kg) 30 min prior to
ischemia induction. At the end of the 3-h ischemic period (or 24 h
for infarct size), the heart was quickly excised and the cardiac
tissue was processed according to the procedures described below;
the mortality rate was ∼5%.
Determination of the infarction size
The determination of the infarction size was
performed as previously described (18,19).
We injected 1 ml 2% Evans blue dye into the left ventricular cavity
24 h after MI induction. The normally perfused region was stained
blue and the area at risk (Ar) was outlined. The heart was then
removed, frozen at −20°C and sliced into 1-mm thick transverse
sections. The sections were incubated for 15 min at 37°C in a 1%
solution of triphenyltetrazolium chloride (TTC) (SCR, Shanghai,
China) to differentiate necrotic (pale) from non-necrotic Ar. The
Ar as a percentage of the left ventricle (Lv) (Ar/Lv), and the area
of necrosis (An) as a percentage of the Ar (An/Ar) were calculated
using Image Pro Plus 6.0 software.
Inflammatory factors assay
For the assays of the serum levels of C-reactive
protein (CRP), interleukin-6 (IL-6) and tumour necrosis factor-α
(TNF-α), a commercial ELISA kit (Uscnlife, Zhengzhou, Henan, China)
was used according to the recommended protocol. At the end of the
3-h ischemic period, we used a serum separator tube and allowed
whole blood to clot for 30 min before centrifugation for 15 min at
1,000 × g. We then removed the serum. According to the kit
instructions, 100 μl of standard, blank or sample were added per
well and incubated for 2 h at 37°C, after which the liquid was
removed. Detection reagent A (100 μl) was added to each well,
incubated for 1 h at 37°C, and we used wash buffer to wash each
well. We then added 100 μl of detection reagent B to each well,
incubated for 1 h at 37°C, and washed each well again. After adding
90 μl of substrate solution, followed by incubation for 20 min, 50
μl of stop solution were added at room temperature. The optical
density of each well was determined using a microplate
spectrophotometer (BioTek, Winooski, VT, USA) at a 450 nm
wavelength. Finally, we created a standard curve to interpret the
results.
Antioxidant assay
For the assays of superoxide dismutase (SOD),
glutathione (GSH) and malondialdehyde (MDA) activities, blood was
sampled from the abdominal aorta and serum was obtained after
centrifugation at 3,000 rpm for 10 min. We used diagnostic kits
(Jiancheng, Nanjing, China) to determine the levels of SOD, MDA and
GSH according to the manufacturer’s instructions.
Statistical analysis
All data were expressed as the means ± standard
deviation (SD). A Dunnett’s t-test was used to compare the data
obtained before and after occlusion. In the animal study, one-way
ANOVA was used to analyse differences in parameters. A probability
of <0.05 was considered to be statistically significant.
Results
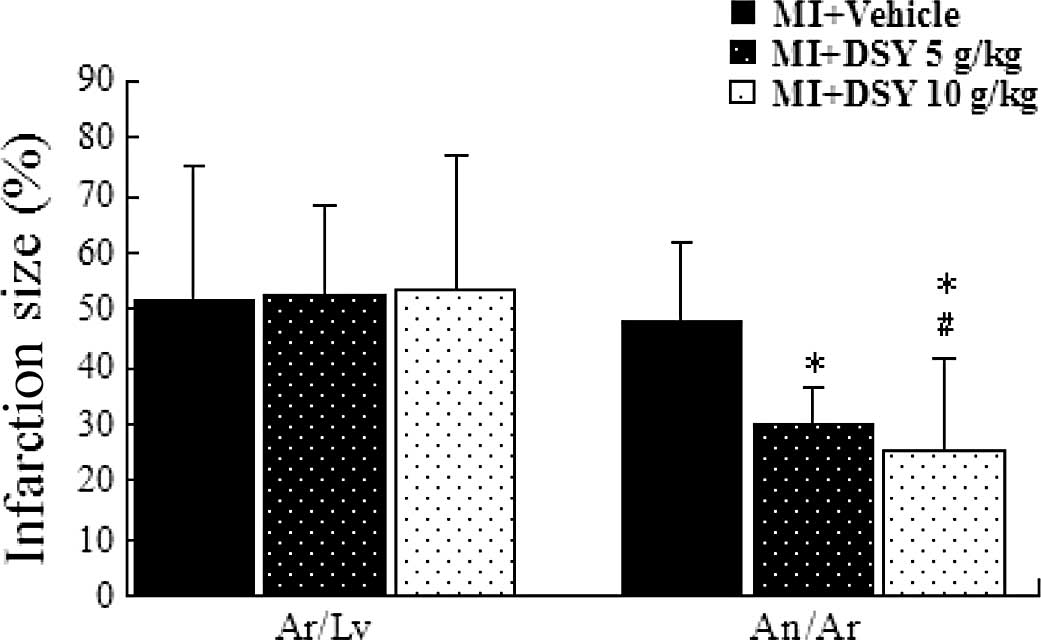
Effect of DSY on infarction size
Images of MI size in the sham-, vehicle- and
DSY-treated groups are shown in Fig.
1. The An (white area), the Ar (red staining and white area)
and the area of the Lv were measured using Image Pro Plus 6.0
software. The effects of DSY on Ar/Lv and An/Ar are shown in
Fig. 1. There was no significant
difference in Ar/Lv among the groups. The administration of 5 g/kg
DSY significantly reduced the An/Ar compared to the vehicle control
group (vs. 49.85±9.13%, P<0.01; 25.53±11.13 vs. 49.85±9.13%,
P<0.01). The administration of 10 g/kg DSY significantly reduced
the An/Ar compared to the DSY 5 g/kg group (25.53±11.13 vs.
29.86±6.27%, P<0.05)
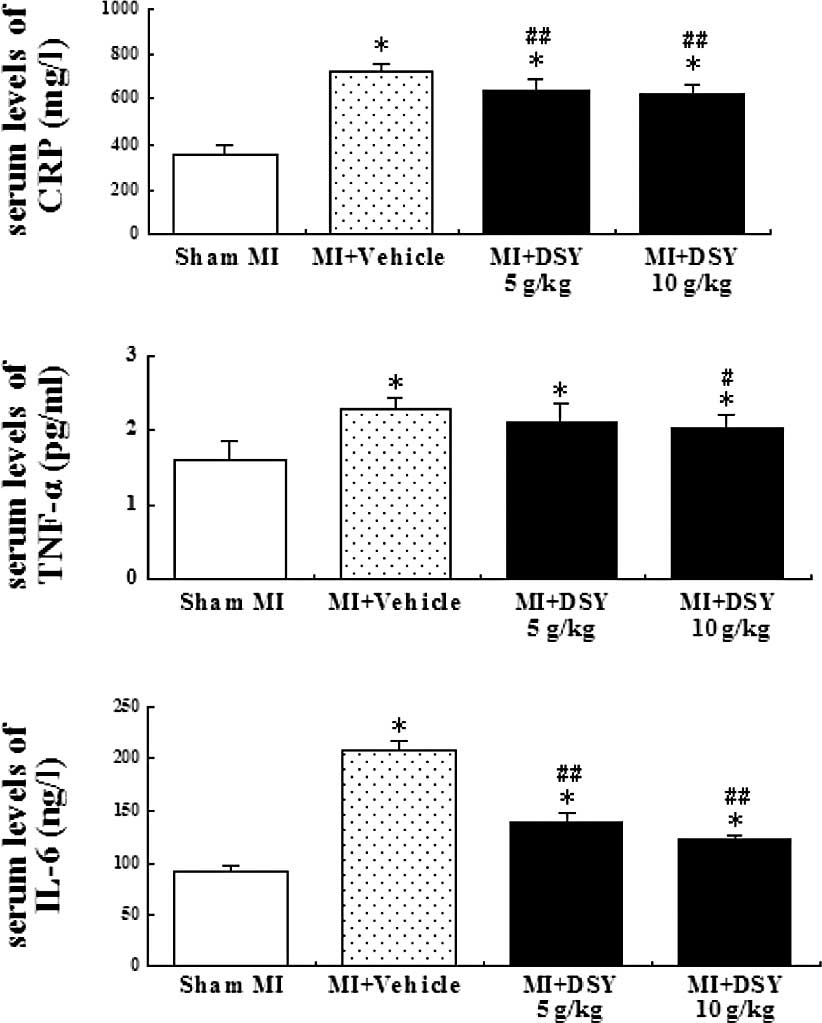
Effect of DSY on the serum levels of CRP,
TNF-α and IL-6
To determine if DSY has an anti-inflammatory effect,
the serum levels of CRP, TNF-α and IL-6 were determined by ELISA.
As shown in Fig. 2A-C, coronary
occlusion significantly increased the serum levels of CRP, TNF-α
and IL-6 compared to the sham group (all P<0.01). As shown in
Fig. 2A and C, the serum levels of
CRP and IL-6 in the DSY-treated group were significantly lower than
those in the vehicle group (P<0.01). The serum levels of TNF-α
in the DSY (10 g/kg)-treated group were significantly lower
compared to those in the vehicle group (P<0.05); there was no
significant difference between 5 g/kg DSY and the vehicle groups
(P>0.05). However, there were no significant differences in the
serum levels of CRP, TNF-α and IL-6 between the 10 and 5 g/kg DSY
groups (P>0.05).
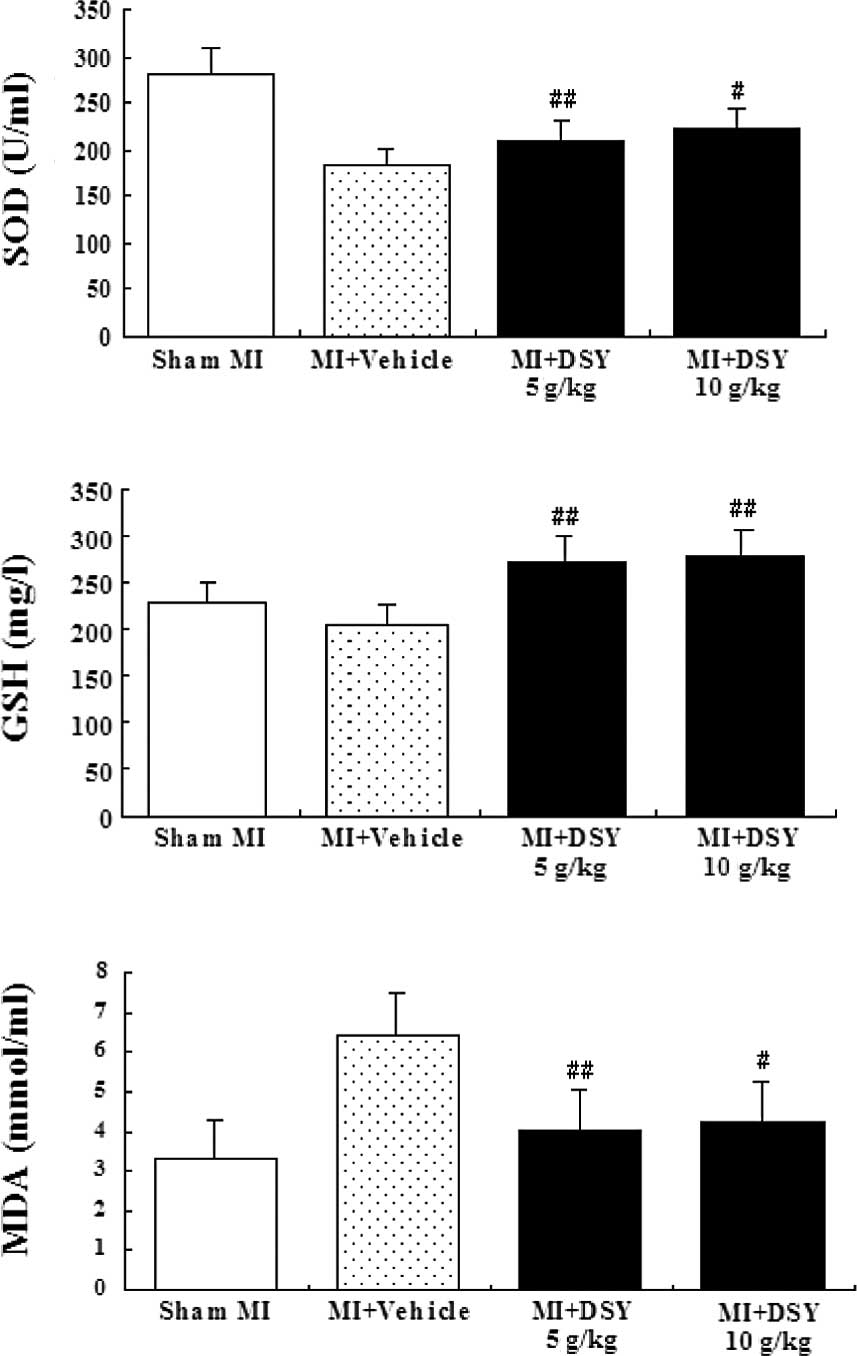
Anti-oxidant assay
The serum levels of SOD and GSH in the DSY-treated
groups were significantly higher (P<0.01 or 0.05; Fig. 3A and B), while the serum MDA levels
in the DSY-treated groups were significantly lower compared to the
vehicle groups (P<0.01 or 0.05; Fig. 3C). There were also significant
differences between the DSY-treated groups and sham groups in the
serum levels of SOD, GSH and MDA (P<0.01 or 0.05). In addition,
there were no significant differences between the 5 g/kg
DSY-treated group and the 10 g/kg DSY-treated group in the serum
levels of SOD, GSH and MDA (all P>0.05)
Discussion
In this study, 2 out of 10 rats in the vehicle group
died, while in the DSY-treated groups all survived. This is the
most direct evidence that AMI has a high mortality risk and DSY has
a protective role. Notably, we found that DSY can reduce infarction
size (Fig. 1). Infarction size is
a major determinant of mortality in AMI (20) and an important determinant of the
prognosis of MI. Therefore, the limitation of the infarction size
must be considered a major therapeutic goal. Numerous
investigations have focused on targeting and exploring several
pharmacological agents in an attempt to reduce experimental MI size
(21). Over the years, Chinese
medicinal herbs and their extracts have received great attention
(22,23). Further studies have found that DSY
decreases the concentrations of CRP, TNF-α and IL-6 and reduces the
amounts of MDA, but enhances SOD and GSH activities in AMI
rats.
CRP is a prototypic acute-phase protein produced in
the liver in response to inflammatory signals (24). Evidence has linked CRP to risk of
cardiovascular events, a reflection of the involvement of
inflammation in atherosclerosis and its complications (25,26).
CRP is a sensitive, non-specific systemic marker of inflammation
(27), and strong evidence
indicates that CRP is associated with CHD events (28). CRP concentrations in the acute
phase have been suggested to reflect pre-existing coronary plaque
instability associated with the onset of AMI (29). Also, CRP has been known for more
than 25 years to bind to LDL (30,31),
and it has been detected in atherosclerotic plaques (32). Laboratory and clinical evidence has
demonstrated that CRP has been examined as a surrogate marker of
other inflammatory mediators, such as IL-6 and TNF-α, to better
understand the inflammatory component of atherosclerosis (33). TNF-α and IL-6 are antigen
non-specific glycoproteins that are synthesized rapidly and
released locally by immune cells in response to injury (34). Additionally, they are
pro-inflammatory cytokines with crucial roles in cardiomyocyte
apoptosis (35). Cardiomyocytes
express functional TNF type-1 receptors and have been shown to
undergo apoptosis after stimulation with TNF-α in vitro
(36). In the present study, we
found significantly reduced levels of CRP, TNF-α and IL-6 in the
DSY-treated groups compared to the vehicle groups after AMI
induction. Thus, DSY, through its anti-inflammatory properties,
protects the cardiomyocytes from the effects of AMI.
Oxidative stress is the leading cause of the worst
outcome of MI (37). Oxidative
stress is increased in the myocardium following infarction, playing
significant roles in cardiac myocyte death and loss of cardiac
function (38). SOD is a very
important enzyme and its activity reflects the cellular capability
of scavenging/quenching free radicals (39). MDA, the degradation product of the
oxygen-derived free radicals and lipid oxidation, interferes with
the metabolism of proteins, glucose and nucleic acid, which results
in a decrease in enzyme activity, template dysfunction of nucleic
acid and injury of tissues and cells (39). The levels of MDA are then regarded
as the degree of lipid peroxidation. In view of evaluating the
basal metabolism of free radicals, the activity of SOD and the
levels of MDA are the principal pathophysiological parameters. GSH,
being an important cellular reductant, is involved in the
protection against free radicals, peroxides and toxic compounds
(40). Depletion of GSH is one of
the primary factors that permit lipid peroxidation (41). DSY reduced the serum levels of MDA,
increased the activities of SOD and the serum levels of GSH. DSY
increased the activities of anti-oxidant defense enzymes, including
SOD and GSH, and reduced MDA in the serum levels of AMI rats in
this study. These results indicate that DSY scavenges various free
radicals effectively through enhancing the activities of the
anti-oxidant enzymes in AMI rats. Furthermore, the anti-oxidant
enzymes in the DSY-treated groups were found to be enhanced by
DSY.
This study, thus, shows that DSY reduces
inflammatory factors and scavenges free radicals through enhancing
antioxidant defense enzymes, and reduces free radical production.
These could be major factors contributing to the cardioprotective
effects of DSY.
In conclusion, our results suggest that Dan-Shen-Yin
has beneficial effects on oxidative stress and inflammation that
are related to the infarction size in AMI rats, and may thus
produce a higher survival rate. Thus, it is valuable to develop
this formula into a potential therapeutic reagent according to
modern pharmacological standards for use in the treatment of
cardiovascular disease.
References
|
1.
|
L RazzoukV MathewRJ LennonAspirin use is
associated with an improved long-term survival in an unselected
population presenting with unstable anginaClin
Cardiol33553558201010.1002/clc.2076920842739
|
|
2.
|
RA KlonerNatural and unnatural triggers of
myocardial infarctionProg Cardiovasc
Dis48285300200610.1016/j.pcad.2005.07.00116517249
|
|
3.
|
JL AndersonCD AdamsEM AntmanACC/AHA 2007
Guidelines for the Management of Patients with Unstable Angina/
Non-ST-Elevation Myocardial Infarction: a report of the American
College of Cardiology/American Heart Association Task Force on
Practice Guidelines (Writing Committee to Revise the 2002
Guidelines for the Management of Patients with Unstable Angina/
Non-ST-Elevation Myocardial Infarction): developed in collaboration
with the American College of Emergency Physicians, the Society for
Cardiovascular Angiography and Interventions, and the Society of
Thoracic Surgeons: endorsed by the American Association of
Cardiovascular and Pulmonary Rehabilitation and the Society for
Academic Emergency MedicineCirculation116e148e3042007
|
|
4.
|
S UedaS YamagishiT MatsuiAdministration of
pigment epithelium-derived factor inhibits left ventricular
remodeling and improves cardiac function in rats with acute
myocardial infarctionAm J
Pathol2591598201110.1016/j.ajpath.2010.10.01821281791
|
|
5.
|
MK MisraM SarwatP BhakuniOxidative stress
and ischemic myocardial syndromesMed Sci
Monit15RA209RA219200919789524
|
|
6.
|
H ZhangWR WangR LinBuyang Huanwu decoction
ameliorates coronary heart disease with Qi deficiency and blood
stasis syndrome by reducing CRP and CD40 in ratsJ
Ethnopharmacol13098102201010.1016/j.jep.2010.04.01720420893
|
|
7.
|
HQ YinB WangJD ZhangEffect of traditional
Chinese medicine Shu-Mai-Tang on attenuating TNFα-induced
myocardial fibrosis in myocardial ischemia ratsJ
Ethnopharmacol1881331392008
|
|
8.
|
Y LiuR LinXL ShiThe roles of buyang huanwu
decoction in anti-inflammation, antioxidation and regulation of
lipid metabolism in rats with myocardial ischemiaEvid Based
Complement Alternat MedMarch102011(E-pub ahead of print).
|
|
9.
|
CI AsliF RochelleW LorenHerbal and
traditional Chinese medicine for the treatment of cardiovascular
complications in diabetes mellitusCurr Diabetes
Rev4320328200810.2174/15733990878624114218991600
|
|
10.
|
C Ibarra-AlvaradoA RojasS
MendozaVasoactive and antioxidant activities of plants used in
Mexican traditional medicine for the treatment of cardiovascular
diseasesPharm Biol7732739201010.3109/1388020090327128020645769
|
|
11.
|
SU ChonBG HeoYS ParkTotal phenolics level,
antioxidant activities and cytotoxicity of young sprouts of some
traditional Korean salad plantsPlant Foods Hum
Nutr12531200910.1007/s11130-008-0092-x19016328
|
|
12.
|
HQ HuangDan-Shen-Yin and Er-Chen-Tang
treat diabetic gastroparesis 40 casesJ New Chinese Med39682007
|
|
13.
|
MF HuJian-Pi-Wan and Dan-Shen-Yin treat
diabetic gastroparesis 60 casesChin J Tradit Med Sci
Tech161501512009
|
|
14.
|
M XieSheng-Mai-San and Dan-Shen-Yin treat
coronary heart disease 60 casesChin Med Mod Dist Educ
Chin613572008
|
|
15.
|
RS LiuJia-Wei-Dan-Shen-Yin treat coronary
heart disease 68 casesChin J Integr Med on Cardio-/Cerebrovascular
Disease86042010
|
|
16.
|
D PinkelThe use of body surface area as a
criterion of drug dosage in cancer chemotherapyCancer
Res18853856195813573353
|
|
17.
|
F QinYX LiuHW ZhaoChinese medicinal
formula Guan-Xin-Er-Hao protects the heart against oxidative stress
induced by acute ischemic myocardial injury in
ratsPhytomedicine16215221200910.1016/j.phymed.2008.08.00518951001
|
|
18.
|
HW ZhaoF QinYX LiuAntiapoptotic mechanisms
of Chinese medicine formula, Guan-Xin-Er-Hao, in the rat ischemic
heartTohoku J Exp Med216309316200810.1620/tjem.216.30919060445
|
|
19.
|
J ZhaoX HuangW TangEffect of oriental
herbal prescription Guan-Xin-Er-Hao on coronary flow in healthy
volunteers and anti-apoptosis on myocardial ischemia-reperfusion in
rat modelsPhytother Res21926931200710.1002/ptr.219417582591
|
|
20.
|
H ThieleL HildebrandC SchirdewahnImpact of
high-dose N-Acetylcysteine versus placebo on contrast-induced
nephropathy and myocardial reperfusion injury in unselected
patients with ST-segment elevation myocardial infarction undergoing
primary percutaneous coronary intervention. The LIPSIA-N-ACC
(Prospective, Single-Blind, Placebo-Controlled, Randomized Leipzig
Immediate PercutaneouS Coronary Intervention Acute Myocardial
Infarction N-ACC)Trial J Am Coll Cardiol55220122092010
|
|
21.
|
HY LingYJ LouTotal flavones from
Elsholtzia blanda reduce infarct size during acute
myocardial ischemia by inhibiting myocardial apoptosis in ratsJ
Ethnopharmacol1011691752005
|
|
22.
|
JY ZhouY FanJL KongDZ WuZB HuEffect of
components isolated from Astragalus membranaceus Bunge on
cardiac function injured by myocardial ischemia reperfusion in
ratsZhongguo Zhong Yao Za Zhi253003022000
|
|
23.
|
BL QiB ChengEffect of the daidzein on
protect of cardiovascular in unsexed ratsJ Chin Clin
Med318192002
|
|
24.
|
A AgrawalCRP after 2004Mol
Immunol42927930200510.1016/j.molimm.2004.09.028
|
|
25.
|
GK HanssonP LibbyThe immune response in
atherosclerosis: a double-edged swordNat Rev
Immunol6508519200610.1038/nri188216778830
|
|
26.
|
P LibbyJT WillersonE BraunwaldC-reactive
protein and coronary heart diseaseN Engl J
Med351295298200410.1056/NEJM20040715351031815257564
|
|
27.
|
MB PepysGM HirschfieldC-reactive protein:
a critical updateJ Clin
Invest11118051812200310.1172/JCI20031892112813013
|
|
28.
|
DI BuckleyRW FuM FreemanC-reactive protein
as a risk factor for coronary heart disease: a systematic review
and meta-analyses for the U.S. preventive services task forceAnn
Intern
Med151483495200910.7326/0003-4819-151-7-200910060-0000919805771
|
|
29.
|
S KojimaT FunahashiT SakamotoThe variation
of plasma concentrations of a novel, adipocyte derived protein,
adiponectin, in patients with acute myocardial
infarctionHeart89667668200310.1136/heart.89.6.66712748233
|
|
30.
|
F BeerAK SoutarML BaltzLow density and
very low density lipoproteins are selectively bound by aggregated
C-reactive proteinJ Exp
Med156230242198210.1084/jem.156.1.2307086355
|
|
31.
|
MB PepysIF RoweML BaltzC-reactive protein:
binding to lipids and lipoproteinsInt Rev Exp
Pathol278311119853915746
|
|
32.
|
YX ZhangWJ CliffGI SchoeflCoronary
C-reactive protein distribution: its relation to development of
atherosclerosisAtherosclerosis145375379199910.1016/S0021-9150(99)00105-710488966
|
|
33.
|
N RifaiPM RidkerHigh-sensitivity
C-reactive protein: a novel and promising marker of coronary heart
diseaseClin Chem47403411200111238289
|
|
34.
|
AK AbbasAH LichtmanJS PoberCellular and
Molecular ImmunologyWB SaundersPhiladelphia2262421991
|
|
35.
|
P KrishnamurthyE LambersS VermaMyocardial
knockdown of mRNA-stabilizing protein HuR attenuates post-MI
inflammatory response and left ventricular dysfunction in
IL-10-null miceFASEB
J2424842494201010.1096/fj.09-14981520219984
|
|
36.
|
KA KrownMT PageC NguyenTumor necrosis
factor alpha-induced apoptosis in cardiac myocytes: involvement of
the sphingolipid signaling cascade in cardiac cell deathJ Clin
Invest9828542865199610.1172/JCI119114
|
|
37.
|
CD FilippoS CuzzocreaF RossiOxidative
stress as the leading cause of acute myocardial infarction in
diabeticsCardiovasc Drug
Rev247787200610.1111/j.1527-3466.2006.00077.x16961722
|
|
38.
|
ME DavisG SeshadriS DikalovDelivery of SOD
with polyketal particles protects rats from acute myocardial
infarctionCirculation120S7472009
|
|
39.
|
W ZhengLZ HuangL ZhaoSuperoxide dismutase
activity and malondialdehyde level in plasma and morphological
evaluation of acute severe hemorrhagic shock in ratsAm J Emerg
Med265458200810.1016/j.ajem.2007.02.00718082781
|
|
40.
|
H Gersterβ-Carotene, vitamin E and vitamin
C in different stages of experimental carcinogenesisEur J Clin
Nutr491551681995
|
|
41.
|
D KonukogluO SerinDG KemerliA study on the
carotid artery intima-media thickness and its association with
lipid peroxidationClin Chim
Acta2779198199810.1016/S0009-8981(98)00117-X9776048
|