Introduction
Endothelin-1 (ET-1) is generated by a variety of
cell types, including endothelial cells, vascular smooth muscle
cells, leukocytes, cardiomyocytes, mesangial cells, certain tumor
cell lines, as well as neurons and glia in both the central and
peripheral nervous systems (1–3).
Local administration of ET-1 induces nociceptive behaviors in
animals (4,5) and causes pain in humans (6). ET-1 also elicits pruritus in mice
(7–9) and humans (6,10).
The signaling of ET-1 is mediated by two main membrane G-protein
coupled receptor subtypes, ETA and ETB
(11). Blockade of the
ETA receptor inhibits the scratching response induced by
ET-1 (7,8,12),
while co-injection of ETB antagonists increases
scratching bouts induced by ET-1 (8,12).
By contrast, ETB agonists exert antipruritic effects of
itch induced by both ET-1 and compound 48/80 (8).
ET-1 is secreted in response to inflammation, tissue
injury and other stress stimuli. Therefore, ETB is a
promising target to mitigate ET-1-induced itch symptoms. The
mechanisms that drive the antipruritic effects of ETB
remain to be elucidated. It has been reported that keratinocytes
express ETB receptors, and upon activation may lead to
the release of opium-like substances (13). Peripheral µ- and κ-opioid receptors
(MORs and KORs, respectively) are reported to be involved in the
anti-nociception effect triggered by ETB agonists
(13,14) Peripheral δ-opioid receptors (DORs)
are also considered to be involved in the antinociceptive activity
of opioid peptides, which are released from neutrophils and in
response to endothelin-A receptor antagonists (15,16).
Peripheral MORs and KORs, but not DORs, may play key
roles in pruritus (17,18). For example, topical application of
naltrexone inhibits pruritus in patients with atopic dermatitis
(19). In addition, ICI 204448, a
peripherally restricted KOR agonist, was found to antagonize
chloroquine-induced scratching in mice (20). We note that neuron excitability is
reduced upon opioid receptor activation, which is due to the
inhibition of voltage-dependent Ca2+ channels and adenyl
cyclase, as well as the activation of K+ channels
(21). Notably, SQ22536, a
selective inhibitor of adenyl cyclase, inhibits the scratching
response induced by ET-1 (12).
Therefore, our goal was to investigate the effects of opioid
receptor antagonists on the scratching response induced by
ET-1.
Materials and methods
Animals
Male C57BL/6J mice, weighing 20–22 g, were obtained
from the Center for Laboratory Animals, Sun Yat-Sen University
(Guangzhou, China). The animals were housed at room temperature
(22±1°C) on a 12/12-h light (8 a.m.-8 p.m.)/dark (8 p.m.-8 a.m.)
cycle and had free access to rodent food and water. The
experimental procedures and the animal use and care protocols were
approved by the Committee on Ethical Use of Animals of Guangdong
General Hospital (Guangzhou, China). Our procedures also followed
the National Institutes of Health’s animal use and care guidelines.
All efforts were made to minimize animal suffering and to reduce
the number of animals used.
Drugs and chemicals
Synthetic ET-1, BQ-123 (ETA antagonist)
and BQ-788 (ETB antagonist) were purchased from American
Peptides (Sunnyvale, CA, USA). Naloxone hydro-chloride was supplied
by Kawin Technology Share-Holding Co. (Beijing, China). CTOP
(Phe-Cys-Tyr-Trp-Orn-Thr-Pen-Thr-NH2; MOR antagonist)
and nor-Binaltorphimine dihydrochloride (nor-BNI; KOR antagonist)
were purchased from Sigma Chemical Co. (St. Louis, MO, USA).
Naltrindole hydrochloride (DOR antagonist) was purchased from
Tocris (Bristol, UK). All drugs were diluted in phosphate-buffered
saline (PBS) and the pH was adjusted to 7.4.
Pruritus model and behavioral
analysis
The pruritus model was established as previously
described (12). Briefly, at one
day after shaving the rostral part of the back of the neck, mice
were placed into a small plastic chamber (22×12×20 cm3)
30 min prior to the experiment. For drug administration, mice were
briefly removed from the chamber and 50 μl of each test drug was
intradermally injected with a 30-gauge needle (detailed description
of injection has been described elsewhere) (22). The mice were then returned to the
chamber and hind limb scratching that was directed towards the
shaved area at the back of the neck was observed and recorded for
30 min. One scratch was defined as a lift of the hind limb towards
the injection site and then a reposition of the limb back to the
floor, regardless of the scratching strokes that took place between
the two movements. All testing was conducted between 10:00 and
16:00. To reduce the injection times, antagonists or inhibitors
(BQ-123 50 nmol, BQ-788 15 nmol, naloxone 2 nmol or 0.5 mg/kg, CTOP
10 nmol, nor-BNI 5 nmol, naltrindole 60 nmol) were co-injected with
ET-1 (50 pmol) in a volume of 50 μl. Naloxone (2 nmol or 0.5 mg/kg)
was subcutaneously injected 15 min prior to the administration of
50 μl ET-1 (50 pmol) to investigate the role of systemic naloxone
administration. The specific doses of BQ-123, BQ-788, naloxone,
CTOP and naltrindole used were selected based on previous studies
(12,13,15,23).
Statistical analysis
Minitab 16 for windows (Minitab Inc., State College,
PA, USA) was used for statistical analysis. All results are
expressed as means ± SEM. Data were statistically evaluated by
analysis of variance followed by Bonferroni’s test or, when only
two means were to be compared, unpaired Student’s t-test. P<0.05
was considered to indicate a statistically significant result.
Results
Intradermal injections
Intradermal injections of PBS, BQ-123, BQ-788,
naloxone, CTOP or naltrindole caused no noticeable scratching
response. By contrast, a scratching response was elicited by
intradermal injection of ET-1, or a high concentration of nor-BNI.
However, there was no noticeable increment of hoarsening, grooming
or paw licking with intradermal ET-1 or nor-BNI injections.
BQ-123 and ET-1 injection
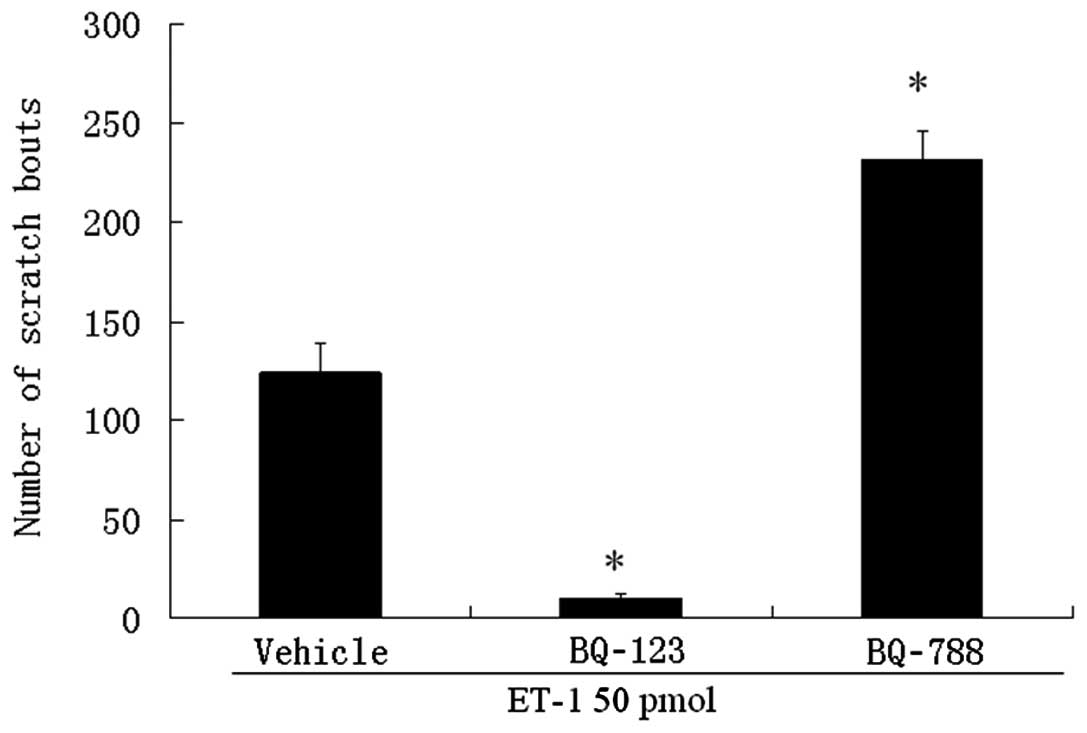
Intradermal injection of 50 pmol ET-1 evoked
scratching bouts (124±14 bouts). Scratching bouts induced by ET-1
were markedly inhibited when they were co-administered locally with
the selective ETA receptor antagonist BQ-123 (Fig. 1). In contrast, local co-injection
of the selective ETB receptor antagonist BQ-788,
substantially augmented the effect of ET-1 (231±14 bouts; Fig. 1).
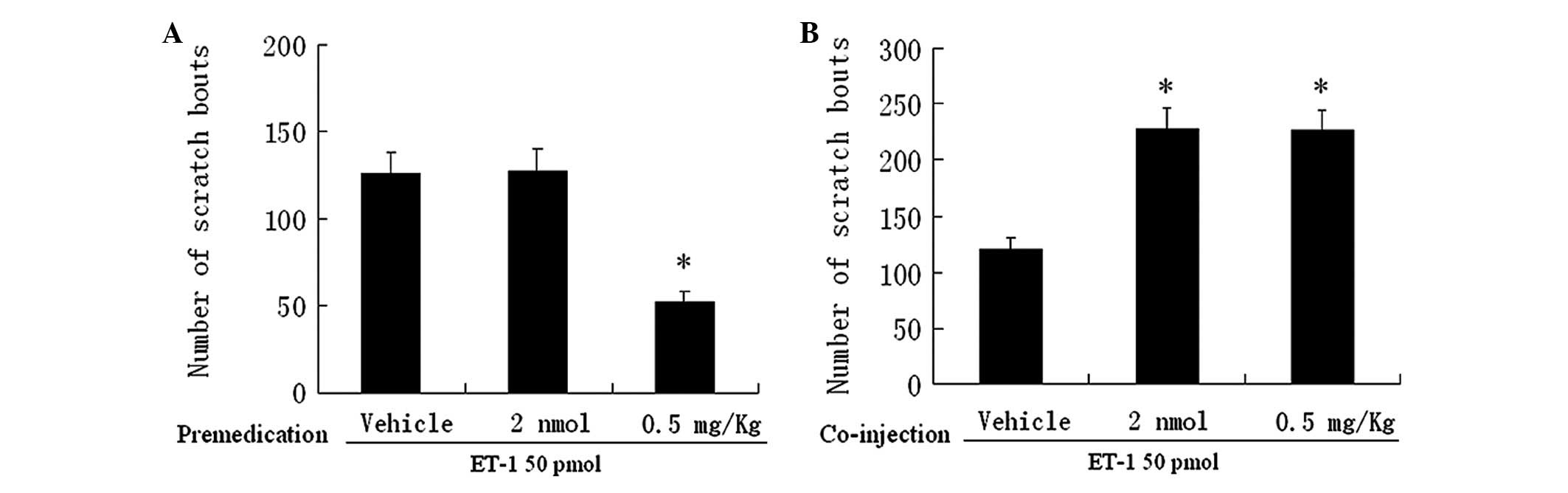
Naloxone and ET-1
We then examined the effects of naloxone, an
antagonist of opioid receptors, on the scratching response induced
by ET-1. Pretreatment with systemically administered naloxone 0.5
mg/kg significantly reduced the number of scratches to 52±6
compared to that induced following pretreatment with PBS (126±12,
Fig. 2A). Co-injection of naloxone
2 nmol or 0.5 mg/kg substantially and similarly augmented the
effect of ET-1 (Fig. 2B).
Pretreatment with a low dose of naloxone (2 nmol), however, was not
able to alter the scratching response (Fig. 2A). Thus, local, but not systemic
naloxone, prevented the antipruritic effect induced by activation
of the ETB receptor, suggesting the involvement of
peripheral opioid receptors in pruritis.
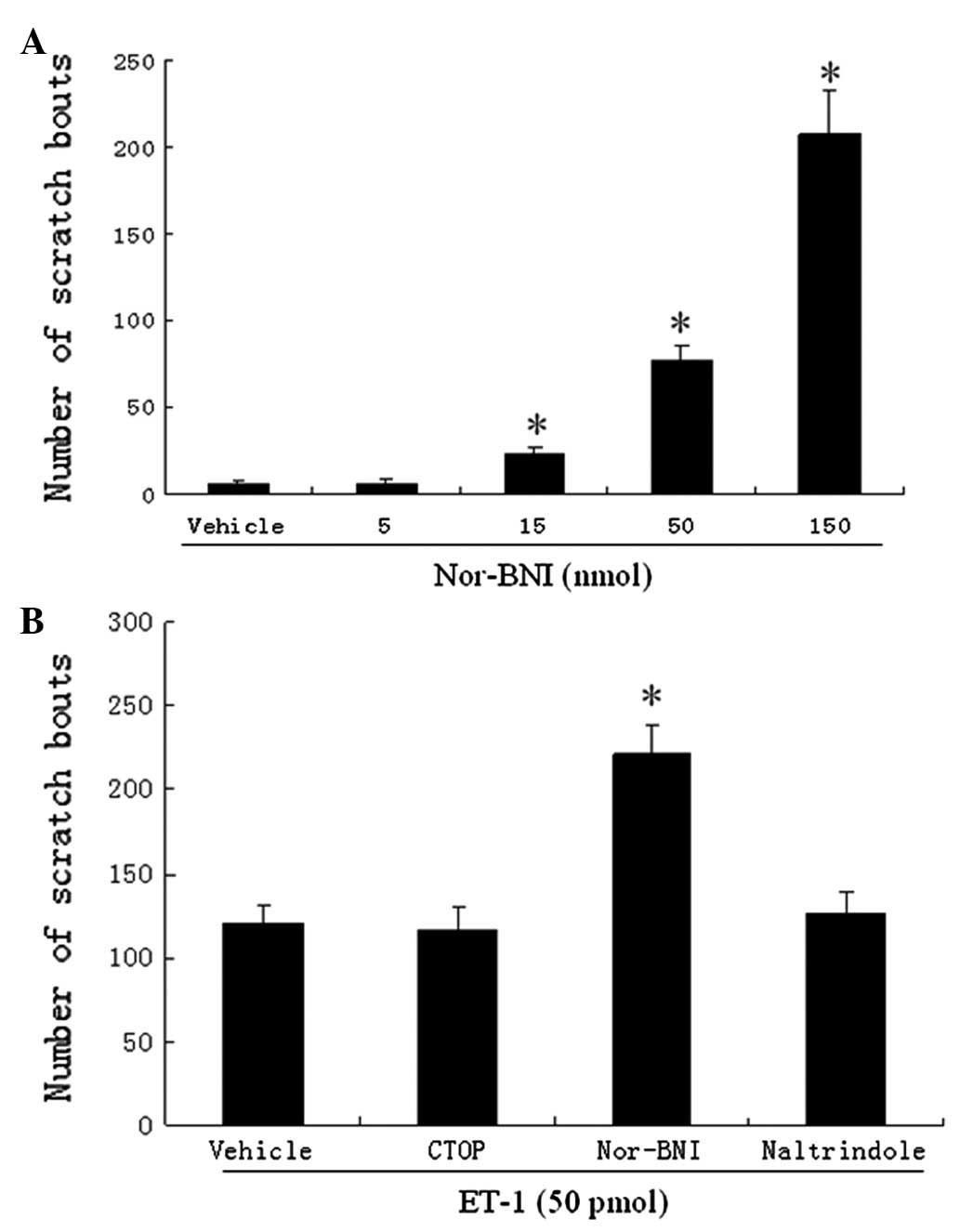
CTOP, nor-BNI, naltrindole and ET-1
We also examined the effects of CTOP, nor-BNI and
naltrindole (MOR, KOR and DOR antagonists, respectively) on the
scratching response induced by ET-1. Intradermal injections of
nor-BNI evoked dose-dependent scratching bouts (Fig. 3A). We note that 5 nmol of nor-BNI
was selected since it did not cause a noticeable scratching
response. Co-injection of nor-BNI significantly increased the
number of scratches (from an average of 121 to 222 bouts; Fig. 3B) induced by ET-1. CTOP and
naltrindole did not alter the scratching response to ET-1 (Fig. 3B).
Discussion
In the present study, we demonstrated that the
activation of peripheral endothelin B receptors exerts antipruritic
effects via peripheral KORs. For example, co-injection of naloxone
(a non-selective opioid receptor antagonist) and nor-BNI (a KOR
antagonist) with ET-1 significantly increased the number of
scratches, while co-injection of CTOP (a MOR antagonist) and
naltrindole (a DOR antagonist) with ET-1 did not alter the
scratching response. Notably the doses of CTOP and naltrin-dole are
sufficient to reduce the analgesic effects evoked by endogenous
opioid peptides (13,15).
The opioid system plays a pivotal role in modulating
pruritus (18). Opioid-induced
pruritus is a well-known side effect of postoperative analgesia
attributed to spinal or epidural morphine, as well as
administration of other MOR agonists (24,25).
Notably, MOR antagonists and KOR agonists have been reported to
treat pruritus effectively in patients with chronic renal failure,
cholestasis and atopic dermatitis (26). Opioid receptors are expressed in
peripheral nerve endings and keratinocytes in human skin (27). The gastrin-releasing peptide
receptor (GRPR) is an itch-specific molecule in the spinal cord
(28). For example, ablation of
lamina I neurons expressing GRPR in the spinal cord of mice
produces profound scratching deficits in response to itching
(pruritogenic) stimuli (29).
Notably, GRP is co-localized with MOR in mouse skin (30). These data suggest that the opioid
receptors involved in itching sensations are peripheral, as well as
central (18).
Naloxone has been shown to inhibit scratching
induced both by capsaicin in inflamed skin and by several
pruritogens (23,31–33).
It is noteworthy that co-injection with naloxone increased
scratches induced by ET-1. Intradermal or subcutaneous injections
of KOR antagonists induced scratching responses both in this study
and in previous research (34),
while intradermal injections of naloxone alone did not induce
scratching, suggesting that the blockade of MORs may inhibit the
pruritogenic effect of KOR antagonists.
Loperamide, a peripherally restricted MOR agonist,
was also found to antagonize scratching evoked by compound 48/80 in
mice (35). However, scratching
responses were evoked by intradermally injected morphine, fentanyl
and loperamide (36). Naloxone
methiodide, a peripherally restricted opioid receptor antagonist,
significantly suppressed scratching behavior induced by MOR
agonists loperamide and DAMGO (37). It has also been demonstrated that
ETB agonists injected into the neck induced scratching
in female BALB/C mice (7), while
no scratching response was induced in male Swiss mice (8). By contrast, ETB
antagonists injected into the neck induced scratching in male Swiss
mice (8), while it did not induce
scratching in this study, as well as previous studies using male
C57BL/6J mice (12). CTOP was not
able to inhibit the scratching response induced by ET-1 in the
current study, indicating the significance of genetic backgrounds
in determining puritogenic sensitivity to peripheral MOR
agonists.
Activation of neuronal KORs reduces neuronal
excitability via various pathways (21). However, it is possible to exert
antipruritic effects via non-neuronal pathways. For example, we
recently observed that activation of transient receptor potential
ankyrin subfamily member 1 (TRPA1) suppresses itch responses
induced by ET-1 (38). Notably,
TRPA1 and KORs are expressed in human keratinocytes (39,40).
It will be valuable to evaluate the role of TRPA1 and KORs in
keratinocytes.
In summary, we investigated the involvement of
peripheral opioid receptors in ET-1-induced pruritus in mice. We
suggest that peripheral KORs mediate the antipruritic effects of
endothelin B receptor activation.
Acknowledgements
This study was supported by the
National Natural Science Foundation (Nos. 30872437 and 81171040),
Natural Science Foundation of Guangdong Province (No.
8151008004000017) and Science and Technology Project of Guangdong
Province (2011), China.
References
|
1.
|
Hans G, Deseure K and Adriaensen H:
Endothelin-1-induced pain and hyperalgesia: a review of
pathophysiology, clinical manifestations and future therapeutic
options. Neuropeptides. 42:119–132. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Giaid A, Gibson SJ, Ibrahim BN, Legon S,
Bloom SR, Yanagisawa M, Masaki T, Varndell IM and Polak JM:
Endothelin 1, an endothelium-derived peptide, is expressed in
neurons of the human spinal cord and dorsal root ganglia. Proc Natl
Acad Sci USA. 86:7634–7638. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
MacCumber MW, Ross CA and Snyder SH:
Endothelin in brain: receptors, mitogenesis, and biosynthesis in
glial cells. Proc Natl Acad Sci USA. 87:2359–2363. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Piovezan AP, D’Orleans-Juste P, Souza GE
and Rae GA: Endothelin-1-induced ETA receptor-mediated nociception,
hyperalgesia and oedema in the mouse hind-paw: modulation by
simultaneous ETB receptor activation. Br J Pharmacol. 129:961–968.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Gokin AP, Fareed MU, Pan HL, Hans G,
Strichartz GR and Davar G: Local injection of endothelin-1 produces
pain-like behavior and excitation of nociceptors in rats. J
Neurosci. 21:5358–5366. 2001.PubMed/NCBI
|
|
6.
|
Hans G, Deseure K, Robert D and De Hert S:
Neurosensory changes in a human model of endothelin-1 induced pain:
a behavioral study. Neurosci Lett. 418:117–121. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
McQueen DS, Noble MA and Bond SM:
Endothelin-1 activates ETA receptors to cause reflex scratching in
BALB/c mice. Br J Pharmacol. 151:278–284. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Trentin PG, Fernandes MB, D’Orleans-Juste
P and Rae GA: Endothelin-1 causes pruritus in mice. Exp Biol Med
(Maywood). 231:1146–1151. 2006.PubMed/NCBI
|
|
9.
|
Imamachi N, Park GH, Lee H, Anderson DJ,
Simon MI, Basbaum AI and Han SK: TRPV1-expressing primary afferents
generate behavioral responses to pruritogens via multiple
mechanisms. Proc Natl Acad Sci USA. 106:11330–11335. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Katugampola R, Church MK and Clough GF:
The neurogenic vasodilator response to endothelin-1: a study in
human skin in vivo. Exp Physiol. 85:839–846. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Lipa JE, Neligan PC, Perreault TM,
Baribeau J, Levine RH, Knowlton RJ and Pang CY: Vasoconstrictor
effect of endothelin-1 in human skin: role of ETA and ETB
receptors. Am J Physiol. 276:H359–H367. 1999.PubMed/NCBI
|
|
12.
|
Liang J, Kawamata T and Ji W: Molecular
signaling of pruritus induced by endothelin-1 in mice. Exp Biol Med
(Maywood). 235:1300–1305. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Khodorova A, Navarro B, Jouaville LS, et
al: Endothelin-B receptor activation triggers an endogenous
analgesic cascade at sites of peripheral injury. Nat Med.
9:1055–1061. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Quang PN and Schmidt BL: Peripheral
endothelin B receptor agonist-induced antinociception involves
endogenous opioids in mice. Pain. 149:254–262. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Rittner HL, Hackel D, Voigt P, Mousa S,
Stolz A, Labuz D, Schäfer M, Schaefer M, Stein C and Brack A:
Mycobacteria attenuate nociceptive responses by formyl peptide
receptor triggered opioid peptide release from neutrophils. PLoS
Pathog. 5:e10003622009. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Quang PN and Schmidt BL: Endothelin-A
receptor antagonism attenuates carcinoma-induced pain through
opioids in mice. J Pain. 11:663–671. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Ikoma A, Steinhoff M, Ständer S,
Yosipovitch G and Schmelz M: The neurobiology of itch. Nat Rev
Neurosci. 7:535–547. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Tominaga M and Takamori K: Recent advances
in pathophysiological mechanisms of itch. Expert Rev Dermatol.
5:197–212. 2010. View
Article : Google Scholar
|
|
19.
|
Bigliardi PL, Stammer H, Jost G, Rufli T,
Büchner S and Bigliardi-Qi M: Treatment of pruritus with topically
applied opiate receptor antagonist. J Am Acad Dermatol. 56:979–988.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Inan S and Cowan A: Kappa opioid agonists
suppress chloroquine-induced scratching in mice. Eur J Pharmacol.
502:233–237. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Satoh M and Minami M: Molecular
pharmacology of the opioid receptors. Pharmacol Ther. 68:343–364.
1995. View Article : Google Scholar
|
|
22.
|
Shimada SG and LaMotte RH: Behavioral
differentiation between itch and pain in mouse. Pain. 139:681–687.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Liang J, Xiao G and Ji W: Capsaicin
induces reflex scratching in inflamed skin. Pharmacology. 88:82–87.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Ballantyne JC, Loach AB and Carr DB:
Itching after epidural and spinal opiates. Pain. 33:149–160. 1988.
View Article : Google Scholar : PubMed/NCBI
|
|
25.
|
Cousins MJ and Mather LE: Intrathecal and
epidural administration of opioids. Anesthesiology. 61:276–310.
1984. View Article : Google Scholar : PubMed/NCBI
|
|
26.
|
Bigliardi PL, Tobin DJ, Gaveriaux-Ruff C
and Bigliardi-Qi M: Opioids and the skin - where do we stand? Exp
Dermatol. 18:424–430. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27.
|
Bigliardi-Qi M, Sumanovski LT, Büchner S,
Rufli T and Bigliardi PL: Mu-opiate receptor and beta-endorphin
expression in nerve endings and keratinocytes in human skin.
Dermatology. 209:183–189. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
28.
|
Sun YG and Chen ZF: A gastrin-releasing
peptide receptor mediates the itch sensation in the spinal cord.
Nature. 448:700–703. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29.
|
Sun YG, Zhao ZQ, Meng XL, Yin J, Liu XY
and Chen ZF: Cellular basis of itch sensation. Science.
325:1531–1534. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30.
|
Tominaga M, Ogawa H and Takamori K:
Histological characterization of cutaneous nerve fibers containing
gastrin-releasing peptide in NC/Nga mice: an atopic dermatitis
model. J Invest Dermatol. 129:2901–2905. 2009. View Article : Google Scholar
|
|
31.
|
Andoh T, Nagasawa T, Satoh M and Kuraishi
Y: Substance P induction of itch-associated response mediated by
cutaneous NK1 tachykinin receptors in mice. J Pharmacol Exp Ther.
286:1140–1145. 1998.PubMed/NCBI
|
|
32.
|
Andoh T, Yageta Y, Takeshima H and
Kuraishi Y: Intradermal nociceptin elicits itch-associated
responses through leukotriene B(4) in mice. J Invest Dermatol.
123:196–201. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
33.
|
Yamaguchi T, Nagasawa T, Satoh M and
Kuraishi Y: Itch-associated response induced by intradermal
serotonin through 5-HT2 receptors in mice. Neurosci Res. 35:77–83.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
34.
|
Kamei J and Nagase H: Norbinaltorphimine,
a selective κ-opioid receptor antagonist, induces an
itch-associated response in mice. Eur J Pharmacol. 418:141–145.
2001.
|
|
35.
|
DeHaven-Hudkins DL, Cowan A, Cortes Burgos
L, Daubert JD, Cassel JA, DeHaven RN, Kehner GB and Kumar V:
Antipruritic and antihyperalgesic actions of loperamide and
analogs. Life Sci. 71:2787–2796. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
36.
|
Yamamoto A, Kuyama S, Kamei C and Sugimoto
Y: Characterization of scratching behavior induced by intradermal
administration of morphine and fentanyl in mice. Eur J Pharmacol.
627:162–166. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
37.
|
Yamamoto A and Sugimoto Y: Involvement of
peripheral mu opioid receptors in scratching behavior in mice. Eur
J Pharmacol. 649:336–341. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
38.
|
Liang J, Ji Q and Ji W: Role of transient
receptor potential ankyrin subfamily member 1 in pruritus induced
by endothelin-1. Neurosci Lett. 492:175–178. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
39.
|
Tominaga M, Ogawa H and Takamori K:
Possible roles of epidermal opioid systems in pruritus of atopic
dermatitis. J Invest Dermatol. 127:2228–2235. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
40.
|
Atoyan R, Shander D and Botchkareva NV:
Non-neuronal expression of transient receptor potential type A1
(TRPA1) in human skin. J Invest Dermatol. 129:2312–2315. 2009.
View Article : Google Scholar : PubMed/NCBI
|