Introduction
In the United States, lung cancer is the leading
cause of cancer-related mortalities (1). Non-small cell lung cancer (NSCLC)
accounts for more than 85% of all lung cancer cases (2). Approximately 40% of lung cancer
patients present with advanced NSCLC (1). For patients with advanced NSCLC with
a favorable performance status, the first-line treatment is a
platinum-based two-drug combination regimen resulting in a slight
increase in survival (3).
Traditionally, 4–6 cycles of platinum-based chemotherapy are
recommended (4) and clinical
trials have demonstrated that prolonged first-line treatment did
not improve survival but increased toxicity (5–7). As
combined chemotherapy is reaching a plateau of efficacy, a ‘watch
and wait’ strategy until disease progression was previously
considered a reasonable therapeutic strategy. The majority of
patients rapidly suffer from disease progression within 2–3 months
of their last chemotherapy cycle (8,9).
Second-line treatments are provided to those who
experience disease progression after first-line therapies. However,
certain studies suggest that 30–50% of patients do not receive
effective second-line therapy after disease progression due to
rapid progression, declining performance status and increased
symptom burden (9–13). Consequently, investigations were
conducted to identify a maintenance strategy as a more active
therapy, which is expected to consolidate the effectiveness of
first-line chemotherapy and improve survival without serious
toxicity. More patients with a favorable condition are able to
receive this regimen due to lack of disease progression.
Maintenance therapy is defined as the prolongation
of treatment duration with the administration of additional drugs
at the end of a defined number of initial chemotherapy cycles after
achieving tumor control. Maintenance therapy has been extensively
investigated in patients with NSCLC. It consists of drugs included
in the first-line treatment or other non-cross-resistant agents.
Although the exact terminology remains unclear, according to the
literature, when drugs included in the induction regimen are used
it is known as ‘continuation maintenance’ and when other
non-cross-resistant agents are used, it is designated as ‘switch
maintenance’ or ‘early second-line’ (13,14).
Previously, many agents were extensively explored for maintenance
therapy in patients with advanced NSCLC, including cytotoxic drugs,
targeted agents and anticancer vaccines. Promising results,
including improvements in progression-free (PFS) or overall
survival (OS), were reported (8,9,15–17).
Erlotinib is a highly potent, orally active epidermal growth factor
receptor (EGFR) tyrosine-kinase inhibitor (TKI) approved worldwide
for advanced NSCLC treatment following chemotherapy failure. In a
large phase III trial, erlotinib monotherapy was proven to
significantly improve OS [6.7 vs. 4.7 months; hazard ratio (HR)=
0.70; P<0.001] vs. placebo and provide significant symptom and
quality-of-life benefits in patients previously treated with
advanced NSCLC (18), which was in
agreement with another large phase IV clinical study (19). Expected to benefit more patients,
this targeted agent was also studied in several trials to test the
efficacy and safety when used concurrently or sequentially with
fist-line chemotherapy in patients who obtained disease control.
However, the PFS and OS results remain controversial. Two early
studies showed that erlotinib did not improve PFS and OS compared
with the placebo when it was combined with chemotherapy as
first-line treatment for advanced NSCLC (20,21).
A multi-centre, randomized, placebo-controlled phase III study
suggested that maintenance therapy with erlotinib for NSCLC
patients is well-tolerated and significantly prolonged PFS and OS
compared with the placebo (16).
Similar discrepancies also exist among other studies. Therefore, a
meta-analysis was performed to examine pooled data of randomized
control trials (RCTs) where erlotinib was compared against placebo
or observation only in the maintenance regimen for patients with
unresectable NCSLC to quantify potential benefits and determine
safety.
Patients and methods
Search strategy and study selection
A wide search of the main electronic databases of
interest was conducted, including PubMed, EMBASE, the Cochrane
Library Trials Register, the National Cancer Institute Clinical
Trials, the Clinical Trials Register of Trials Central and the
abstracts published in the Proceedings of American Society of
Clinical Oncology (ASCO). The reference lists of primary studies
and relevant review articles were also examined manually for
potential eligible studies. An additional search was performed on
the Web of Science database for studies cited if necessary.
The search strategy for PubMed was constructed using
a combination of Medical Subject Headings (MeSH) and text words
relating to erlotinib or Tarceva® for NSCLC. A total of
two searches were performed: i) erlotinib OR Tarceva [All Fields]
and ii) (Lung cancer) OR (pulmonary carcinoma) [All Fields] OR
(Lung neoplasm) [MeSH Terms]. The final search combined i) and ii)
and was limited to humans, clinical trials, meta-analyses, practice
guidelines, RCTs and reviews. The search strategies for the other
databases were based on keywords similar to the terms described
above. The electronic search was conducted until June 2011, without
language limitations.
Inclusion and exclusion criteria
Studies selected from this initial search were
subsequently screened for eligibility using the following criteria:
i) participating patients with unresectable NSCLC at baseline
levels, ii) studies in maintenance therapy with vs. without
erlotinib after the first-line chemotherapy and iii) RCTs with
parallel design. Studies were excluded based on the following
criteria; i) patients previously treated with targeted agents, ii)
phase I clinical trial, iii) retrospective trial or iv) any review,
comment or case report.
Selection, assessment and data
extraction
To select eligible studies for further evaluation,
two independent reviewers screened the title, abstract and keywords
of every study retrieved. Full articles were assessed if the study
conformed to the criteria listed above. Any disagreement in quality
assessment and data collection was discussed and solved by a third
investigator acting as the referee.
Data were extracted from all the included studies by
two independent reviewers. The name of the first author and the
year of publication were used to identify the study. Details of the
study samples (number in each group), interventions (use of
erlotinib, as well as details of other treatments, including
adjuvant chemotherapy) and outcomes [including OS, PFS, ORR and
adverse events (AEs)] were extracted. The data were extracted
directly from the text or calculated from available information.
Whenever reports pertained to sets of patients that overlapped,
only the report with the longest follow-up (having the largest
number of events) was used in the final analysis.
A total of seven criteria were used to evaluate the
quality of included studies: i) whether the allocation method was
completely random, ii) whether there was proper concealment of
allocation, iii) whether there was equality between the two groups
at the baseline in terms of prognostic features, iv) whether the
eligibility criteria were described, v) whether blinding of the
outcome assessors was performed, vi) whether loss to follow-up in
each treatment group was demonstrated and vii) whether
intention-to-treat analysis was considered. A total of seven or six
items were required for a study to be rated as high quality, five
or four items for fair quality and three or fewer for low quality
(22).
Statistical analysis
Outcomes of the included studies were integrated
using the Review Manager 5.12 software provided by the Cochrane
Collaboration (dowloaded from http://www.cochrane.org). Dichotomous clinical
outcomes were reported as risk ratio (RR) or odds ratio (OR), and
time to event data as HR (23).
The corresponding 95% confidence interval (CI) was calculated,
P<0.05 was considered to indicate a statistically significant
result.
The χ2 test was used to test the
heterogeneities of treatment effects between studies, P<0.10 was
considered to indicate a statistically significant result.
Moreover, the I2 method was used to estimate
total variations across studies that were due to heterogeneity
rather than chance in percentage (25% was considered to indicate
low-level heterogeneity, 25–50% as moderate and >50% as high)
(24).
The pooled statistics were first calculated with a
fixed effects model. For dichotomous variables, the
Cochran-Mantel-Haenszel test was used to analyze the significance,
while for survival data, a pooled estimate of the HR was computed
according to the inverse-variance method with P<0.05 being
considered to indicate a statistically significant result. If
statistical heterogeneity was identified, one of the following
techniques was used to explain it: i) random effects model that
provides a more conservative analysis, ii) subgroup analyses
including patients stratified by EGFR immunohistochemistry status
(positive, negative) and smoking history (current, former, ever,
non-smokers) or iii) sensitivity analyses.
To assess the possibility of publication bias, we
performed the funnel plot test described by Egger et al
(25). When the pooled results
were significant, the number of patients needed to treat (NNTs)
were calculated by pooling absolute risk differences in studies
included in our meta-analyses (26–28).
For all the analyses, the forest plots were generated to exhibit
results with point estimates and 95% CIs for each study and the
overall size of the squares is proportional to effect size.
Results
Studies included
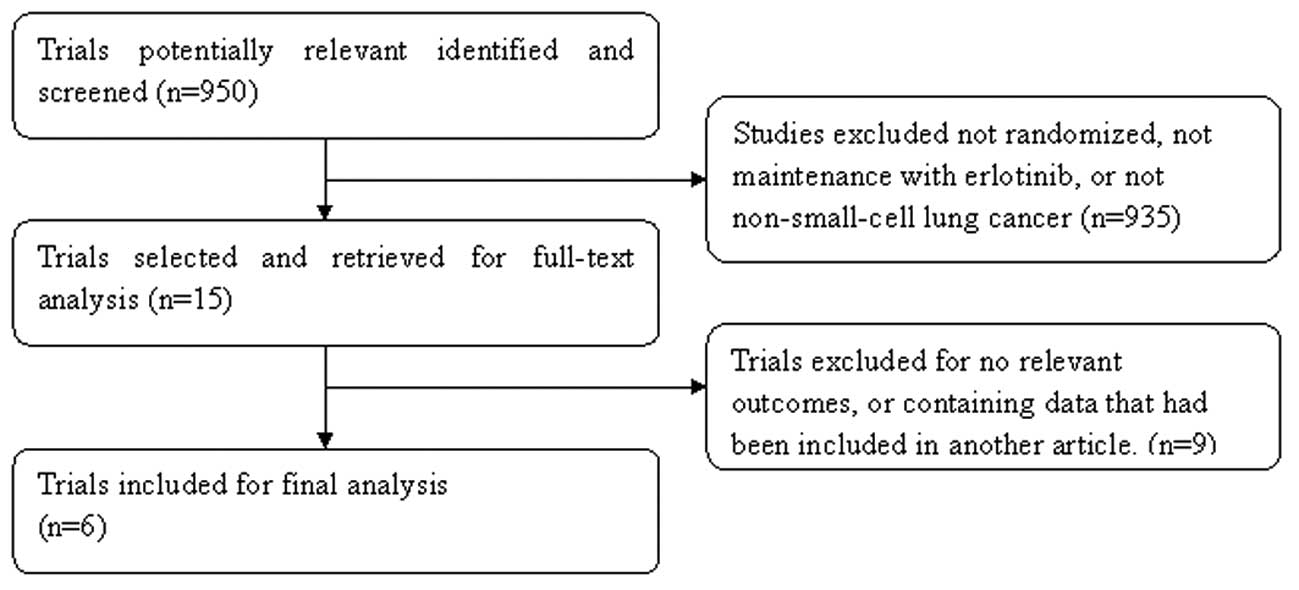
The study selection process was summarized, as
recommended by the QUOROM statement (Fig. 1) (29). In total, 950 studies were
identified and screened. Subsequently, selection was performed
according to the inclusion/exclusion criteria described and 935
items were excluded at the primary selection step by browsing the
retrieved titles and their abstracts. At the secondary selection
step, nine studies were further excluded after reading the full
text of 15 potentially eligible studies. Finally, six studies
comprising 4,372 patients with histologically proven NSCLC met the
inclusion criteria. Of these studies, four were published in full
text (16,20,21,30),
while the other two were reported in the annual meeting of the ASCO
in the form of abstracts (31,32).
There were certain differences in the experimental designs. Of the
included studies, two administered erlotinib concurrently with
chemotherapy followed by a maintenance phase (20,21),
another study adopted a sequential regimen of erlotinib and
chemotherapy (30), while the
remaining three studies continued with erlotinib after chemotherapy
(16,31,32).
The main characteristics of these studies and the evaluations of
the studies are presented in Tables
I and II, respectively. A
total of 4,327 patients were available for analysis and 2,191
patients were randomized to maintain with erlotinib (treatment
group).
 | Table ICharacteristics of included
studies. |
Table I
Characteristics of included
studies.
| Study | Design | n | Patients | Intervention | Outcomes |
|---|
| Herbst et al
(21) | Multi-center,
randomized placebo-controlled phase III trial | 1079 | CT-naive advanced
(stage IIIB or IV) NSCLC | GP concurrent with
Erl or placebo and followed by Erl or placebo | OS, TTP, ORR,
safety, duration of response |
| Gatzemeier et
al (20) | Multi-center,
randomized placebo-controlled, double-blind, phase III trial | 1172 | CT-naive
unresectable or recurrent or advanced (stage III or IV) NSCLC | PC concurrent with
Erl or placebo and followed by Erl or placebo | OS, TTP, ORR, QOL,
safety, duration of response |
| Mok et al
(30) | Multi-center,
randomized placebo-controlled phase II trial | 154 | Previously
untreated advanced (stage IIIB or IV) NSCLC | Sequential Erl or
placebo and CT, followed by Erl or placebo | NPR, RR, OS, PFS,
safety, duration of response |
| Cappuzzo et
al (16) | Multi-center,
randomized placebo-controlled phase III trial | 889 | Unresectable or
advanced (stage IIIB or IV) NSCLC | Maintenance Erl vs.
placebo after 4 cycles of standard platinum-doublet CT | PFS, OS, safety,
QOL |
| Perol et al
(32) | Randomized, three
group phase III trial | 310 | Stage IIIB or IV
NSCLC | Maintenance Erl vs.
Gem vs. observation after 4 cycles | PFS, OS, safety
symptom control of GP |
| Kabbinavar et
al (31) | Randomized,
double-blind, placebo-controlled, phase IIIb trial | 768 | Previously
untreated recurrent or advanced (stage IIIB or IV) NSCLC | Maintenance Erl
plus Bev vs. after 4 cycles of first-line CT combined Bev | PFS, OS,
safety |
 | Table IIQuality of included studies. |
Table II
Quality of included studies.
| Study | Truly random | Random
allocation | Equivalence of
baseline features | Eligibility
criteria | Blinding
assessment | Loss to
follow-up | Intent to
treat | Study quality |
|---|
| Herbst et al
(21) | Yes | Yes | Yes | Yes | Yes | Unclear | Yes | High |
| Gatzemeier et
al (20) | Yes | Yes | Yes | Yes | Yes | Yes | Unclear | High |
| Mok et al
(30) | Yes | Yes | Yes | Yes | Unclear | Yes | Yes | High |
| Cappuzzo et
al (16) | Yes | Yes | Yes | Yes | Yes | Unclear | Yes | High |
| Perol et al
(32) | Yes | No | Yes | Yes | Yes | Unclear | Yes | Fair |
| Kabbinavar et
al (31) | Yes | Yes | Yes | Yes | Unclear | Unclear | Yes | Fair |
Analysis of efficacy
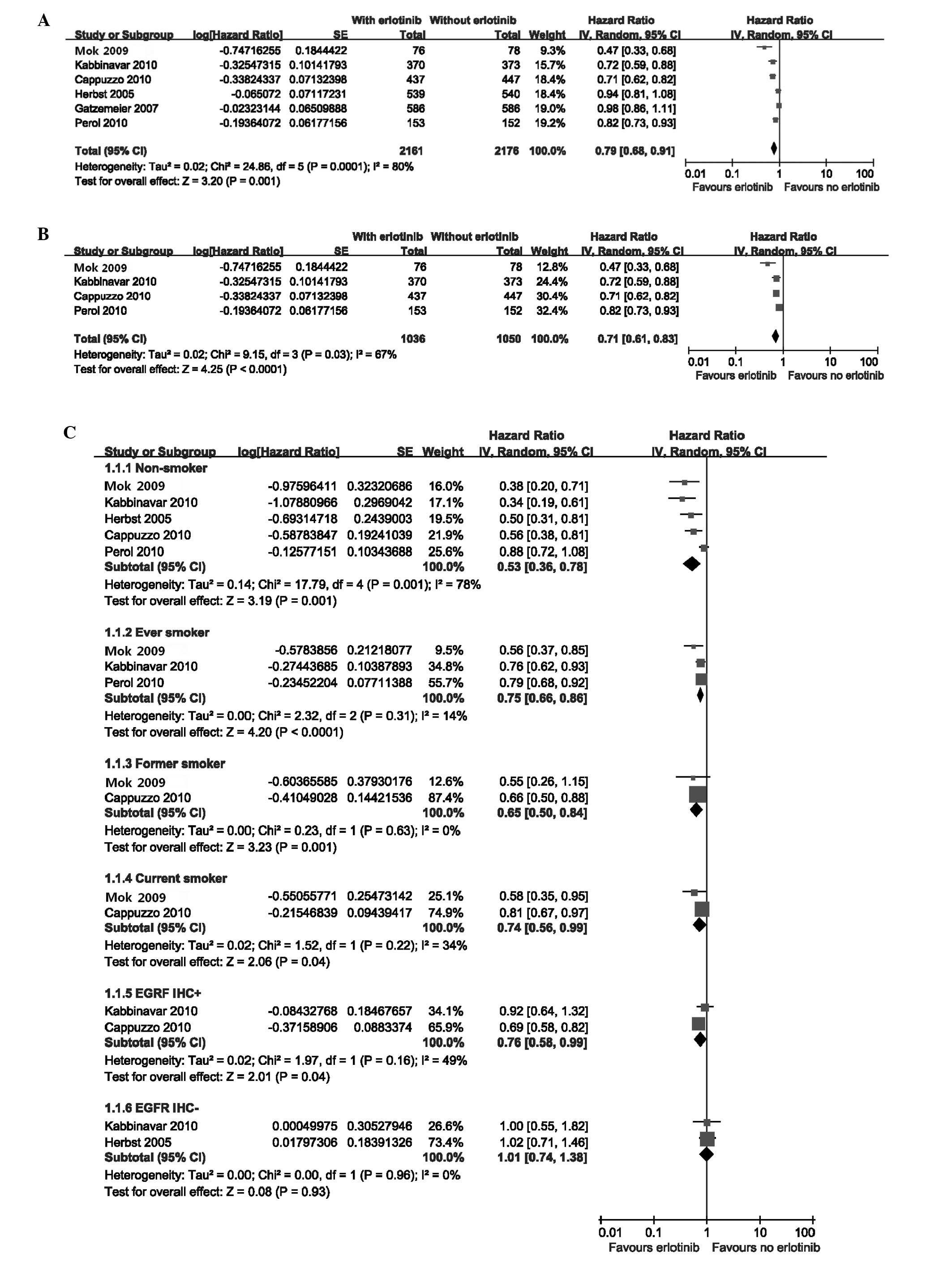
The meta-analysis showed a longer PFS in patients
who received erlotinib as maintenance therapy [random effects: HR=
0.79 (95% CI= 0.68–0.91); P= 0.001; NNT=5], showing a high
heterogeneity level [χ2=24.86, df=5 (P=0.0001);
I2=80%] (Fig.
2A). To further explore this heterogeneity, we excluded the two
studies that used erlotinib concurrently with chemotherapy
(20,21). The benefit to PFS was sustained
[random effects: HR=0.71 (95% CI=0.61–0.83); P<0.001; NNT=3]
with no significant change in the heterogeneity
[χ2=9.15, df=3 (P=0.003); I2=67%]
(Fig. 2B). Planned subgroup
analyses also suggested that the PFS benefit with erlotinib was
consistent across the majority of clinical subgroups with the
exception of the EGFR immunohistochemistry-negative (EGFR IHC−)
population. In addition, smokers did not obtain the greatest
benefit from erlotinib [random effects: HR=0.53 (95% CI=0.36–0.78);
P=0.001; NNT=2] (Fig. 2C).
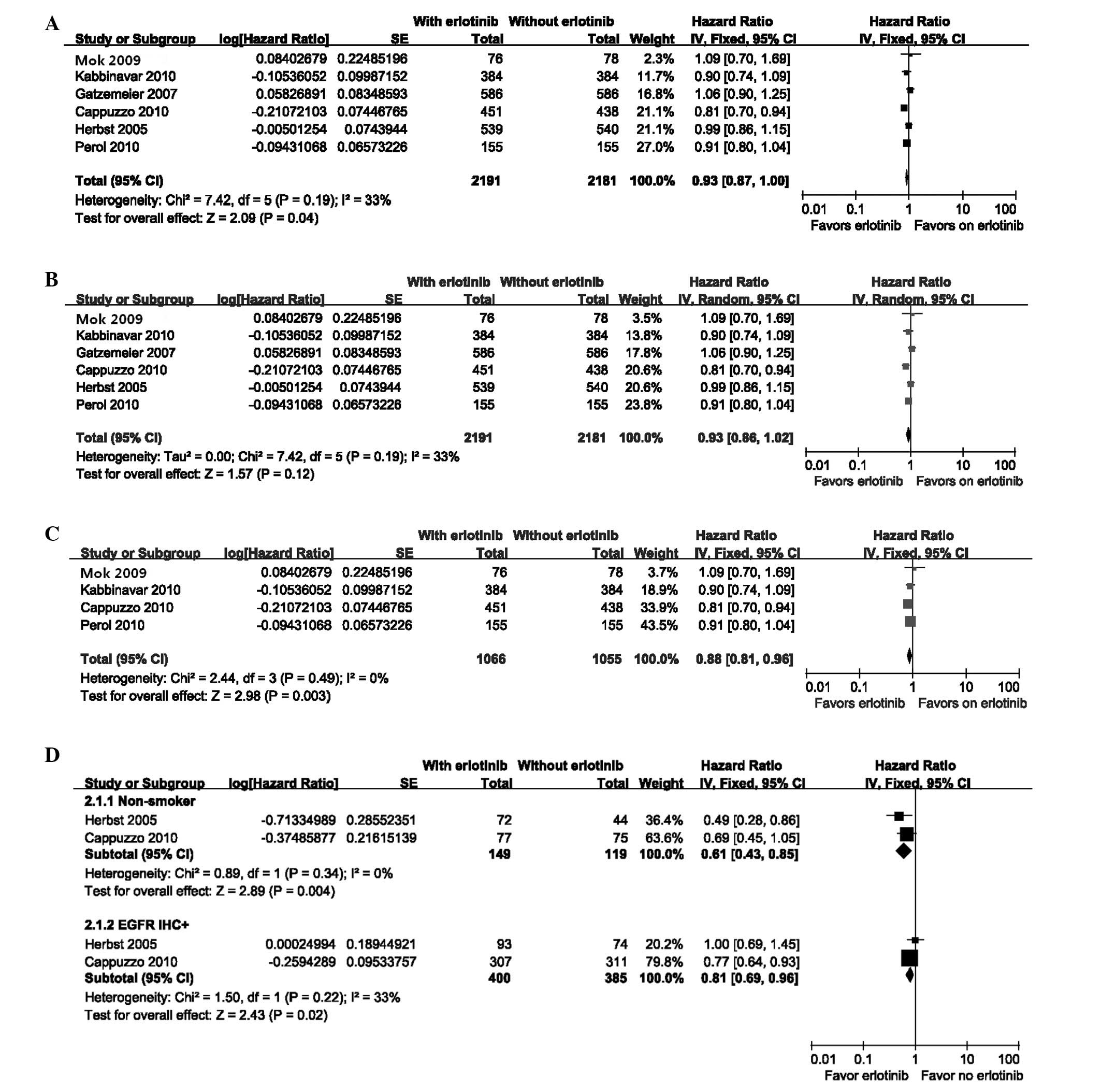
The OS was slightly longer for patients who received
erlotinib as maintenance therapy [fixed effect: HR= 0.93 (95%
CI=0.87–1.00); P=0.04; NNT=15] with moderate heterogeneity
[χ2=7.42, df=5 (P=0.19); I2=33%]
(Fig. 3A). However, the random
effects model indicated no significant difference [random effects:
HR= 0.93 (95% CI= 0.86–1.02); P= 0.12] (Fig. 3B). When the two studies were
excluded (20,21), due to concurrent erlotinib
treatment and chemotherapy, the heterogeneity disappeared
[χ2=2.44, df=3 (P=0.49); I2= 0%]. The
treatment group obtained a greater benefit to OS compared with that
in the control group [fixed effects: HR= 0.88 (95% CI= 0.81–0.96);
P= 0.003; NNT=8] (Fig. 3C). Due to
limited information, there were only two subgroups available for
subset analyses for OS and the results are presented in Fig. 3D.
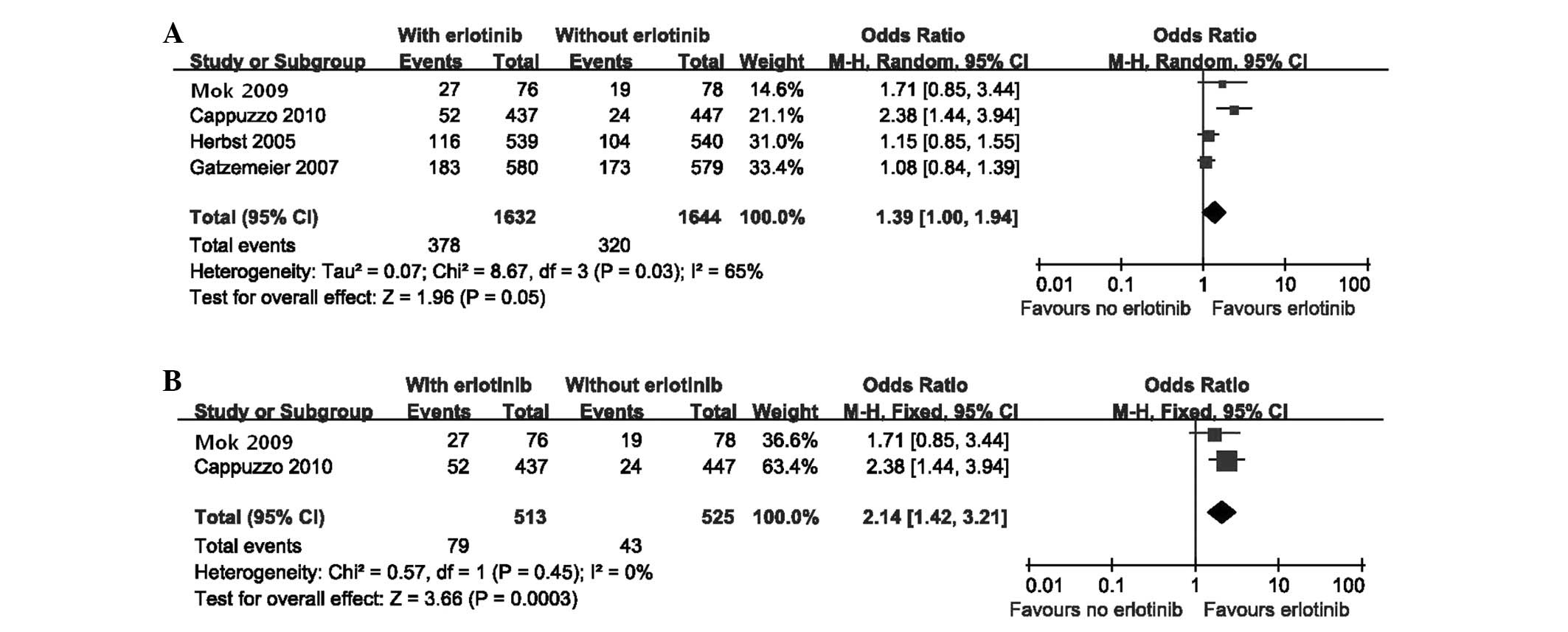
The meta-analysis of ORR is presented in Fig. 4A. The random effects model analysis
indicated that there was no significant difference in ORR between
the erlotinib and control groups [random effects: OR=1.39; (95%
CI=1.00–1.94); P= 0.05]. Due to evident heterogeneity
[χ2=8.67, df=3 (P=0.03); I2=65%], we
also performed a sensitivity analysis by excluding the two studies
(20,21). The results demonstrated a higher
ORR in patients who received erlotinib only after chemotherapy
[fixed effect: OR=2.14; (95% CI=1.42–3.21); P=0.0003], with no
heterogeneity [χ2=0.57, df=1 (P=0.45);
I2=0%] (Fig.
4B).
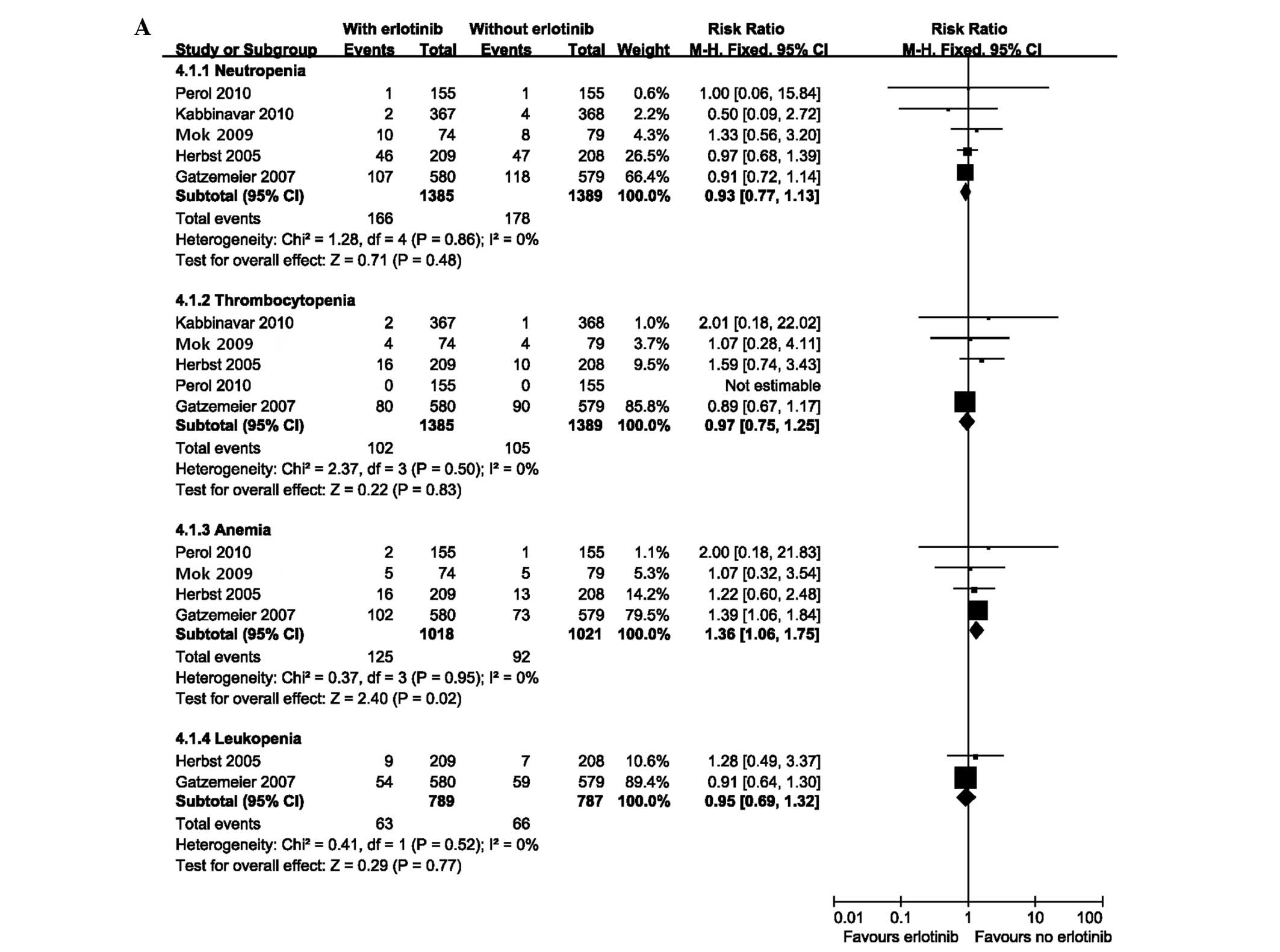
Analysis of safety
Pooled safety analyses of reported grade three or
four AEs of interest, serious adverse events (SAEs) and
treatment-related deaths were performed. The group receiving
erlotinib had a higher incidence of anemia [fixed effect: RR=1.36;
(95% CI=1.06–1.75); P=0.02]. No difference was observed in patients
with other hematological toxicities including neutropenia,
thrombocytopenia and leukopenia (Fig.
5A). With regard to the non-hematological toxicities, patients
receiving erlotinib experienced a significantly higher incidence of
diarrhea, skin toxicity and renal impairment with a pooled HR of
5.10 [fixed effect: (95% CI=3.20–8.14); P<0.00001], 17.67 [fixed
effect: (95% CI=9.22–33.86); P<0.00001] and 4.84 [fixed effect:
(95% CI=2.09–11.18); P=0.0002], respectively (Fig. 5B). Although the SAEs occurred more
frequently in the experimental group [fixed effect: RR=1.38 (95%
CI=1.09–1.75); P=0.007] (Fig. 5C),
there was no significant difference in the incidence of
treatment-related deaths [fixed effect: RR=1.51 (95% CI=
0.73–3.12); P= 0.27] (Fig. 5D).
The funnel plot test did not reveal a significant publication bias
(Egger test; P>0.05).
Discussion
At present, whether maintenance therapy is necessary
for advanced NSCLC remains controversial. It is also unclear which
population of patients may gain the greatest benefit from this new
approach. In the present study, we examined the efficacy and safety
of maintenance with erlotinib for unresectable NSCLC. As shown by
the meta-analysis, PFS was greatest with maintenance with erlotinib
for patients with NSCLC. In the OS analysis, we observed a small
increase in the maintenance group in a fixed effects model with a
moderate-level heterogeneity. When a random effects model analysis
was performed, this difference disappeared. There was no
significant difference in ORR between the two groups in the
original analysis. To explain the causes of heterogeneity among
these studies, we conducted sensitivity and subgroup analyses.
Preclinical evidence suggested that there may be a potential
antagonism between the constituents of the combination therapy.
EGFR TKIs induce G1 phase cell cycle arrest, which
protects cells from the cytotoxic effects of cell-cycle
phase-dependent chemotherapeutic agents, while alternative dosing
schedules, including sequential or pulse dosing of erlotinib,
proved to be more effective than concurrent administration
(33,34). Based on these observations, the
sensitivity analysis was performed by excluding the two studies
that administered erlotinib concurrently with chemotherapy before
the maintenance phase (20,21).
The results showed the benefit to PFS was sustained, and
maintenance erlotinib sequential with or just after first-line
chemotherapy may improve OS and ORR. This fact indirectly proved
that the concurrent administration of erlotinib and chemotherapy is
not suitable for patients with NSCLC and may counteract the effect
of maintenance therapy. By contrast, a sequential schedule has been
successfully used to avoid the potential cell-cycle-based
antagonism between EGFR TKIs and chemotherapy (30). However, the optimal sequential
schedule of erlotinib with chemotherapy remains unclear.
It is worth noting that although erlotinib in
combination with chemotherapy showed no benefit to survival
compared with the placebo group for all the patients in the two
excluded studies, the non-smoker subgroup experienced substantial
prolongation in survival associated with erlotinib. There was no
apparent pharmacokinetic interaction between erlotinib and
cytotoxic drugs (20,21). The subset analyses in our study
also demonstrated that maintenance with erlotinib prolonged PFS vs.
control across the majority of clinical subgroups stratified by
EGFR status and smoking history, with the exception of patients
with EGFR IHC−. This result contradicted the opinion that an
unidentified negative interaction exists between targeted agents
and chemotherapy. In mice-bearing human NSCLC xenografts, a
combination of erlotinib with cytotoxic drugs produced additive or
synergistic antitumor activity (35). This contradiction suggests that the
exact interaction between erlotinib and chemotherapy remains
unclear, and more large-scale randomized clinical trials and
preclinical studies are required to reveal their potential
associations. Therefore, it appears that administration of
erlotinib only after completion of first-line chemotherapy rather
than a combination regimen should be the optimal maintenance
setting.
The pooled results of side-effects were almost
consistent with other studies. There was a small toxicity increase
in patients with erlotinib. The most common side-effects were
diarrhea and rash, which were usually controllable. Renal
impairment was also higher in the treatment group due to
insufficient hydration as indicated by the authors (20), however, this was not reported in
previous erlotinib studies. Due to the similar incidence of
treatment-related mortalities in the two groups, we supposed that
maintenance erlotinib in unresectable NSCLC is tolerated.
Based on the pooled analysis of a large number of
patients, we concluded that maintenance with erlotinib immediately
after chemotherapy for unresectable NSCLC patients is associated
with significant improvement in PFS, OS and ORR, as compared with
placebo or observation only. However, this meta-analysis has its
limitations. Due to limited data, we failed to perform pooled
analyses of quality-of-life and cost-effectiveness, which are
useful for doctors to determine whether the involved patients
should receive maintenance therapy or a ‘treatment holiday’.
Subsequent therapy may affect the OS of patients, but this issue
was not analyzed in the present study. In addition, the number of
included studies is small with little difference in design and one
study did not achieve the mature OS data (32).
In an era of individualized treatment, continuing
RTCs are required to identify patients who may derive greater
benefit from erlotinib in this setting, and to compare the efficacy
of erlotinib used as maintenance therapy with second-line
treatment. Due to the fast development of molecular biology,
genotyping is likely to replace traditional histopathological
classification in the future and is likely to be more effective in
treatment prediction and more prognostic of survival rates of
patients with advanced cancer. Therefore, gene expression profiling
on microarrays, including EGFR-activating mutations should be
investigated. Thus, this meta-analysis should be updated in the
future to clarify the effects of erlotinib in patients with
advanced NSCLC. The incremental cost and toxicity associated with
the adoption of this regimen should also be evaluated.
References
|
1
|
Jemal A, Siegel R, Ward E, et al: Cancer
statistics. CA Cancer J Clin. 58:71–96. 2008.
|
|
2
|
Govindan R, Page N, Morgensztern D, et al:
Changing epidemiology of small-cell lung cancer in the United
States over the last 30 years: analysis of the surveillance,
epidemiologic, and end results database. J Clin Oncol.
24:4539–4544. 2006.PubMed/NCBI
|
|
3
|
Stinchcombe TE, Bogart J, Wigle DA and
Govindan R: Annual review of advances in lung cancer clinical
research: a report for the year 2009. J Thorac Oncol. 5:935–939.
2010.PubMed/NCBI
|
|
4
|
Pfister DG, Johnson DH, Azzoli CG, et al:
American Society of Clinical Oncology treatment of unresectable
non-small cell lung cancer guideline: update 2003. J Clin Oncol.
22:330–353. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Park JO, Kim SW, Ahn JS, et al: Phase III
trial of two versus four additional cycles in patients who are
nonprogressive after two cycles of platinum-based chemotherapy in
non small-cell lung cancer. J Clin Oncol. 25:5233–5239. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Socinski MA, Schell MJ, Peterman A, et al:
Phase III trial comparing a defined duration of therapy versus
continuous therapy followed by second-line therapy in
advanced-stage IIIB/IV non-small cell lung cancer. J Clin Oncol.
20:1335–1343. 2002. View Article : Google Scholar
|
|
7
|
von Plessen C, Bergman B, Andresen O, et
al: Palliative chemotherapy beyond three courses conveys no
survival or consistent quality-of-life benefits in advanced
non-small cell lung cancer. Br J Cancer. 95:966–973.
2006.PubMed/NCBI
|
|
8
|
Ciuleanu T, Brodowicz T, Zielinski C, et
al: Maintenance pemetrexed plus best supportive care versus placebo
plus best supportive care for non-small cell lung cancer: a
randomised, double-blind, phase 3 study. Lancet. 374:1432–1440.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Fidias PM, Dakhil SR, Lyss AP, et al:
Phase III study of immediate compared with delayed docetaxel after
front-line therapy with gemcitabine plus carboplatin in advanced
non-small cell lung cancer. J Clin Oncol. 27:591–598. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Belani CP, Barstis J, Perry MC, et al:
Multicenter, randomized trial for stage IIIB or IV non-small-cell
lung cancer using weekly paclitaxel and carboplatin followed by
maintenance weekly paclitaxel or observation. J Clin Oncol.
21:2933–2939. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Pirker R, Pereira JR, Szczesna A, et al:
Cetuximab plus chemotherapy in patients with advanced
non-small-cell lung cancer (FLEX): an open-label randomised phase
III trial. Lancet. 373:1525–1531. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Scagliotti GV, Parikh P, von Pawel J, et
al: Phase III study comparing cisplatin plus gemcitabine with
cisplatin plus pemetrexed in chemotherapy-naive patients with
advanced-stage non-small cell lung cancer. J Clin Oncol.
26:3543–3551. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Stinchcombe TE and Socinski MA: Treatment
paradigms for advanced stage non-small cell lung cancer in the era
of multiple lines of therapy. J Thorac Oncol. 4:243–250. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Gridelli C, Maione P, Rossi A, et al:
Potential treatment options after first-line chemotherapy for
advanced NSCLC: maintenance treatment or early second-line?
Oncologist. 14:137–147. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Butts C, Murray N, Maksymiuk A, et al:
Randomized phase IIB trial of BLP25 liposome vaccine in stage IIIB
and IV non-small cell lung cancer. J Clin Oncol. 23:6674–6681.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Cappuzzo F, Ciuleanu T, Stelmakh L, et al
SATURN investigators: Erlotinib as maintenance treatment in
advanced non-small cell lung cancer: a multicentre, randomised,
placebo-controlled phase 3 study. Lancet Oncol. 11:521–529. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sandler A, Gray R, Perry MC, et al:
Paclitaxel-carboplatin alone or with bevacizumab for non-small cell
lung cancer. N Engl J Med. 355:2542–2550. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Shepherd FA, Rodrigues Pereira J, Ciuleanu
T, et al National Cancer Institute of Canada Clinical Trials Group:
Erlotinib in previously treated non-small cell lung cancer. N Engl
J Med. 353:123–132. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Reck M, van Zandwijk N, Gridelli C, et al:
Erlotinib in advanced non-small cell lung cancer: efficacy and
safety findings of the global phase IV tarceva lung cancer survival
treatment study. J Thorac Oncol. 5:1616–1622. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Gatzemeier U, Pluzanska A, Szczesna A, et
al: Phase III study of erlotinib in combination with cisplatin and
gemcitabine in advanced non-small-cell lung cancer: the Tarceva
Lung Cancer Investigation Trial. J Clin Oncol. 25:1545–1552. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Herbst RS, Prager D, Hermann R, et al
TRIBUTE Investigator Group: TRIBUTE: a phase III trial of erlotinib
hydrochloride (OSI-774) combined with carboplatin and paclitaxel
chemotherapy in advanced non-small cell lung cancer. J Clin Oncol.
23:5892–5899. 2005. View Article : Google Scholar
|
|
22
|
NHS Centre for Reviews and Dissemination:
Undertaking systematic reviews of research on effectiveness: CRD’s
guidance for carrying out or commissioning reviews. CRD Report 4.
2nd edition. University of York; York: 2001
|
|
23
|
Parmar MK, Torri V and Stewart L:
Extracting summary statistics to perform meta-analyses of the
published literature for survival endpoints. Stat Med.
17:2815–2834. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Higgins JP, Thompson SG, Deeks JJ and
Altman DG: Measuring inconsistency in meta-analyses. BMJ.
327:557–560. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Egger M, Davey Smith G, Schneider M and
Minder C: Bias in meta-analysis detected by a simple, graphical
test. BMJ. 315:629–634. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Altman DG and Deeks JJ: Meta-analysis,
Simpson’s paradox, and the number needed to treat. BMC Med Res
Methodol. 2:32002.
|
|
27
|
McQuay HJ and Moore RA: Using numerical
results from systematic reviews in clinical practice. Ann Intern
Med. 126:712–720. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Smeeth L, Haines A and Ebrahim S: Numbers
needed to treat derived from meta-analyses–sometimes informative,
usually misleading. BMJ. 318:1548–1551. 1999.
|
|
29
|
Moher D, Cook DJ, Eastwood S, Olkin I,
Rennie D and Stroup DF: Improving the quality of reports of
meta-analyses of randomised controlled trials: the QUOROM
statement. Quality of Reporting of Meta-analyses. Lancet.
354:1896–1900. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Mok TS, Wu YL, Yu CJ, et al: Randomized,
placebo-controlled, phase II study of sequential erlotinib and
chemotherapy as first-line treatment for advanced non-small-cell
lung cancer. J Clin Oncol. 27:5080–5087. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kabbinavar FF, Miller VA, Johnson BE,
O’Connor PG and Soh C; ATLAS Investigators: Overall survival (OS)
in ATLAS, a phase IIIb trial comparing bevacizumab (B) therapy with
or without erlotinib (E) after completion of chemotherapy (chemo)
with B for first-line treatment of locally advanced, recurrent, or
metastatic non-small cell lung cancer (NSCLC). J Clin Oncol
(meeting abstracts). 28:75262010.
|
|
32
|
Perol M, Chouaid C, Milleron BJ, et al:
Maintenance with either gemcitabine or erlotinib versus observation
with predefined second-line treatment after cisplatin-gemcitabine
induction chemotherapy in advanced NSCLC: IFCT-GFPC 0502 phase III
study. J Clin Oncol (meeting abstracts). 28:75072010.
|
|
33
|
Davies AM, Ho C, Lara PN Jr, Mack P,
Gumerlock PH and Gandara DR: Pharmacodynamic separation of
epidermal growth factor receptor tyrosine kinase inhibitors and
chemotherapy in non-small cell lung cancer. Clin Lung Cancer.
7:385–388. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Piperdi B, Ling YH and Perez-Soler R:
Schedule-dependent interaction between the proteosome inhibitor
bortezomib and the EGFR-TK inhibitor erlotinib in human
non-small-cell lung cancer cell lines. J Thorac Oncol. 2:715–721.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Higgins B, Kolinsky K, Smith M, et al:
Antitumor activity of erlotinib (OSI-774, Tarceva) alone or in
combination in human non-small cell lung cancer tumor xenograft
models. Anticancer Drugs. 15:503–512. 2004. View Article : Google Scholar : PubMed/NCBI
|