Introduction
Multiple organ dysfunction syndrome (MODS) is
defined as simultaneous or sequential dysfunction of two or more
organs or systems and normally develops following an acute and
severe injury. It develops as follows: primary injury, stress
response of the body, systemic inflammatory response syndrome
(SIRS) and the dynamic evolution process of MODS (1), wherein SIRS is the main cause of MODS
(2). Since MODS was identified in
1991, scholars in the field of trauma surgery have concentrated on
studying and investigating the disorder. Studies into traumatic and
infectious MODS have accomplished notable achievements (3,4);
however, not enough attention is paid to cerebrogenic multiple
organ dysfunction syndrome (CMODS), in the field of neurology.
CMODS, an important branch of MODS, may be caused by a variety of
cerebral injuries (5). CMODS
caused by acute cerebrovascular diseases (ACVD) have the highest
incidence of approximately 10.7% and a mortality rate of 58.4%.
Compared with MODS induced by other causes, organ and system damage
in CMODS occurs more rapidly, with a higher mortality rate
(6,7). At present, MODS is reported as a
clinical phenomenon or a complication of ACVD, with very few
studies researching the disorder. In a previous study, we
investigated the expression of endotoxin and inflammatory factors
in a model of CMODS caused by ischemic cerebrovascular disease, as
well as the function of the hypothalamus-hypophysis axis of three
target glands (adrenal gland, thyroid gland and the gonads)
(7). However, the neural control
mechanism of CMODS has not yet been clarified.
Previous studies (8,9) have
shown that when ACVD occurs, the hypothalamus plays a central role
in the functional control of the neuroendocrine system. It not only
participates in neuroendocrine-immune modulation through the
hypothalamus-hypophysis-target gland axis and the
hypothalamus-spinal cord-sympathetic nerve axis, but may also act
through the hypothalamus-medullary visceral zone (MVZ)-vagus nerve
route. As an important hub for regulating visceral activity and as
a relay station for neuro-immunomodulation (10), the MVZ (11), an arc-shaped band from the
dorsomedial to ventrolateral area in the middle-caudal segment of
the medulla oblongata, is involved in the cholinergic
anti-inflammatory pathways of the vagus nerve and plays an
important role in MODS (12).
At present, MODS therapy includes protopathy,
anti-infective treatment, immune regulation, organ support and
protection, but with a limited effect and a high mortality rate
(13). As a result, the search for
effective treatment methods is continuing. The discovery of the
anti-inflammatory effects of the cholinergic anti-inflammatory
pathways undoubtedly provided new ideas and methods for the
treatment of MODS (14–16). This study aimed to observe the
expression of FOS protein in the rat MVZ following subarachnoid
hemorrhage (SAH) and the severing of the celiac branches of the
vagus nerve, as well as provide experimental evidence to clarify
the neuroendocrine and immunological mechanisms of MODS caused by
ACVD.
Materials and methods
Animal grouping
Adult male Wistar rats (n=90, clean grade), each
weighing 250–300 g, were provided by the Experimental Animal Center
of Shandong University, China. Rats were divided into 15 groups as
follows: the normal control group; the sham surgery group; the
severed-down vagus nerve group (SDV group); the subarachnoid
hemorrhage group (SAH group), which was equally divided into 6
subgroups at the time-phased points of 4, 12, 24, 36, 48 and 72 h;
and the subarachnoid hemorrhage and severed-down vagus nerve group
(SAH+SDV group), which was also divided into 6 subgroups in the
same way. Each of the 15 groups contained 6 rats. The animal use
protocols used were approved by the Institutional Animal Care and
Use Committee at Shandong University (Shandong, China). All animal
experiments were carried out in accordance with the National
Research Council Guide for the Care and Use of Laboratory Animals,
as adopted by the National Institutes of Health.
Preparation of the animal model
The SDV procedure was as follows: the rats were
given a laparotomy after being narcotized with 1% pentobarbital
solution. The right and left vagus nerves were exposed in the
lesser curvature of the stomach, ligated with silk thread and cut
off ∼5 mm away from every branch of the vagus nerve (gastric,
hepatic and celiac branches). The postoperative animals were given
solid food and those in the SAH+SDV group were given the SAH
surgery after four weeks (17).
The sham surgery was performed at the same time as
the SDV and SAH surgeries. Right and left vagus nerves were exposed
in the SDV surgery, without ligation or cutting. In the SAH
surgery, 100 μl normal saline was injected by intubation.
Inspection items
Postoperative vital signs were observed at every
time-phased point, at which femoral vein blood was assayed by
routine blood tests, liver function tests [alanine transaminase
(ALT) and aspartate transaminase (AST)], renal function tests
[blood urea nitrogen (BUN), serum creatinine (Cr)] and measurement
of myocardial enzyme levels [creatinine kinase (CK)]. The brain,
lung, liver, kidney and small intestine were monitored for
pathological changes. FOS protein expression in frozen brain
sections from the MVZ was assayed using the immunohistochemical ABC
method (18).
Diagnostic criteria of SIRS and MODS
Diagnosis of SIRS and MODS was performed according
to the diagnosis criteria of SIRS and MODS in experimental animals
proposed by Bone et al(1).
Statistical analysis
All indexes were expressed as the mean ± standard
deviation (SD) and processed by SPSS 10.0 software with Student’s
t-test and variance analysis. P<0.05 was considered to indicate
a statistically significant difference.
Results
Animal model
In the sham surgery and SDV groups, animals
expressed mild listlessness and increased respiration within 12 h
of surgery, which improved after 12 h. Abdominal distension,
slightly reduced feeding and normal activities were observed in the
SDV group. In the SAH group there were 11 cases (30.6%) of
postoperative coma, cyanosis and hyperventilation; 19 cases (52.8%)
of epileptic seizure, with listlessness, loss of appetite,
increased respiration and a slowed reaction time to pinprick pain
in postoperative woken rats and 14 cases (38.9%) of mortality prior
to the time-phased points. In the SAH+SDV group, all rats displayed
serious abdominal distension (Fig.
1). There were also 14 cases (38.9%) of postoperative coma,
cyanosis and hyperventilation; 20 cases (55.6%) of epileptic
seizure, with listlessness, loss of appetite, increased respiration
and a slowed reaction time to pinprick pain in postoperative woken
rats and 17 cases (47.2%) of mortality prior to the time-phased
points.
Compared with the normal control group, there was no
significant difference in breathing rate, heart rate, body
temperature or white blood cell count (WBC), ALT, AST, BUN, Cr and
CK of peripheral blood between the sham surgery group and the SDV
group (P>0.05). The above parameters were higher in the SAH
group and the SAH+SDV group than those in the normal control, sham
surgery and SDV groups (P<0.01). There was no significant
difference in breathing rate, heart rate or CK between the SAH+SDV
group and the SAH group (P>0.05); however, there was a
significant difference in body temperature and WBC, ALT, AST, BUN
and Cr of peripheral blood between the SAH+SDV group and the SAH
group, with the SAH+SDV group producing significantly higher
results than the SAH group (P<0.01).
Pathological changes
Organ and tissue structure in the normal control
group and sham surgery group was essentially normal; however, in
the sham surgery group the alveolar septa was broadened,
capillaries were congested with blood and some neutrophil
granulocytes infiltrated the lung tissue. The lung and kidney
tissues in the SDV group were the same as in the sham surgery
group. We noted edema and thickening in the mucous layer in the
small intestine, and a cloudy swelling of hepatocytes appeared in
the hepatic tissue in the SDV group.
The pathological changes of the surrounding visceral
organs at every time-phased point in the SAH group included
considerable nonspecific inflammation. In lungs the following
changes were observed: angiotelectasis and congestion, endovascular
neutrophilia as well as intra-alveoli and perivascular exudation
appeared at 4 h; the alveolar cavity was expanded and fibrin was
exuded in bronchial lumen and tracheal lumen at 12 h; and mild and
chronic lymphocytic bronchopneumonia occurred at 24 and 36 h. These
inflammatory responses were relieved at 48 h. Inflammatory cell
infiltration and alveolar expansion appeared at 72 h. In the small
intestine, the following changes were observed: edema and
thickening in the mucosa and submucosa appeared at 24 h; edema,
thickening and congestion between the muscular layer and mucous
layer as well as light inflammatory cell infiltration occurred at
36 and 48 h; and congestion and edema appeared in the mucosa and
submucosa at 72 h. In the liver, the following changes were
observed: a number of liver cells were swollen at 8 h; spotty
necrosis of liver cells, liver sinus expansion and markedly swollen
liver cells around the central vein and individual ballooning
degeneration occurred at 24 h; chronic inflammatory cell
infiltration and acidophilic degeneration of liver cells appeared
in stroma at 36 h; and acidophilic degeneration of liver cells
disappeared at 48 h. In the kidney, the following changes were
observed: the renal interstitium was hyperemic, a number of
proximal tubular epithelial cells were swollen and the cytoplasm
became empty at 12 h; an atrophied glomerulus, renal tubular
expansion and swollen proximal tubular epithelial cells appeared at
24 and 36 h; individual necrosis of the renal proximal tubule
appeared at 48 h; and no changes occurred at later time-phased
points.
The inflammatory pathological changes of the small
intestine, liver and kidneys at each time-phased point in the
SAH+SDV group were more apparent and lasted longer than those in
the SAH group. The changes that appeared in the lungs at 4 h
(angiotelectasis, congestion and endovascular neutrophilia) were
more evident at 24 and 36 h. A number of animals showed signs of
lobar pneumonia at 48 h and inflammatory cell infiltration and
alveolar expansion were still present at 72 h. The inflammatory
changes observed in the small intestine (edema and thickening in
mucosa and submucosa) at 4 h and at each time-phased point were
more apparent in the SAH+SDV group than in the SAH group. The
congestion and edema in the mucosa and submucosa and lymphocyte
infiltration remained at 72 h. Inflammatory lesions of the liver
lasted longer in the SAH+SDV group than in the SAH group and
lymphocyte infiltration remained at 72 h. Kidney damage was more
apparent in the SAH+SDV group than in the SAH group. Protein and
cellular casts appeared in the proximal tubule at 36 h and proximal
tubular epithelial cells remained swollen at 72 h.
Incidence of SIRS and MODS in the SAH and
SAH+SDV groups
The incidence of SIRS following SAH in rats was
100%. There were 25 cases with MODS in the SAH group, with an
incidence of 69.4%. Fourteen animals were dead prior to the
time-phased point, accounting for 38.9% in the SAH group. There
were 27 cases with MODS in the SAH+SDV group, with an incidence of
75.0%. Seventeen animals were dead prior to the time-phased point,
accounting for 47.2% in the SAH+SDV group.
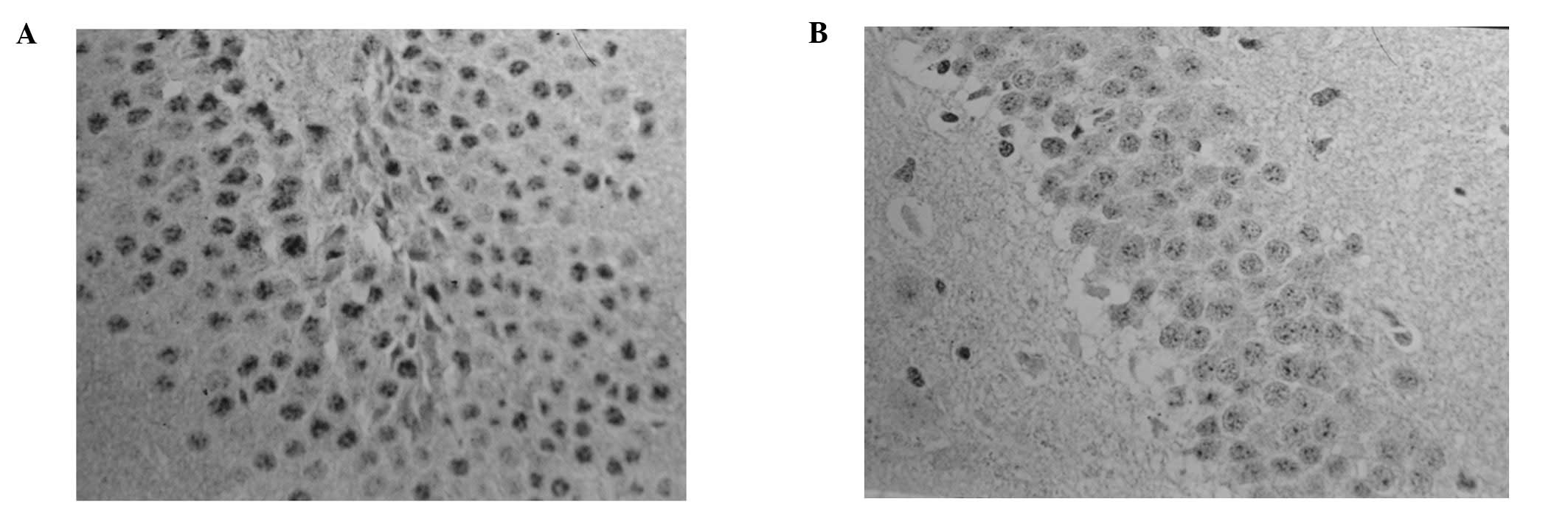
Positive protein expression of FOS
A number of hypochromatic FOS-positive cells were
found in the MVZ in the normal control and sham surgery groups;
however, more FOS-positive cells were found in the MVZ in the SDV
group, but the difference was not statistically significant
(P>0.05). The number of FOS-positive cells in the MVZ in the the
SAH and SAH+SDV groups was significantly higher than those in the
normal control, sham surgery and SDV groups (P<0.01); however,
the number of FOS-positive cells in the MVZ in the SAH+SDV group
was significantly lower than that in the SAH group (P<0.01;
Table I). In the SAH group,
FOS-positive cells were almost hyperchromatic, with a concentrated
distribution in the nucleus tractus solitarius (NTS) of the
dorsomedial area (DM), the dorsal motor nucleus of the vagus nerve
(DMV) and the lateral reticular nucleus (LRN) between the area
postrema (AP) and the ventrolateral medulla (VLM). In the SAH+SDV
group, FOS-positive cells were mainly distributed in the NTS and
LRN. The expression level peaked at 24 h and then gradually reduced
at 36–48 h, but still existed at 72 h (Fig. 2).
 | Table IFOS protein expression in the
medullary visceral zone at different time points in each group. |
Table I
FOS protein expression in the
medullary visceral zone at different time points in each group.
| Group | n | Number of FOS
protein-positive cells (mean ± SD, x10−3
μm2) |
|---|
| Normal | 6 | 5.83±1.17 |
| Sham surgery | 6 | 6.33±1.21 |
| SDV | 6 | 7.17±1.47 |
| SAH | | |
| 4 h | 6 |
49.66±4.63ab–c |
| 12 h | 6 |
76.50±4.72ab–c |
| 24 h | 6 |
115.16±13.79ab–c |
| 36 h | 6 |
90.84±12.86ab–c |
| 48 h | 6 |
62.10±8.19ab–c |
| 72 h | 6 |
39.67±5.24ab–c |
| SAH + SDV | | |
| 4 h | 6 |
5.51±5.01abc–d |
| 12 h | 6 |
52.01±4.82abc–d |
| 24 h | 6 |
93.57±10.46abc–d |
| 36 h | 6 |
82.33±9.15ab–c,e |
| 48 h | 6 |
64.83±7.63ab–c |
| 72 h | 6 |
42.15±5.34ab–c |
Discussion
ACVD complicated with MODS is common in the clinic;
however, there are very few studies using related animal models.
This study was completed according to the diagnosis criteria of
SIRS and MODS in experimental animals proposed by Bone et
al(1). Our results correlated
with the criteria for an MODS animal model, with SIRS incidence of
100%, MODS incidence of 69.4% and mortality of 38.9% following SAH.
Therefore, our model of MODS following SAH, produced by injecting
arterial blood into the Willis’ circle, was successful.
FOS protein in the MVZ in ACVD is the expression
product of immediate early gene c-fos. As a regulation factor in
cell transcription, it participates in the transcriptional
processes of various effect enzymes. A number of studies (19,20)
confirm that FOS protein plays a role of ‘tracer’ and may be used
to locate neuronal activity and monitor function. Another study
(21) found that different dosages
(0.4/0.8 units) of collagenase in a rat cerebral hemorrhage model,
increased the positive expression of FOS in the MVZ, compared with
the levels in a control group. The number of FOS-positive cells in
the 0.8 unit group was significantly higher than that in the 0.4
unit group. Therefore, serious ACVD may cause changes in FOS
protein expression in the MVZ and the amount of induced FOS is
consistent with the intensity of the stimulus, to a certain extent.
As one of the important control centers for stress reaction, the
MVZ may be related to the fibers in the hypothalamus, limbic system
and surrounding visceral organs and therefore participate in the
regulation of various somatic and visceral sensations and
activities (including breathing, heartbeat, blood pressure,
gastrointestinal motility, endocrine responses and immunoreaction)
through its NTS, DMV, nucleus ambiguus (NA), ventrolateral
reticular nucleus (VLR) and other internal nuclear groups. A
previous study has shown (20)
that electronic damage to rat NTS in the MVZ may cause neuronal
pulmonary edema. It is considered that the uplink fiber of the
caudal NTS has a wide fiber connection with catecholaminergic
neurons in the brainstem reticular formation and that neuronal
pulmonary edema may be caused by regulation of the release of
catecholamine. The current study revealed that the respiratory
frequency increased at each time-phased point in the SAH group
compared with the control group, particularly at 24, 36 and 48 h.
Nonspecific inflammatory damage in the lungs appeared 4 h after
SAH, peaked at 24–36 h and gradually reduced at 48 h. At the same
time, the positive FOS protein expression in the MVZ followed the
same pattern. The dense positive protein expression of FOS in the
caudal NTS and VLM appeared at 4 h in the SAH group, peaked at 24
h, with continuously high levels at 36 h and then gradually reduced
at 48 h with some positive expression still shown at 72 h. This
suggests that a series of physiological pathological changes in the
brain following SAH may influence the function of the NTS in the
MVZ and VLM, cause imbalance in the respiratory function of the
lungs in normal conditions and lead to neurogenic pulmonary
edema.
Previous studies (21,22)
have shown that in a model of cardiovascular stress reaction,
hypertension and acute myocardial ischemia induced by intravenous
injection of pituitrin, FOS protein expression in the medulla
oblongata was limited to the MVZ, with all surrounding nucleus
groups displaying negative expression. FOS proteins in the MVZ were
densely expressed in the NTS and VLM, 50% of which were FOS/TH
(thyrosine hydroxylase) double-labeled positive neurons, which
indicates that catecholaminergic neurons in the MVZ participate in
the stress reaction of cardiovascular noxious stimulation. Changes
in rat blood pressure and electrocardiogram following SAH were not
measured in this study; however, the changes in heart rate and
myocardial enzyme may reflect the rat’s myocardial ischemic
condition. The dense expression of FOS protein in the MVZ of the
SAH group was mainly observed in the NTS, VLM and DMV, with a
reduced amount shown in the AP, at 4 h after hemorrhage and peaking
at 24 h. Dense expression was still observed at 36 h, which
gradually reduced at 72 h. The positive expression of FOS protein
was consistent with changes in heart rate and myocardial enzyme,
which indicates that the positive expression of NTS and VLM in the
MVZ is related to peripheral myocardial ischemia and that the MVZ
participates in the regulation of cardiac function following
SAH.
Ge et al(23) reported a large amount of FOS
protein expression in the muscle plexus and submucosal neurons of
the NTS and colon wall in the MVZ of the rat model of
gastrointestinal stress reaction, which indicates that NTS may
participate in the gastrointestinal stress reaction by influencing
the activity of the colon muscle plexus and submucosal neurons. In
the current study, rats in the SAH group suffered inflammatory
damage to the intestine 24 h after SAH, which peaked at 36–48 h and
was significantly relieved at 72 h. The consistent expression of
FOS in the MVZ following SAH, particularly the dense expression of
FOS in the DMV, indicates that inflammatory damage to the
intestinal mucosa and submucosa is a result of influence on the
function of the NTS and DMV following SAH.
Studies (24,25)
have shown that, similar to MODS caused by trauma, infection and
shock, SIRS is also an important pathological basis of ACVD
complicated with MODS and adjustment of the nerve-endocrine-immune
system is a fundamental cause of ACVD with MODS. Additionally, a
number of studies found that as well as the
hypothalamus-pituitary-target gland axis and the
hypothalamus-spinal cord-sympathetic nerve axis, the
hypothalamus-MVZ-vagus nerve is another nerve-endocrine-immune
route (25). Borovikova et
al(26) found that
lipopolysaccharides (LPS) and cytokines stimulate the
anti-inflammatory pathway of the hypothalamus-pituitary-adrenal
gland axis after activating the afferent vagus nerve fiber. As a
main transmitter in the vagus nerve, acetylcholine (ACh)
significantly reduces inflammatory factors [tumor necrosis factor,
(TNF) and interleukin (IL)-1D, IL-6, IL-18] secreted by human
macrophages. Furthermore, severing the subphrenic vagus nerve in
rats challenged with LPS significantly weakens the
adrenocorticotrophic hormone reaction and prevents hypothalamic
norepinephrine activation and fever reaction aggravating animal
injuries (27). Yang et
al(10) injected LPS into rat
abdominal cavities and found a large amount of FOS expression in
the cerebral frontoparietal cortex, limbic forebrain, hypothalamic
paraventricular nucleus (PVN), supraoptic nucleus (SON) and the
MVZ. The current study revealed that FOS expression in the MVZ
induced by LPS decreased after severing the subphrenic vagus nerve,
which indicates that peripheral immunologic information may be
transmitted though the vagus nerve-MVZ to the hypothalamus and
other control regions. Additionally, the positive expression of FOS
in the MVZ in the SAH+SDV group was significantly reduced compared
with the SAH group, which indicates that severing the subphrenic
vagus nerve may prevent the inflammatory information of abdominal
visceral organs from being transmitted to the control regions when
MODS occurs following SAH.
To date, studies have focused on how humoral factors
or autocrine and paracrine factors influence the release of
anti-inflammatory mediators or pro-inflammatory mediators when SIRS
occurs (28); however, the
anti-inflammatory effects of nervous-endocrine anti-inflammatory
pathways (i.e., the vagus nerve and its transmitter ACh) have been
rarely studied. In fact, compared with the humoral
anti-inflammatory pathways, cholinergic anti-inflammatory pathways,
with shorter reaction times, quickly and directly regulate the
systemic inflammatory responses and inhibit the lethal effect of
biotoxin (29). Tracey et
al(27) observed in
vitro that ACh has a stronger inhibitory effect on TNF-α as
well as the release of inflammatory factors IL-1, IL-6 and IL-18
generated as a result of macrophage stimulation by LPS. Hansen
et al(30) found that
severing the subphrenic vagus nerve in endotoxic shock rat models
results in increased shifts of intestinal flora and tolerance of
endotoxin. Furthermore, the levels of endotoxin, IL-1 and IL-6 in
the blood significantly increased following intraperitoneal
injection of LPS. In the SAH group and the SAH+SDV group, the
incidence of SIRS was 100%; however, in the SAH group the incidence
of MODS was 69.4% and animals dead prior to the time-phased points
accounted for 38.9%; while in the SAH+SDV group the incidence of
MODS was 75.0% and animals dead prior to the time-phased points
accounted for 47.2%, significantly higher than those in the SAH
group. The body temperature, WBC number, kidney function and liver
function in the SAH+SDV group were significantly higher than those
in the SAH group. In the SAH+SDV group, the inflammatory damage to
the peripheral visceral organs including the lungs, small
intestine, liver and kidneys appeared earlier, lasted longer and
was more serious compared with those in SAH group. In addition,
although the mean breathing rate, heart rate and myocardial enzymes
in the SAH+SDV group were slightly higher than those in the SAH
group, there was no significant difference beween the two groups.
This may be related to the completeness of the vagus nerve pathway
controlled by the visceral organs of the thoracic cavity.
Therefore, severing the subphrenic vagus nerve increased the
incidence of MODS following SAH and enhanced inflammatory damage to
the surrounding visceral organs caused by SAH. The mechanism of
this may be related to the severing of the cholinergic
anti-inflammatory pathways. Our results indicate that the vagus
nerve may protect surrounding visceral organs in MODS following
SAH.
The mechanism of MODS caused by ACVD is complicated.
In this study we found that serious inflammatory damage and SIRS
appeared in rat organs, such as the lungs, small intestine and
liver, following SAH and that the pathological process was
consistent with the positive expression of FOS in the MVZ. Severing
the subphrenic vagus nerve may increase the incidence of MODS
following SAH and enhance the inflammatory damage to surrounding
visceral organs caused by SAH, which indicates that SIRS is the
pathologic basis of MODS following SAH. Our study showed that MVZ
is involved in the functional regulation of surrounding visceral
organs following SAH and is one of the direct control centers of
MODS following SAH. Finally, the vagus nerve has a potential
protective effect on the surrounding visceral organs in MODS
following SAH.
Acknowledgements
This study was supported by the
National Natural Science Foundation of China (No. 30970991,
30971216), and the Natural Science Foundation of Shandong Province
(No. Y2007C043).
References
|
1
|
Bone RC, Balk RA, Cerra FB, et al:
Definitions for sepis and organ failure and guidelines for the use
of innovative therapies in sepsis. The ACCP/SCCM Consensus
Conference Committee American College of Chest Physicians/Society
of Critical Care Medicine. Chest. 101:1644–1655. 1992. View Article : Google Scholar
|
|
2
|
Bhatia M, Wong FL, Cao Y, et al:
Pathophysiology of acute pancreatitis. Pancreatology. 5:132–144.
2005. View Article : Google Scholar
|
|
3
|
Kaczorowski DJ, Mollen KP, Edmonds R and
Billiar TR: Early events in the recognition of danger signals after
tissue injury. J Leukoc Biol. 83:546–552. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Romero CM, Downey P and Hernández G: High
volume hemofiltration in septic shock. Med Intensiva. 34:345–352.
2010.(In Spanish).
|
|
5
|
Reinhart K, Menges T, Gardlund B, et al:
Randomized, placebo-controlled trial of the anti-tumor necrosis
factor antibody fragment afelimomab in hyper inflammatory response
during severe sepsis: the RAMSES study. Crit Care Med. 29:765–769.
2006. View Article : Google Scholar
|
|
6
|
Jiang LY, Yang ZF and Yang LH: Analysis of
acute cerebral arterial thrombosis with multiple organ dysfunction
syndrome. Nan Fang Yi Ke Da Xue Xue Bao. 27:1215–1217. 2007.(In
Chinese).
|
|
7
|
Qu C, Guo S, Guo H, et al: Increased serum
endotoxin and elevated CD14 and IL-1beta expression in a rat model
of cerebrogenic multiple organ dysfunction syndrome. Med Chem.
5:462–467. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bitto A, Polito F, Altavilla D, et al:
Melanocortins protect against multiple organ dysfunction syndrome
in mice. Br J Pharmacol. 162:917–928. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Goldstein RS, Gallowitsch-Puerta M, Yang
L, et al: Elevated high-mobility group box 1 levels in patients
with cerebral and myocardial ischemia. Shock. 25:571–574. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yang ZJ, Rao ZR and Ju G: Evidence for the
medullary visceral zone as a neural station of
neuroimmunomodulation. Neurosci Res. 38:237–247. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Jia HG, Rao ZR and Shi JW: Projection from
the ventrolateral medullary neurons containing tyrosine hydroxylase
to the central amygdaloid nucleus in rat brain. Brain Res.
589:167–172. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Mioni C, Bazzani C and Giuliani D:
Activation of efferent cholinergic pathway produces strong
protection against myocardial ischemia/reperfusion injury in rats.
Crit Care Med. 33:2621–2628. 2005. View Article : Google Scholar
|
|
13
|
Dellinger RP, Levy MM, Cadet JM, et al:
Surviving sepsis campaign: international guidelines for management
of severe sepsis and septic shock: 2008. Crit Care Med. 36:296–327.
2008. View Article : Google Scholar
|
|
14
|
Van Der Zanden EP, Boeckxstaens GE and de
Jonge WJ: The vagus nerve as a modulator of intestinal
inflammation. Neurogastroenterol Motil. 21:6–17. 2009.PubMed/NCBI
|
|
15
|
Parrish WR, Rosas-Ballina M,
Gallowitsch-Puerta M, et al: Modulation of TNF release by choline
requires alpha7 subunit nicotinic acetylcholine receptor-mediated
signaling. Mol Med. 6:232–241. 2008.PubMed/NCBI
|
|
16
|
Prunell GF, Mathiesen T, Diemer NH and
Svendgaard NA: Experimental subarachnoid hemorrhage; subarachnoid
blood volume, mortality rate, neuronal death, cerebral blood flow
and perfusion pressure in three different rat models. Neurosurgery.
52:165–175. 2003.
|
|
17
|
Bullitt E: Expression of c-fos-like
protein as a marker for neuronal activity following noxious
stimulation in the rat. J Comp Neurol. 296:517–530. 1990.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Jedynak JP, Cameron CM and Robinson TE:
Repeated methamphetamine administration differentially alters Fos
expression in caudate-putamen patch and matrix compartments and
nucleus accumbens. PLoS One. 7:e342272012. View Article : Google Scholar
|
|
19
|
Sun YN, Luo JY, Rao ZR, Lan L and Duan L:
GFAP and Fos immunoreactivity in lumbo-sacral spinal cord and
medulla oblongata after chronic colonic inflammation in rats. World
J Gastroenterol. 11:4827–4832. 2005.PubMed/NCBI
|
|
20
|
Zhao DQ, Lu CL and Ai HB: The role of
catecholaminergic neurons in the hypothalamus and medullary
visceral zone in response to restraint water-immersion stress in
rats. J Physiol Sci. 61:37–45. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Richerson GB and Getting PA: Medullary
respiratory neurons in the guinea pig: localization and firing
patterns. Brain Res. 591:79–87. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Gao W, Liu H, Rao Z and Ju G: Distribution
of SPR-like immunoreactivity in the medullary visceral zone of the
rat and changes following acute myocardial ischemia induced by
intravenous injection of vasopressin. J Hirnforsch. 39:129–135.
1998.PubMed/NCBI
|
|
23
|
Ge X, Yang Z, Duan L and Rao Z: Evidence
for involvement of the neural pathway containing the peripheral
vagus nerve, medullary visceral zone and central amygdaloid nucleus
in neuroimmunomodulation. Brain Res. 914:149–158. 2001. View Article : Google Scholar
|
|
24
|
Hildebrand F, Pape HC and Krettek C: The
importance of cytokines in the posttraumatic inflammatory reaction.
Unfallchirurg. 108:793–794. 796–803. 2005.(In German).
|
|
25
|
Kawada T, Yamazaki T, Akiyama T, et al:
Vagal stimulation suppresses ischemia induced myocardial
interstitial norepinephrine release. Life Sci. 78:882–887. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Borovikova LV, Ivanova S, Zhang M, et al:
Vagus nerve stimulation attenuates the systemic inflammatory
response to endotoxin. Nature. 405:458–462. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Tracey KJ: The inflammatory reflex.
Nature. 420:853–859. 2002. View Article : Google Scholar
|
|
28
|
Barnum SR, Ames RS, Maycox PR, et al:
Expression of the complement C3a and C5a receptors after permanent
focal ischemia: an alternative interpretation. Glia. 38:169–173.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kim HM, Shin HJ, Jeong HJ, et al: Reduced
IL-2 but elevated IL-4, IL-6 and IgE serum levels in patients with
cerebral infarction during the acute stage. J Mol Neurosci.
14:191–196. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hansen MK, Nguyen KT, Fleshner M, et al:
Effects of vagotomy on serum endotoxin, cytokines and
corticosterone after intraperitoneal lipopolysaccharide. Am J
Physiol Regul Integr Comp Physiol. 278:R331–R336. 2000.
|