Introduction
Invasion and metastasis are dynamic, complex and
multistep processes, and are the leading causes of mortality among
breast cancer patients (1).
Previous studies have suggested that matrix metalloproteinases
(MMP-2 and MMP-9) and their inhibitors (TIMP-1 and TIMP-2) play
important roles in the invasion and metastasis of breast cancer
(2–4). Exploring the upstream regulator of
MMPs in breast cancer and the underlying mechanism is important for
understanding the invasion and metastasis of tumors. Twist, a basic
helix-loop-helix (bHLH) transcription factor, was originally
reported as a master regulator of embryonic morphogenesis (5). However, in previous studies, the
Twist gene as an oncogene has been shown to play an essential role
in diverse pathways, including tumor cell apoptosis, angiogenesis,
invasion and metastasis, which are involved in carcinogenesis and
cancer progression (6–8). To explore the functions of Twist in
breast cancer and investigate whether the alteration of Twist has
an effect on the expression of MMP-2 and MMP-9, we determined the
expression of Twist, MMP-2 and MMP-9 proteins in 200 breast cancer
tissue specimens by immunohistochemical (IHC) assay, and studied
the correlation between Twist expression and clinicopathological
characteristics in the breast cancer tissue samples. Moreover, we
further investigated the correlation between Twist and gelatinase
(MMP-2 and MMP-9) expression in breast cancer.
Materials and methods
Patients and specimens
The patient population consisted of 200 breast
cancer patients at the Second People’s Hospital of Hefei, the First
Affiliated Hospital of Anhui Medical University and the First
People’s Hospital of Huainan between April 2001 and April 2002. The
200 patients had a median age of 50 years (range, 27–78 years).
Patients who had undergone chemotherapy or radiation therapy prior
to surgery were excluded, as were patients with rheumatic disease,
acute infection, human immunodeficiency virus (HIV) or other types
of cancer. The pathological tumor stage was defined according to
the sixth edition of the tumor-node-metastasis (TNM) classification
of the International Union against Cancer. Tumor differentiation
was defined according to the 2003 World Health Organization (WHO)
classification of tumors (9).
Complete follow-up data were obtained from 126 breast cancer
patients. Primary study endpoints were post-operative overall
survival (OS) and post-operative relapse-free survival (RFS). OS
and RFS were defined as the time from the date of surgery to the
date of mortality from breast cancer or to the date of local
recurrence or detection of distant metastasis, respectively. All
tissue diagnoses were confirmed by permanent histology. A protocol
for the use of tissue samples from patients and follow-up study was
approved by the Institutional Review Boards of the Second People’s
Hospital of Hefei, the First Affiliated Hospital of Anhui Medical
University and the First People’s Hospital of Huainan. Every
patient had signed a consent form.
Tissue microarray construction
All the hematoxylin and eosin (H&E)-stained
sections from each formalin-fixed, paraffin-embedded block were
assessed to identify target areas. Three to five representative
1-mm cores were obtained from each case and embedded in a grid
pattern into a recipient paraffin block using a tissue arrayer
(Hengtai Instruments Inc., Liaoning, China). Consecutive 3-im
sections were cut from the paraffin block and then attached to 10%
polylysine pre-treated slides.
IHC analyses
IHC analyses of Twist, MMP-2, MMP-9, estrogen
receptor (ER), progesterone receptor (PR) and human epidermal
growth factor receptor 2 (HER-2) protein expression were performed
using a Two-Step Histostaining kit (Changdao Biotech Co., Ltd.,
Shanghai, China) with a polyclonal antibody against Twist (1:200;
Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA) and monoclonal
antibodies against human MMP-2 (1:200; Maixin, Fuzhou, China),
MMP-9 (1:200; Maixin), ER (working solution; Changdao Biotech Co.,
Ltd.), PR (working solution; Changdao Biotech Co., Ltd.) and HER-2
(working solution; Changdao Biotech Co., Ltd.). The sections were
deparaffinized in xylene and rehydrated in a graded series of
ethanol solutions. For antigen retrieval, slides were heated in a
microwave oven in 0.01 M sodium citrate buffer (pH 6.0) for 20 min.
Then, the slides were allowed to cool in the same buffer and were
subsequently immersed in 3% hydrogen peroxide in methanol for 10
min to block endogenous peroxidase activity. After rinsing with
phosphate-buffered saline (PBS; 2 min, 3 times), slides were
incubated with primary antibody at 4°C overnight. Then, slides were
rinsed in PBS as above, incubated for 20 min with universal
horseradish peroxidase-conjugated detection reagent (Changdao
Biotech Co., Ltd.), rinsed in PBS as above, incubated with
3,3′-diaminobenzidine tetrahydrochloride (Changdao Biotech Co.,
Ltd.) and then all IHC slides were counterstained with hematoxylin
staining solution. Known positive samples were used as positive
controls. For negative controls, the primary antibody was replaced
with 0.01 mol/l PBS.
Scoring of stained sections
Immunostaining signals were reviewed and scored
independently by two expert pathologists under double-blind
conditions. The sum of the extent and intensity score was used as
the final staining score for Twist, MMP-2 and MMP-9. The extent of
staining, defined as the percentage of positively stained areas of
tumor cells in relation to the whole tissue area, was scored on a
scale of 0–3 as follows: 0, no staining; 1, less than one-third; 2,
one-third to two-thirds; and 3, greater than two-thirds. The
staining intensity was scored as 0, no staining; 1, weakly stained;
2, moderately stained; and 3, strongly stained. For the evaluation
of Twist expression, a final staining score <6 was considered to
be weak expression and ≥6 was considered to be high expression
(10). For the evaluation of MMP-2
and MMP-9, a final staining score ≥3 was considered to be positive
(11). For the evaluation of ER
and PR expression, a percentage of stained tumor cells >10% was
considered to be positive. For the evaluation of HER-2, membrane
staining intensity and pattern were evaluated as follows: 0,
completely negative or <10% of tumor cells had membrane
positivity; +, >10% of tumor cells had incomplete faint membrane
positivity; ++, >10% of tumor cells had complete moderate
membrane positivity; and +++, >10% of tumor cells had complete
strong circumferential membrane positivity (12).
Statistical analysis
All statistical analyses were performed using SPSS
software system for Windows (version 10.0; SPSS, Inc., Chicago, IL,
USA). P<0.05 was considered to indicate a statistically
significant difference. The Chi-square test was used to examine the
difference in the positive expression rate between the groups. The
correlation between the positive expression rate and the different
clinicopathological parameters was examined using the
non-parametric Spearman’s rank correlation analysis. Variables
associated with OS and RFS rates were tested using Kaplan-Meier
estimates and compared by log-rank test.
Results
Expression of Twist, MMP-2 and MMP-9
proteins in breast cancer specimens
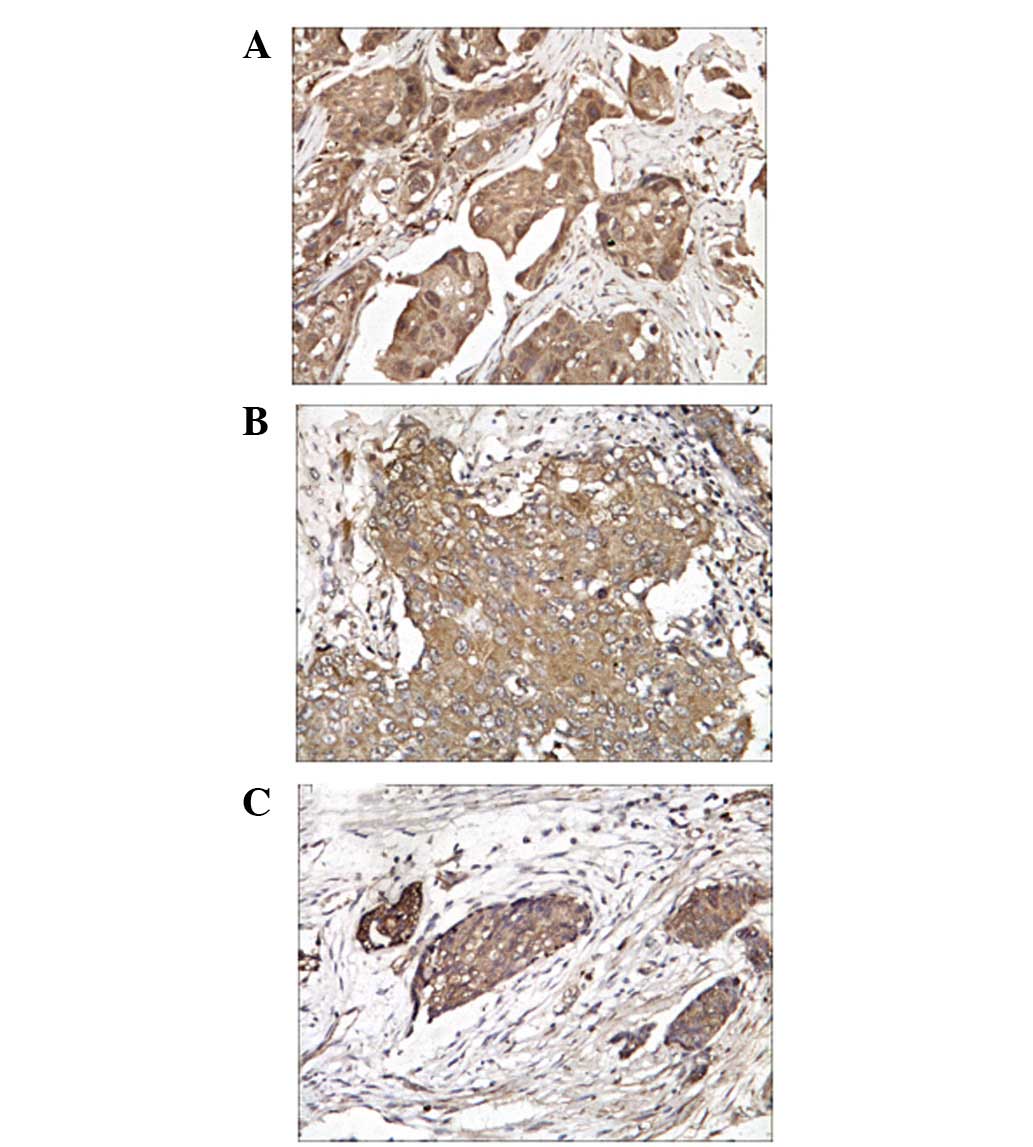
The positive signals of Twist, MMP-2 and MMP-9
protein expression were predominantly located in the cytoplasm
and/or nucleus of breast cancer cells (Fig. 1).
IHC analyses were performed on 200 breast cancer
tissues specimens. Twist protein expression was detected in 151
(75.5%) of the breast cancer tissues, while the expression of MMP-2
and MMP-9 were detected in 194 (97.0%) and 192 (96.0%)
specimens.
Association of the expression of ER, PR,
HER-2 and Twist with clinicopathological features of breast
cancer
As shown in Table
I, increased Twist expression was associated with increased
lymph node involvement (P=0.001) and higher TNM stage (P=0.001).
Twist expression was correlated with the expression of ER and PR,
although these did not reach statistical significance (P=0.063 and
0.055, respectively). There was no significant association of Twist
with HER-2 protein expression (P=0.745).
 | Table I.Correlation between Twist protein
expression and clinicopathological parameters of breast cancer
patients. |
Table I.
Correlation between Twist protein
expression and clinicopathological parameters of breast cancer
patients.
| Clinical and
pathological features | n | Twist, n (%) | P-value |
|---|
| Age (years) | | | 0.514 |
| ≤35 | 21 | 18 (85.7) | |
| 36–55 | 112 | 83 (74.1) | |
| ≥56 | 67 | 50 (74.6) | |
| Tumor size (cm) | | | 0.055 |
| ≤2 | 14 | 12 (85.7) | |
| >2–5 | 148 | 105 (70.9) | |
| >5 | 38 | 34 (89.5) | |
| Lymph node
metastasis | | | 0.001 |
| 0 | 69 | 34 (49.3) | |
| 1–3 | 69 | 58 (84.1) | |
| >3 | 62 | 59 (95.2) | |
| Histological
grading | | | 0.483 |
| I | 18 | 15 (83.3) | |
| II | 125 | 91 (72.8) | |
| III | 57 | 45 (78.9) | |
| TNM stage | | | 0.001 |
| I–II | 106 | 68 (64.2) | |
| III–IV | 94 | 83 (88.3) | |
| Estrogen
receptor | | | 0.063 |
| Negative | 116 | 82 (70.7) | |
| Positive | 84 | 69 (82.1) | |
| Progesterone
receptor | | | 0.055 |
| Negative | 111 | 78 (70.3) | |
| Positive | 89 | 73 (82.0) | |
| HER-2 | | | 0.745 |
| Low | 135 | 101 (74.8) | |
| High | 65 | 50 (76.9) | |
Correlation between Twist expression and
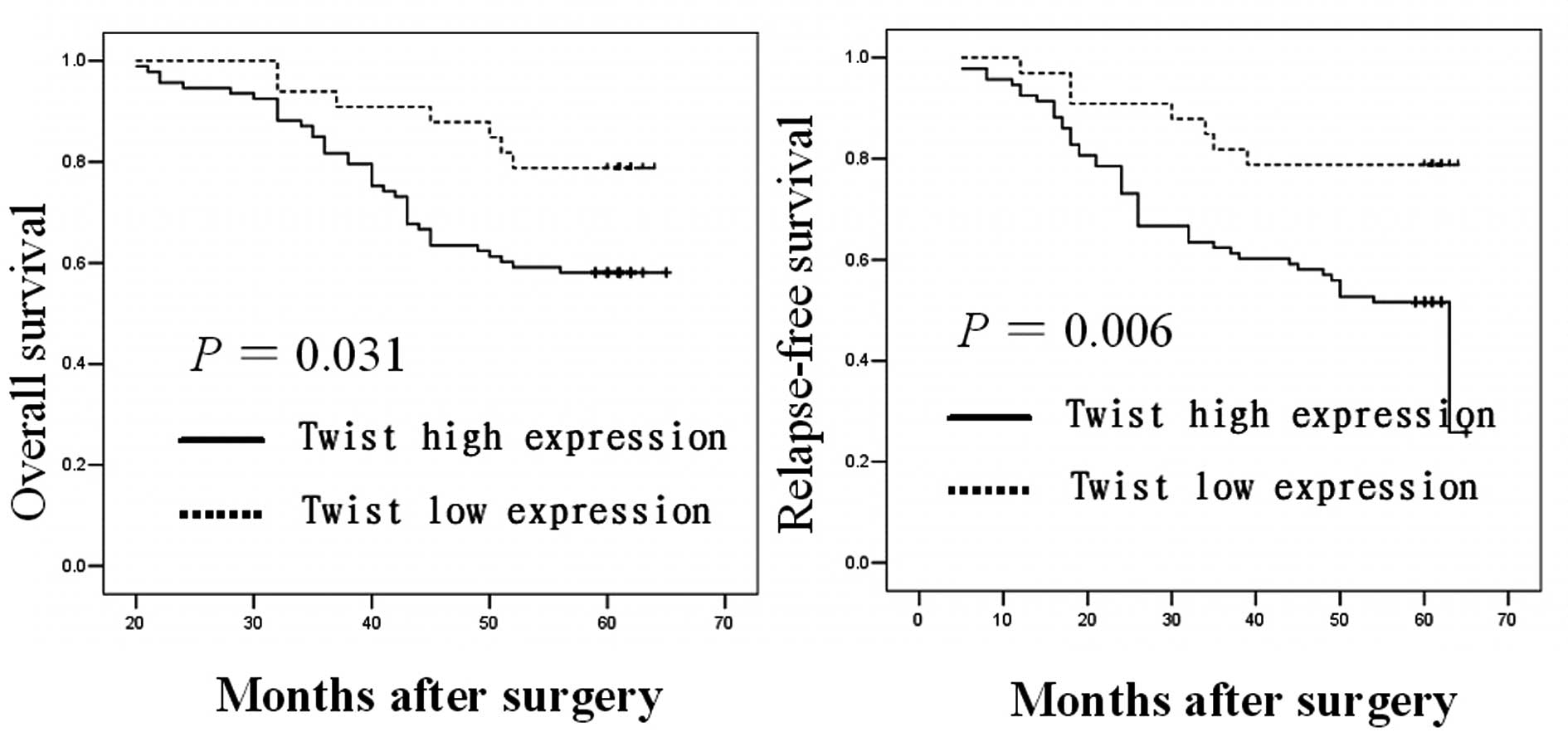
patient survival
We performed Kaplan-Meier estimates and Log-rank
test to determine whether the expression of Twist is associated
with OS and RFS of breast cancer patients. Among the 126 breast
cancer patients with complete follow-up data, those with primary
tumors expressing higher levels of the Twist protein had a
significantly poorer OS and RFS compared with those with lower
Twist protein expression (P=0.031 and 0.006, respectively; Fig. 2).
Association of the expression of MMP-2
and MMP-9 with clinicopathological features of breast cancer
The protein expression of MMP-2 and MMP-9 was
positively associated with the status of lymph node metastasis and
TNM stage (P<0.001). However, there was no significant
association of MMP-2 and MMP-9 protein expression with patient age,
tumor size, histological grading and ER, PR and HER-2 protein
expression (P>0.05).
Correlation of MMP-2, MMP-9 and Twist
protein expression in breast cancer tissue
Spearman’s correlation analysis demonstrated that
Twist protein expression was positively correlated with MMP-2 and
MMP-9 protein expression (rs=0.828, P<0.001 and rs=0.500,
P<0.001, respectively).
Discussion
In the present study, we demonstrated that increased
Twist, MMP-2 and MMP-9 protein expression levels are associated
with increased lymph node involvement and higher TNM stage.
Furthermore, Twist protein expression correlated with MMP-2 and
MMP-9 protein expression in the breast cancer tissue specimens.
The gelatinases, MMP-2 (gelatinase A) and MMP-9
(gelatinase B), are two members of the MMP family and play a
critical role in tumor invasion and metastasis (13,14).
Several studies have demonstrated that gelatinases induce
proteolytic degradation of extracellular matrix (ECM) components
and basement membranes to facilitate the invasion of tumors
(15–17). In the present study and a previous
study (3), we demonstrated that
MMP-2 and MMP-9 protein expression is associated with increased
lymph node involvement and higher TNM stage. Thus, our data suggest
that MMP-2 and MMP-9 may play fundamental roles in breast cancer
invasion and metastasis.
Epithelial-mesenchymal transition (EMT) is a
characteristic of the most aggressive metastatic cancer cells and
is critical for the induction of invasiveness and metastasis of
human cancers (18,19). Increasing evidence suggests that
Twist acts as one of the major EMT inducers by regulating
E-cadherin expression to promote cancer progression (8,20–22).
Kyo et al (10) detected
the expression of Twist in 70 cases of endometrial carcinoma and
observed that 51% of the patients presented high Twist expression
and the increased expression of Twist was positively associated
with local tumor invasion and poor OS. Yang et al (23) detected Twist expression in several
human breast tumor cell lines. The authors observed that invasive
and metastatic cell lines expressed Twist, while non-metastatic
breast tumor cell lines did not. In addition, the authors
demonstrated that suppression of Twist expression inhibits tumor
metastasis and reduces the presence of tumor cells in the blood
circulation in a mouse model. Consistent with their results, the
present study demonstrated that Twist protein expression is
correlated with lymph node involvement and TNM stage, suggesting
that Twist may be involved in the invasion and metastasis of breast
cancer.
Moreover, our data also suggest that Twist protein
expression is positively associated with gelatinase expression in
breast cancer. Lee et al (24) identified that EMT is induced by
transforming growth factor (TGF)-β and Twist in mammary epithelial
cells via a MMP-dependent mechanism. Yu et al (25) explored the functions of Twist in
hypopharyngeal cancer tissue samples by IHC assays and the results
indicated that alteration of Twist has an effect on EMT, c-fos and
MMP-9 expression. Luo et al (26) transfected the Twist gene into human
gastric carcinoma MKN28 cells with a Twist sense plasmid. The
authors demonstrated that the migration and invasion ability of
Twist-MKN28 cells was clearly increased. Moreover, overexpression
of Twist in MKN28 cells promoted the expression of cyclin D1 and
MMP-2.
The current study suggests that the Twist gene may
play an essential role in breast cancer invasion and metastasis.
Twist may serve as a potential novel prognostic factor for breast
cancer patients. Furthermore, there is a significant association of
Twist and gelatinases with breast cancer progression and it is
possible that Twist serves as a potential regulator of gelatinases.
Further studies are required to explore the regulatory mechanisms
between Twist and gelatinases.
Acknowledgements
This study was funded by grants from
the Scientific and Technological Program of Hefei (No. 2010-37),
the Scientific Research of BSKY and the Program for Excellent
Talents from Anhui Medical University.
References
|
1.
|
Eccles SA and Welch DR: Metastasis: recent
discoveries and novel treatment strategies. Lancet. 369:1742–1757.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Wu ZS, Wu Q, Yang JH, et al: Prognostic
significance of MMP-9 and TIMP-1 serum and tissue expression in
breast cancer. Int J Cancer. 122:2050–2056. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Xue S, Li SX, Wu ZS, et al: Expression of
CD147, matrix metalloproteinases and transforming growth factor
beta1 in breast cancer. Zhonghua Bing Li Xue Za Zhi. 38:524–528.
2009.(In Chinese).
|
|
4.
|
Wu ZS, Wu Q and Yang F: Expression of
gelatinase and its inhibitors in breast carcinoma on tissue chip
platform. Acta Universitatis Medicinalis Anhui. 41:608–611.
2006.(In Chinese).
|
|
5.
|
Thisse B, el Messal M and Perrin-Schmitt
F: The twist gene: isolation of a Drosophila zygotic gene necessary
for the establishment of dorsoventral pattern. Nucleic Acids Res.
15:3439–3453. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Stasinopoulos IA, Mironchik Y, Raman A, et
al: HOXA5-twist interaction alters p53 homeostasis in breast cancer
cells. J Biol Chem. 280:2294–2299. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Puisieux A, Valsesia-Wittmann S and
Ansieau S: A twist for survival and cancer progression. Br J
Cancer. 94:13–17. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Matsuo N, Shiraha H, Fujikawa T, et al:
Twist expression promotes migration and invasion in hepatocellular
carcinoma. BMC Cancer. 9:2402009. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Tavassoli FA and Devilee P: Tumours of the
breast. World Health Organization Classification of Tumours:
Pathology and Genetics of Tumours of the Breast and Female Genital
Organs. IARC Press; Lyon: pp. 13–59. 2003
|
|
10.
|
Kyo S, Sakaguchi J, Ohno S, et al: High
Twist expression is involved in infiltrative endometrial cancer and
affects patient survival. Hum Pathol. 37:431–438. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Shimizu M, Saitoh Y and Itoh H:
Immunohistochemical staining of Ha-ras oncogene product in normal,
benign, and malignant human pancreatic tissue. Hum Pathol.
21:607–612. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Jacobs TW, Gown AM, Yaziji H, et al:
Specificity of HercepTest in determining HER-2/neu status of breast
cancers using the United States Food and Drug
Administration-approved scoring system. J Clin Oncol. 17:1983–1987.
1999.PubMed/NCBI
|
|
13.
|
Stetler-Stevenson WG: Type IV collagenases
in tumor invasion and metastasis. Cancer Metastasis Rev. 9:289–303.
1990. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Liotta LA, Steeg PS and Stetler-Stevenson
WG: Cancer metastasis and angiogenesis: an imbalance of positive
and negative regulation. Cell. 64:327–336. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Dragutinović V, Izrael-Zivković L and
Radovanović N: Relation of matrix metalloproteinase-9 to different
stages of tumors in the serum of gastric cancer. Dig Dis Sci.
54:1203–1207. 2009.PubMed/NCBI
|
|
16.
|
Kleiner DE and Stetler-Stevenson WG:
Matrix metalloproteinases and metastasis. Cancer Chemother
Pharmacol. 43(Suppl): S42–S51. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Bourboulia D and Stetler-Stevenson WG:
Matrix metalloproteinases (MMPs) and tissue inhibitors of
metalloproteinases (TIMPs): Positive and negative regulators in
tumor cell adhesion. Semin Cancer Biol. 20:161–168. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Bates RC and Mercurio AM: The
epithelial-mesenchymal transition (EMT) and colorectal cancer
progression. Cancer Biol Ther. 4:365–370. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Xue C, Plieth D, Venkov C, et al: The
gatekeeper effect of epithelial-mesenchymal transition regulates
the frequency of breast cancer metastasis. Cancer Res.
63:3386–3394. 2003.PubMed/NCBI
|
|
20.
|
Smit MA, Geiger TR, Song JY, et al: A
Twist-Snail axis critical for TrkB-induced epithelial-mesenchymal
transition-like transformation, anoikis resistance, and metastasis.
Mol Cell Biol. 29:3722–3737. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Yang MH, Chen CL, Chau GY, et al:
Comprehensive analysis of the independent effect of twist and snail
in promoting metastasis of hepatocellular carcinoma. Hepatology.
50:1464–1474. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Foubert E, De Craene B and Berx G: Key
signalling nodes in mammary gland development and cancer. The
Snail1-Twist1 conspiracy in malignant breast cancer progression.
Breast Cancer Res. 12:2062010. View
Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Yang J, Mani SA, Donaher JL, et al: Twist,
a master regulator of morphogenesis, plays an essential role in
tumor metastasis. Cell. 117:927–939. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Lee YH, Albig AR, Regner M, et al:
Fibulin-5 initiates epithelial-mesenchymal transition (EMT) and
enhances EMT induced by TGF-beta in mammary epithelial cells via a
MMP-dependent mechanism. Carcinogenesis. 29:2243–2251. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
25.
|
Yu L, Lu S, Tian J, et al: TWIST
expression in hypopharyngeal cancer and the mechanism of
TWIST-induced promotion of metastasis. Oncol Rep. 27:416–422.
2012.PubMed/NCBI
|
|
26.
|
Luo GQ, Li JH, Wen JF, et al: Effect and
mechanism of the Twist gene on invasion and metastasis of gastric
carcinoma cells. World J Gastroenterol. 14:2487–2493. 2008.
View Article : Google Scholar : PubMed/NCBI
|