Introduction
Interruption of the blood supply to tissues results
in rapid changes in the environment of cells. The resulting absence
of oxygen and nutrients creates a condition in which the
restoration of blood flow causes oxidation and inflammation.
Intestinal ischemia-reperfusion injury (IRI) is an important factor
associated with high morbidity and mortality in patients (1). Intestinal IRI is associated with the
exacerbation of intestinal injury and a systemic inflammatory
response leading to progressive distal organ impairment, finally
resulting in cardiocirculatory, respiratory, hepatic and renal
failure (2,3). Intestinal IRI is also associated with
other diseases, including septic hypovolemic shock (4,5). The
process involved in intestinal IRI and protective treatment
strategies have been studied for a number of years. Several trials
have provided successful methods to attenuate the injury effect of
intestinal IRI. Ischemic preconditioning, antioxidants, NO
supplementation, anticomplement therapy, antileukocyte therapy,
perfluorocarbons, enteral feeding, glutamine supplementation and
glycine supplementation have been well studied (6).
Dexmedetomidine hydrochloride is an
S-enantiomer of medetomidine, chemically described as
(+)-4-(S)-[1-(2,3-dimethylphenyl)ethyl]-1H-imidazole
monohydrochloride. This drug remains the first and the only
α2 agonist indicated for sedation (7), and is often used by Intensive Care
Units and Anesthesiology Departments. Dexmedetomidine hydrochloride
has sedative, analgesic, sympatholytic and anxiolytic effects that
blunt a number of the cardiovascular responses in the perioperative
period. The sedative reduces the requirements for volatile
anesthetics, sedatives and analgesics without causing significant
respiratory depression (8).
Dexmedetomidine hydrochloride may be useful for the deleterious
cardiovascular effects of acute cocaine intoxication (9). Dexmedetomidine hydrochloride may also
offer a new paradigm in the pharmacological treatment of symptoms
of distress at the end of life. Previous studies have shown that
dexmedetomidine hydrochloride exhibits a protective effect in a
number of tissues with IRI (10–12).
Gu et al(13) observed that
renal IR significantly induced pulmonary injuries, increased the
wet/dry (W/D) ratio, enhanced MPO activities and increased ICAM-1
and TNF-α mRNA levels in mice. Pre- and post-treatment with
dexmedetomidine was demonstrated to markedly reduce lung edema and
inflammatory response and lower MPO activity and ICAM-1 and TNF-α
mRNA expression. Kiliç et al(14) reported that dexmedetomidine
treatment leads to biochemical and histopathological benefits by
preventing the IR-associated cellular damage of intestinal and
renal tissues, as shown in rabbits. In the current study, the
potential effects of dexmedetomidine hydrochloride on lung injury
caused by intestinal IRI were investigated in rats.
Materials and methods
Animal models and surgery
Male Sprague-Dawley rats (8 weeks-old; 250–270 g;
n=36) were provided by the Second Xiangya Hospital of Central South
University (Changsha, China). All surgical procedures were
performed according to the Regulations for the Administration of
Affairs Concerning Experimental Animals (Approved by the State
Council on October 31, 1988 and promulgated by Decree No. 2 of the
State Science and Technology Commission on November 14, 1988). Rats
were acclimatized for one week following transfer to the Department
of Anesthesiology, Second Xiangya Hospital. During this period,
rats received food and water ad libitum. Prior to surgery,
rats were fasted for 24 h with free access to water. Rats were
injected with 2% pentobarbital sodium (50 mg/kg). During surgery,
incandescence was used to maintain the rectal temperature at
37–38°C. All rats were mechanically ventilated with a standard
tidal volume ventilation protocol (tidal volume, 10 ml/kg;
respiratory rate, 50–60 breaths/min; inspiratory/expiratory ratio,
1:2). The intestine was exteriorized by midline laparotomy and the
intestinal IRI was established by occluding the superior mesenteric
artery with a microvessel clip for 1 h, followed by 2 h of
reperfusion, as described previously (15,16).
The study was approved by the Second Xiangya Hospital, Central
South University.
Groups and drug treatment
Thirty-six rats were randomly divided into six
groups (n=6/group). The detailed definitions of each group were as
follows: Sham, rats received continuous intravenous infusion of
normal saline and sham surgical preparation, including isolation of
the superior mesenteric artery without occlusion;
ischemia-reperfusion (IR), rats received continuous intravenous
infusion of normal saline and intestinal IR was induced by clamping
the superior mesenteric artery for 1 h followed by declamping
(reperfusion) for 2 h; low dose dexmedetomidine hydrochloride
(LDH), rats were pretreated with dexmedetomidine hydrochloride in
the tail vein at a dose of 2.5 μg/kg/h for 1 h prior to IR; high
dose dexmedetomidine hydrochloride (HDH), rats were pretreated with
dexmedetomidine hydrochloride in the tail vein at a dose of 5
μg/kg/h for 1 h prior to IR; yohimbine and dexmedetomidine
hydrochloride (Y+D), rats were treated with yohimbine at a dose of
1 mg/kg (>15 min) in the tail vein, followed by 5 μg/kg/h
dexmedetomidine hydrochloride for 1 h prior to IR; yohimbine only
(Y), rats were treated with yohimbine only at a dose of 1 mg/kg
(>15 min) in the tail vein prior to IR. Yohimbine is an
antagonist of α2 receptors. We used it to block the effect of
dexmedetomidine hydrochloride. All the rats were sacrificed by
bleeding in the right ventricular after 2 hours after IR
Parameters
Arterial blood gas (ABG)
Following reperfusion in the intestine, the
pressures of oxygen and carbon dioxide and the pH were analyzed in
the arterial blood from the left ventricle.
W/D ratio of the lung
Two hours following reperfusion, the left lung was
harvested, cleaned by removing the cover blood and water and then
weighed. Next, the sample was incubated at 70°C for 48 h and
weighed. The W/D ratio was calculated as the ratio of the wet
weight of the lung to the dry weight.
Histopathological evaluation
The lung tissues were embedded in paraffin and then
sectioned into 5-μm sections. Slides were stained with hematoxylin
and eosin and examined under a microscope (TE300; Nikon, Tokyo,
Japan).
Histopathology scores
Semi-quantitative analysis of lung histopathology
was performed by scoring the tissues based on lung edema,
infiltration of inflammatory cells, alveolar hemorrhage, hyaline
membrane and atelectasis: no lesion, 0; injured area ≤25%, 1;
injured area 26–50%, 2; injured area 51–70%, 3; injured area
70–90%, 4; injured area >90%, 5. A total of three fields were
randomly selected for each slide and the average was used as the
histopathology score.
Enzyme-linked immunosorbent assay
(ELISA)
After the rats were sacrificed, the trachea was
separated at the bifurcation. Below the tracheal cannula, a
‘V’-shaped gap was cut and a sterile 20G trocar was inserted into
the left bronchus. The other end was ligated with a 5-ml syringe.
Sterile cold graded saline (10 ml) was poured into the left lung
(≤3 ml each time) under the pressure of 20 cm H2O. The
bronchoalveolar lavage fluid (BALF) was collected in a 10-ml
sterile dry centrifuge tube (recovery ratio, >75%), placed on
ice and then centrifuged at 500 × g for 10 min. The supernatant was
collected and the concentrations of TNF-α and IL-6 were measured by
ELISA (Bio-Swamp Life Science, Wuhan, China).
RNA isolation and qPCR
Lung tissue was harvested 2 h following reperfusion.
Total RNA was isolated using the TRIzol reagent and was then
reverse transcribed to cDNA. Real-time quantification of genes was
performed using three-step qPCR. The following primers were used:
TLR4, forward: GAATGAGGACTGGGTGAGAAAC and reverse:
ACCAACGGCTCTGGATAAAGT; MyD88, forward: ACC GCATCGAGGAGGACTG and
reverse: CTGTGGGAC ACTGCTCTCCA; and β-actin, forward: AGGCCCCTCTGA
ACCCTAAG and reverse: CCAGAGGCATACAGGGACAAC. All primers are
purchased from BGI (Shenzhen, China). The reaction conditions were
as follows: step 1, 95°C for 2 min; step 2, 95°C for 15 sec, 60°C
for 30 sec and 72°C for 1 min; repeat step 2 for additional 39
cycles and 72°C for 10 min.
Protein extraction and western
blotting
Lung tissue (50 mg) from liquid nitrogen was ground
into a powder using a mortar. The powder was diluted in 500 μl RIPA
lysis buffer plus protease inhibitors for 15 min on ice. The
samples were then sonicated for 10 sec followed by a 10-sec rest.
This step was repeated three times. Next, 125 μl 5X protein loading
buffer was added to the samples and the samples were boiled for 5
min. The samples were then subjected to sodium dodecyl
sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and
transferred to polyvinylidene difluoride (PVDF) membranes.
Following blocking with 5% BSA, the anti-IκBα, anti-AKT,
anti-P-IκBα, anti-P-AKT and anti-β-actin primary antibodies (Santa
Cruz Biotechnology, Inc. Santa Cruz, CA, USA) were added and
incubation was conducted overnight at 4°C. Secondary antibodies
(Sigma St. Louis, MO, USA) were then added to the membranes for 1 h
at room temperature after washing with TBST three times. Finally,
the membranes were detected using an X-ray film following enhanced
chemiluminescence (ECL) reaction.
Statistical analysis
All data are presented as mean ± SD (SPSS 17.0;
SPSS, Inc., Chicago, IL, USA). All parameters are based on one-way
ANOVA analysis and comparison between groups followed by Bonferroni
or Dunnett method. P<0.05 was considered to indicate a
statistically significant difference.
Results
Body weight, ABG and W/D ratio of the
lungs
The average body weight of the rats was 260.16±7.60
g (250–270 g). The rats were randomly divided into six groups. The
average body weight, pH and pressure of O2 and
CO2 among the groups were not observed to be
significantly different (P>0.05; Table I). Lung injury was measured by lung
histopathology scores. IR surgery caused an increase in the ratio
of W/D and lung histopathology score. As shown in Table I, dexmedetomidine significantly
reduced the W/D ratio and the lung injury caused by IRI.
Pretreatment with dexmedetomidine prior to IR (LDH and HDH group)
was demonstrated to have a protective effect against lung
injury.
 | Table IWeight, ABG and W/D ratio of
experiment rats. |
Table I
Weight, ABG and W/D ratio of
experiment rats.
| Groups | n | Weight (g) | pH | Pressure of
O2 (mmHg) | Pressure of
CO2 (mmHg) | W/D ratio | Histopathological
score |
|---|
| Sham | 6 | 257.97±8.01 | 7.41±0.09 | 153.50±23.87 | 37.50±4.18 | 5.71±0.41 | 2.39±0.83 |
| IR | 6 | 260.53±6.0 | 7.40±0.07 | 146.17±18.45 | 35.67±5.43 |
7.28±0.50b |
4.61±0.39b |
| LDH | 6 | 258.02±9.78 | 7.43±0.09 | 141.17±29.03 | 35.50±4.64 |
6.13±0.82c |
4.00±0.70b |
| HDH | 6 | 259.93±9.91 | 7.38±0.07 | 156.50±34.80 | 35.33±5.05 |
5.84±0.53d |
3.61±0.25a,c |
| Y+D | 6 | 262.38±5.28 | 7.40±0.07 | 130.33±13.87 | 36.50±3.89 |
6.46±0.62a,c |
4.12±0.59b |
| Y | 6 | 262.10±7.76 | 7.37±0.04 | 137.50±20.01 | 37.50±5.05 |
7.20±0.76b |
4.28±0.49b |
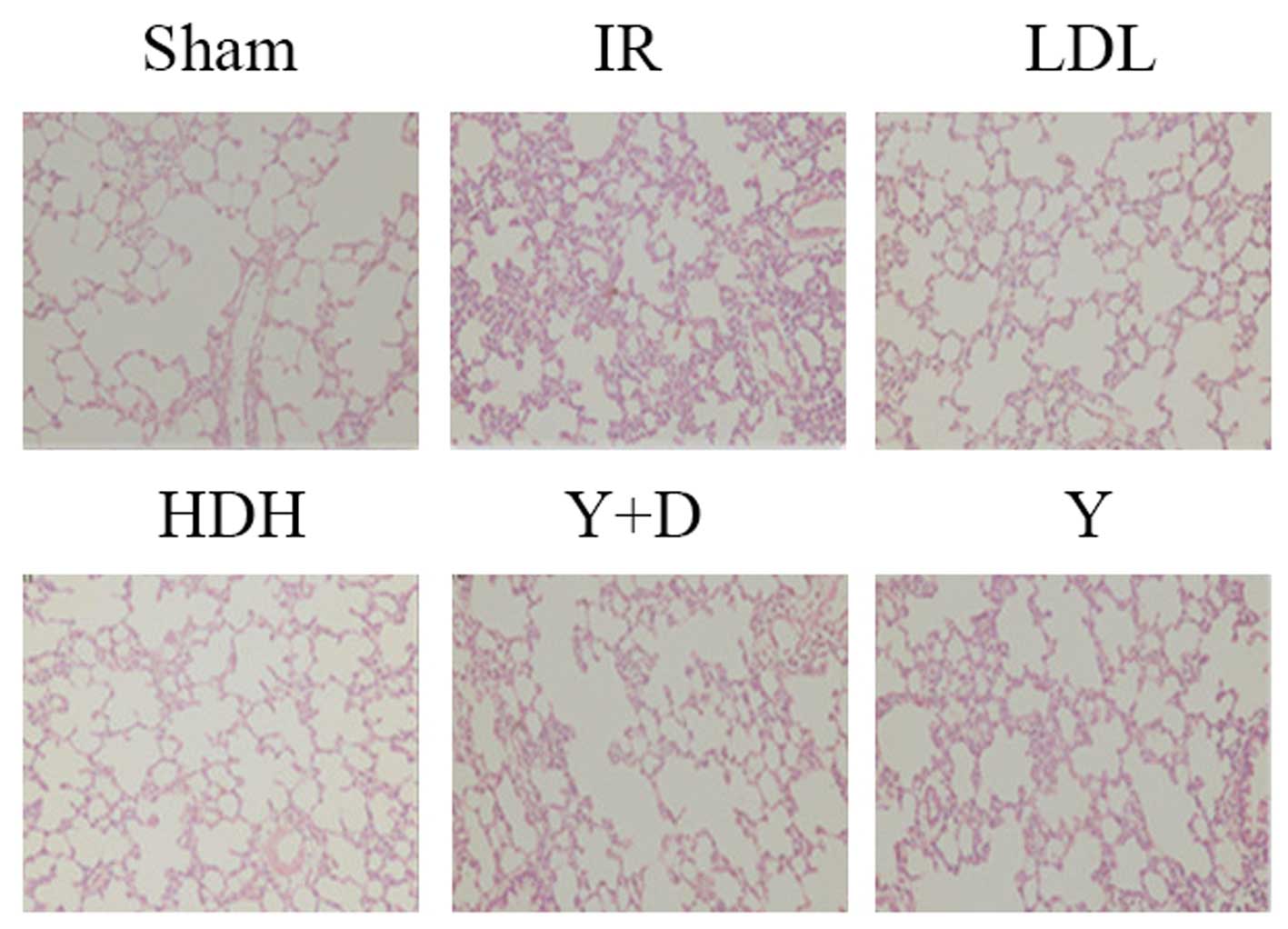
Histopathological evaluation
Lungs were harvested 2 h following reperfusion,
embedded in paraffin and sectioned into 5-μm sections. Slides were
stained with hematoxylin and eosin and examined under a microscope
(Fig. 1). In the sham group, the
integrity of the pulmonary interstitial and alveolar structures was
preserved with clear alveolar space and no evident bleeding. The
alveolar septa were thick with no edema, and no inflammatory cells
had infiltrated into the alveolar septa. In the IR and Y groups,
the alveolar structure was severely damaged. The alveolar septa
were thick and were infiltrated by a large number of inflammatory
cells. A large amount of exudate, red blood cells and inflammatory
cells filled the alveolar space. In the LDH group, the alveolar
structure exhibited a small amount of damage. A mild edema was
observed in the lung interval and exudate was visible as well as a
small amount of inflammatory cell infiltration in specific alveolar
cavities. In the HDH group, the alveolar structure was rarely
destroyed. No thickening or edema was observed in the alveolar
septa with little capillary congestion and infiltration of
inflammatory cells into the alveolar wall. In the Y+D group, the
alveolar structure was partially destroyed with alveolar septal
thickening and edema. Parts of the alveolar cavity contained
exudate and inflammatory cell infiltration. These observations
indicate that pretreatment with dexmedetomidine prior to IR has a
protective effect on lung injury, particularly at high doses.
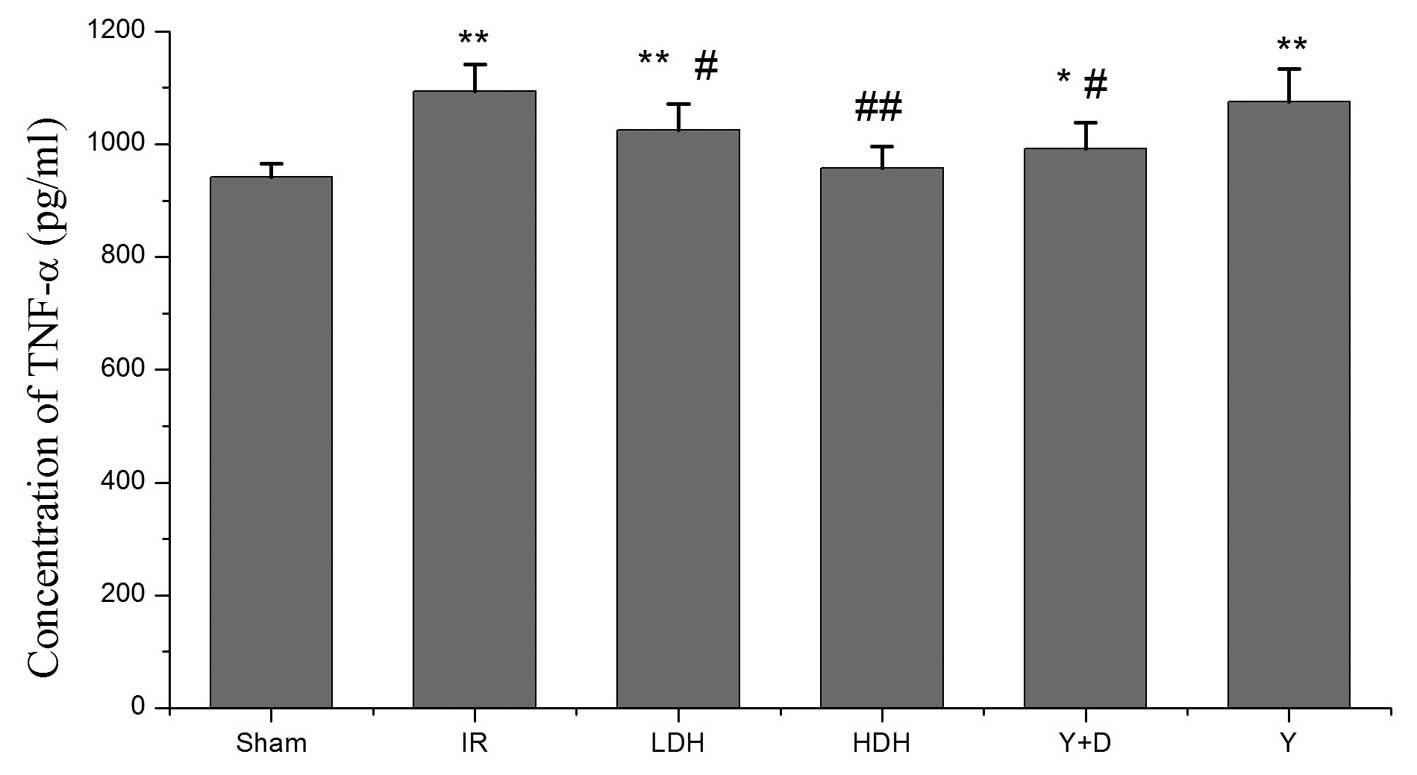
TNF-α and IL-6 levels in BALF
Intestinal IRI is associated with the exacerbation
of intestinal injury and a systemic inflammatory response leading
to progressive distal organ impairment. To evaluate the lung injury
caused by IRI, BALF was collected following reperfusion and TNF-α
and IL-6 levels were examined by ELISA. Four groups (IR, LDH, Y+D
and Y) had a higher concentration of TNF-α than the sham group. The
differences were statistically significant. The TNF-α levels in the
LDH, HDH and Y+D groups were lower than those in the IR group. The
HDH group had lower TNF-α levels than the LDH group (Fig. 2). High dose dexmedetomidine
hydrochloride appeared to have an stronger effect than a low dose.
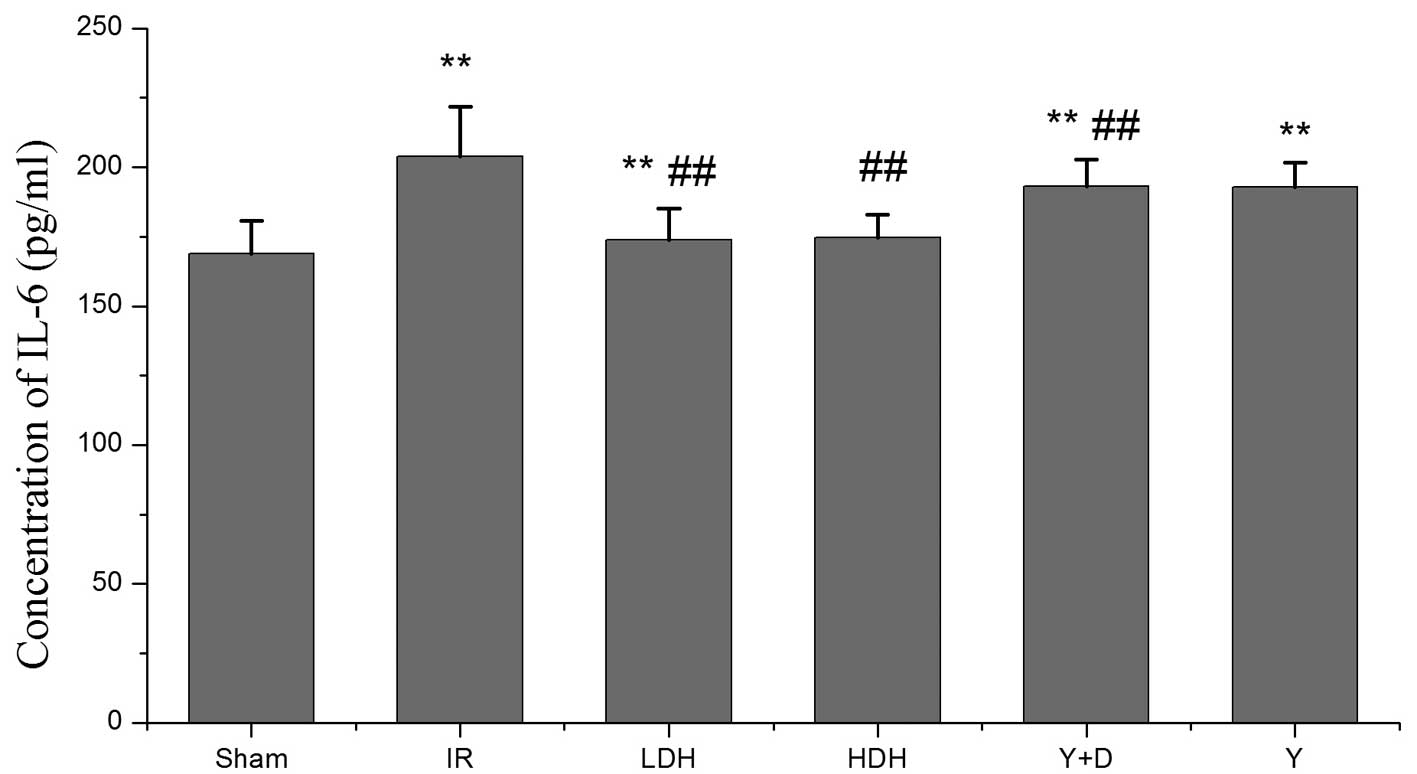
the IL-6 levels were consistent with the TNF-α levels and showed a
similar trend among the groups (Fig.
3). Pro-inflammatory factor production in the lung was
considered to indicate the extent of lung injury. The levels of
TNF-α and IL-6 among the groups varied, which was consistent with
the histopathological evaluation. Therefore, these results indicate
that dexmedetomidine hydrochloride pretreatment is a protective
method to avoid lung injury by reducing cytokine production.
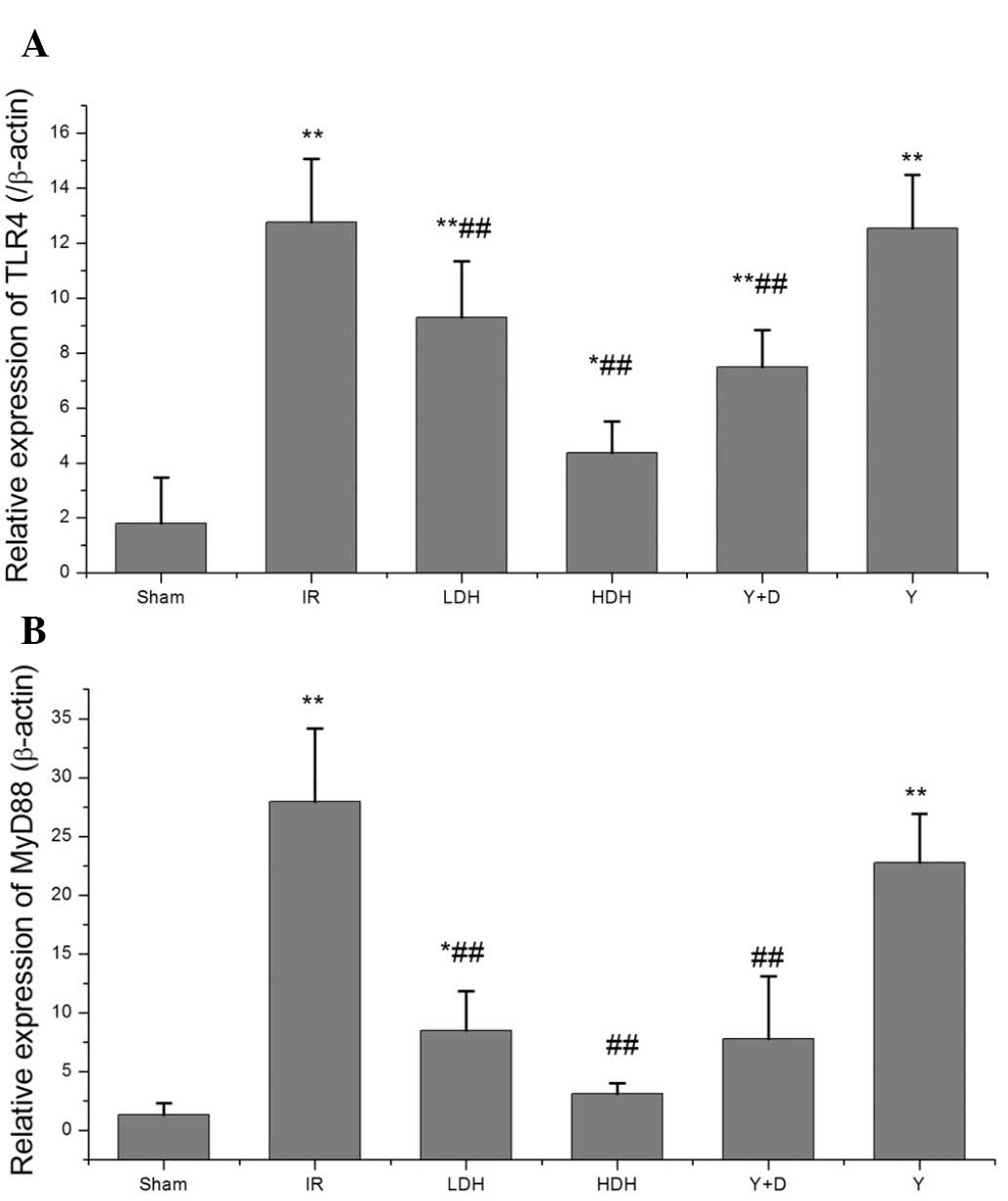
TLR4 and MyD88 expression in the lung at
2 h following reperfusion
Since dexmedetomidine hydrochloride was observed to
mediate a protective effect against lung injury following
intestinal ischemia-reperfusion, molecules upstream of the
cytokines were analyzed, including the TLR4/MyD88 pathway which
mediates cytokine production. qPCR was performed to determine the
levels of TLR4 and MyD88 expression. Compared with their levels in
the sham group, the TLR4 and MyD88 levels were increased in the IR,
LDH, Y+D and Y groups and the differences were statistically
significant (Fig. 4). In addition,
the TLR4 and MyD88 levels were decreased in the HDH, LDH and Y+D
groups when compared with those in the IR group. This result was
consistent with the findings for TNF-α and IL-6 production,
indicating that pretreatment with dexmedetomidine hydrochloride may
affect the production of TNF-α and IL-6 by altering the expression
levels of TLR4 and MyD88.
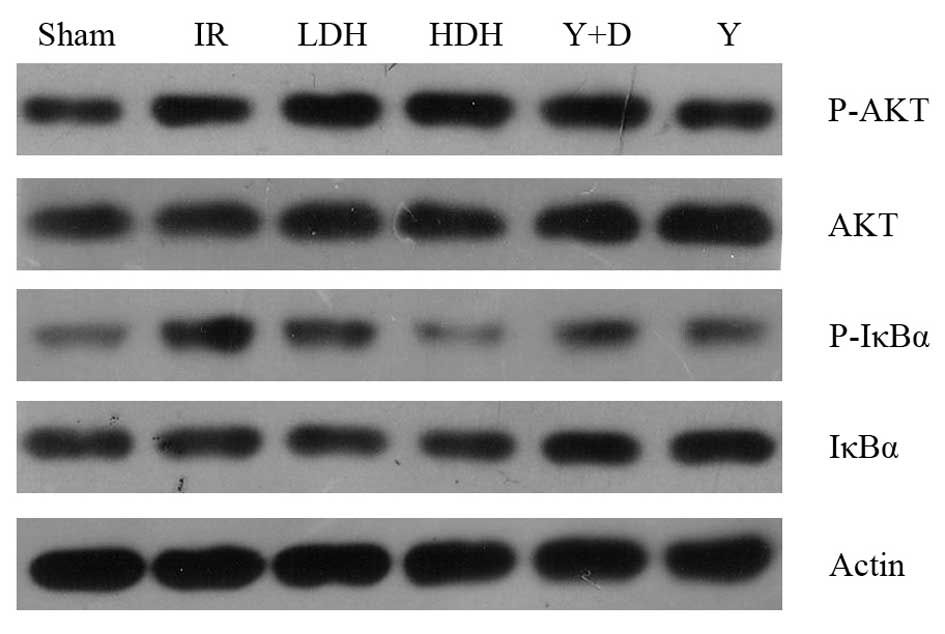
IκBα and AKT phosphorylation levels
NF-κB is an important factor downstream of
TLR4/MyD88 and plays a key role in regulating the immune response.
Following the observation that TLR4 and MyD88 mRNA levels were
reduced in the HDH and LDH groups compared with those in the IR
group, western blot analysis of NF-κB activation was performed.
IκBα is an inhibitor of NF-κB and blocks its nuclear localization
(17,18). Activation of NF-κB is initiated by
the signal-induced degradation of IκB proteins. Phosphorylation of
IκB is the signal for degradation and therefore, the
phosphorylation levels of IκBα were examined (Fig. 5). As the results show,
phosphorylation of IκB in the LDH and HDH groups was lower than in
the IR group, which was consistent with the mRNA levels of
TLR4/MyD88. In addition, the AKT phosphorylation levels were
examined, as previous studies have shown that the PI3K/AKT pathway
is involved in acute lung injury (19,20).
No marked changes in AKT phosphorylation were observed.
Discussion
IRI of the intestine may cause multi-organ injury
and harm to human health. It is associated with a rate of high
morbidity and mortality in patients (1). IRI in the intestine results in the
production of molecules, including hydrogen peroxide, superoxide
and inflammatory cytokines, that may harm distant organs, . This
leads to the development of systemic inflammatory response
syndrome, which may progress to MOF (21). Intestinal IRI also causes pulmonary
infiltration of neutrophils, which contributes to the development
of acute respiratory distress syndrome (ARDS) (22,23).
Therefore, it is important to reduce the immune response to avoid
multi-organ injury. TNF-α release caused by intestinal IRI occurs
at the earliest stage of inflammation. It plays a central role in
endogenous inflammation to induce endothelial cell injury by
promoting IL-1 and IL-6 production. In the acute phase of ARDS,
IL-6 promotes inflammatory reaction leading to neutrophil
aggregation, infiltration, tissue damage and pulmonary edema
(24,25).
Few studies have determined the effect of
dexmedetomidine hydrochloride on the immune response. Taniguchi
et al(26) observed that
dexmedetomidine reduced the mortality rate and had an inhibitory
effect on the inflammatory response during endotoxemia. The authors
identified that endotoxemia, together with dexmedetomidine
administration, had an inhibitory effect on hypotension, the
production of cytokines and the infiltration of neutrophils into
the lungs. Moreover, dexmedetomidine administration, following
endotoxin injection, reduced the mortality rate. The inhibitory
mechanism of dexmedetomidine on the production of TNF-α and IL-6
remains unclear. There are several studies that have studied the
effects of dexmedetomidine and α2 agonist-adrenergic
receptor agonists on cytokines (27–30).
The α2 adrenoceptor agonist, paminoclonidine, is known
to suppress IL-6 production (27)
and clonidine suppresses the production of TNF-α by monocytes
(28). α2-adrenoceptor
agonists have also been observed to modulate LPS-induced TNF-α
production by macrophages (29).
In a clinical study, dexmedetomidine attenuated IL-6 elevation in
post-operative patients (30).
In the current study, dexmedetomidine hydrochloride
was shown to have a protective effect on the immune response
following IRI in the intestine. Pretreatment with dexmedetomidine
hydrochloride significantly reduced the lung injury caused by IRI.
In addition, pretreatment with dexmedetomidine hydrochloride prior
to IRI reduced IL-6 and TNF-α production in the lung, which was
consistent with previous observations of the inhibitory effects of
dexmedetomidine hydrochloride on cytokines. To further explore the
mechanism, TLR4 and MyD88, upstream factors of IL-6 and TNF-α, were
examined. The mRNA levels of TLR4 and MyD88 were decreased,
compared with those in the IR group, in the groups pre-treated with
dexmedetomidine hydrochloride. In addition, NF-κB activation was
attenuated in the dexmedetomidine hydrochloride treatment group,
which was consistent with changes in the expression of TLR4 and
MyD88. TLR4/MyD88 pathways have been reported to be important for
tissue injury following IRI. TLR4- or MyD88-deficient mice exhibit
reduced injury levels following IR. Ben et al(31) reported that TLR4-deficient mice had
significantly reduced lung tissue damage, capillary leakage, lung
tissue MPO expression, neutrophil aggregation and reduced TNF-α,
IL-6, MCP-1 and MIP-2 mRNA and protein expression levels in lung
tissue following intestinal ischemia-reperfusion. Victoni et
al(32) studied the function
of the TLR4/MyD88 signaling pathway in the remote organ acute lung
injury caused by intestinal ischemia-reperfusion. The authors
observed that MyD88−/− mice had significantly reduced
pulmonary vascular permeability leakage, lung tissue MPO expression
and neutrophil aggregation and decreased levels of TNF-α and IL-1β
expression in the lung tissue, which reduced the mortality rate of
the mice (32). The current
results in rats also verified the role of TLR4/MyD88 in lung injury
due to IRI.
The current study and previous studies indicate that
pretreatment with dexmedetomidine may provide useful and effective
protection against the harmful effects of IRI. The results also
indicate that dexmedetomidine directly suppresses the immune
response by modulating the expression of TLR4 and MyD88, which may
explain the inhibitory effect of dexmedetomidine on cytokine
production. Our results provide a new insight for the clinical use
of dexmedetomidine and suggest that a higher dose of
dexmedetomidine treatment before ischemic insult produces effective
intestinal protection. The effect of dexmedetomidine on other organ
injuries caused by IRI requires further investigation.
References
|
1
|
Koike K, Moore FA, Moore EE, Read RA, Carl
VS and Banerjee A: Gut ischemia mediates lung injury by a xanthine
oxidase-dependent neutrophil mechanism. J Surg Res. 54:469–473.
1993. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Biffl WL and Moore EE: Splanchnic
ischaemia/reperfusion and multiple organ failure. Br J Anaesth.
77:59–70. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Deitch EA: Role of the gut lymphatic
system in multiple organ failure. Curr Opin Crit Care. 7:92–98.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Moore EE, Moore FA, Franciose RJ, Kim FJ,
Biffl WL and Banerjee A: The postischemic gut serves as a priming
bed for circulating neutrophils that provoke multiple organ
failure. J Trauma. 37:881–887. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Swank GM and Deitch EA: Role of the gut in
multiple organ failure: bacterial translocation and permeability
changes. World J Surg. 20:411–417. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Mallick IH, Yang W, Winslet MC and
Seifalian AM: Ischemia-reperfusion injury of the intestine and
protective strategies against injury. Dig Dis Sci. 49:1359–1377.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kamibayashi T and Maze M: Clinical uses of
alpha2-adrenergic agonists. Anesthesiology. 93:1345–1349. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Paris A and Tonner PH: Dexmedetomidine in
anaesthesia. Curr Opin Anaesthesiol. 18:412–418. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Jackson KC III, Wohlt P and Fine PG:
Dexmedetomidine: a novel analgesic with palliative medicine
potential. J Pain Palliat Care Pharmacother. 20:23–27.
2006.PubMed/NCBI
|
|
10
|
Okada H, Kurita T, Mochizuki T, Morita K
and Sato S: The cardioprotective effect of dexmedetomidine on
global ischaemia in isolated rat hearts. Resuscitation. 74:538–545.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kocoglu H, Ozturk H, Ozturk H, Yilmaz F
and Gulcu N: Effect of dexmedetomidine on ischemia-reperfusion
injury in rat kidney: a histopathologic study. Ren Fail. 31:70–74.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Engelhard K, Werner C, Eberspacher E, et
al: The effect of the alpha 2-agonist dexmedetomidine and the
N-methyl-D-aspartate antagonist S(+)-ketamine on the
expression of apoptosis-regulating proteins after incomplete
cerebral ischemia and reperfusion in rats. Anesth Analg.
96:524–531. 2003.PubMed/NCBI
|
|
13
|
Gu J, Chen J, Xia P, Tao G, Zhao H and Ma
D: Dexmedetomidine attenuates remote lung injury induced by renal
ischemia-reperfusion in mice. Acta Anaesthesiol Scand.
55:1272–1278. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kiliç K, Hanci V, Selek S, et al: The
effects of dexmedetomidine on mesenteric arterial
occlusion-associated gut ischemia and reperfusion-induced gut and
kidney injury in rabbits. J Surg Res. 178:223–232. 2012.PubMed/NCBI
|
|
15
|
Liu KX, Rinne T, He W, Wang F and Xia Z:
Propofol attenuates intestinal mucosa injury induced by intestinal
ischemia-reperfusion in the rat. Can J Anaesth. 54:366–374. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Liu KX, Chen SQ, Huang WQ, Li YS, Irwin MG
and Xia Z: Propofol pretreatment reduces ceramide production and
attenuates intestinal mucosal apoptosis induced by intestinal
ischemia/reperfusion in rats. Anesth Analg. 107:1884–1891. 2008.
View Article : Google Scholar
|
|
17
|
Huxford T, Huang DB, Malek S and Ghosh G:
The crystal structure of the IkappaBalpha/NF-kappaB complex reveals
mechanisms of NF-kappaB inactivation. Cell. 95:759–770. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Jacobs MD and Harrison SC: Structure of an
IkappaBalpha/NF-kappaB complex. Cell. 95:749–758. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
James IA, Chen CL, Huang G, Zhang HY,
Velten M and Besner GE: HB-EGF protects the lungs after intestinal
ischemia/reperfusion injury. J Surg Res. 163:86–95. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yum HK, Arcaroli J, Kupfner J, et al:
Involvement of phosphoinositide 3-kinases in neutrophil activation
and the development of acute lung injury. J Immunol. 167:6601–6608.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ceppa EP, Fuh KC and Bulkley GB:
Mesenteric hemodynamic response to circulatory shock. Curr Opin
Crit Care. 9:127–132. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Köksoy C, Kuzu MA, Kuzu I, Ergün H and
Gürhan I: Role of tumour necrosis factor in lung injury caused by
intestinal ischaemia-reperfusion. Br J Surg. 88:464–468.
2001.PubMed/NCBI
|
|
23
|
Xiao F, Eppihimer MJ, Young JA, Nguyen K
and Carden DL: Lung neutrophil retention and injury after
intestinal ischemia/reperfusion. Microcirculation. 4:359–367. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Köksoy C, Kuzu MA, Kuzu I, Ergün H and
Gürhan I: Role of tumour necrosis factor in lung injury caused by
intestinal ischaemia-reperfusion. BR J Surg. 88:464–468.
2001.PubMed/NCBI
|
|
25
|
Mallick IH, Yang W, Winslet MC and
Seifalian AM: Ischemia-reperfusion injury of the intestine and
protective strategies against injury. Dig Dis Sci. 49:1359–1377.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Taniguchi T, Kidani Y, Kanakura H,
Takemoto Y and Yamamoto K: Effects of dexmedetomidine on mortality
rate and inflammatory responses to endotoxin-induced shock in rats.
Crit Care Med. 32:1322–1326. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Straub RH, Herrmann M, Berkmiller G, et
al: Neuronal regulation of interleukin 6 secretion in murine
spleen: adrenergic and opioidergic control. J Neurochem.
68:1633–1639. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Maes M, Lin A, Kenis G, Egyed B and
Bosmans E: The effects of noradrenaline and alpha-2 adrenoceptor
agents on the production of monocytic products. Psychiatry Res.
96:245–253. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Szelényi J, Kiss JP and Vizi ES:
Differential involvement of sympathetic nervous system and immune
system in the modulation of TNF-alpha production by alpha2- and
beta-adrenoceptors in mice. J Neuroimmunol. 103:34–40.
2000.PubMed/NCBI
|
|
30
|
Venn RM, Bryant A, Hall GM and Grounds RM:
Effects of dexmedetomidine on adrenocortical function, and the
cardiovascular, endocrine and inflammatory responses in
post-operative patients needing sedation in the intensive care
unit. Br J Anaesth. 86:650–656. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ben DF, Yu XY, Ji GY, et al: TLR4 mediates
lung injury and inflammation in intestinal ischemia-reperfusion. J
Surg Res. 174:326–333. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Victoni T, Coelho FR, Soares AL, et al:
Local and remote tissue injury upon intestinal ischemia and
reperfusion depends on the TLR/MyD88 signaling pathway. Med
Microbiol Immunol. 199:35–42. 2010. View Article : Google Scholar : PubMed/NCBI
|