Introduction
Primary pulmonary lymphoma (PPL) is an uncommon type
of non-Hodgkin’s lymphoma (NHL). The vast majority of PPLs are of
low-grade, mucosa-associated lymphoid tissue (MALT) type. Primary
pulmonary diffuse large B-cell lymphoma (DLBCL) is particularly
rare and occurs only in 10% cases of primary pulmonary NHL
(1). The rapid diagnosis of
primary pulmonary DLBCL is challenging since the clinical symptoms
and signs are nonspecific. Although the clinical features,
diagnostic procedure, optimal management and prognostic factors of
this disease have has yet to be clearly elucidated, previous
studies have indicated that open thoracotomy or chest computed
tomography (CT)-guided percutaneous biopsy are the preferred
methods of diagnosis (2). To the
best of our knowledge, the positive rate of diagnosis via direct
bronchoscopic biopsy is low, and has been reported in only ~10% of
patients in previous studies (3–5). In
the present case study, the diagnosis of a patient with primary
pulmonary DLBCL via transbronchial needle aspiration (TBNA) is
reported.
Case report
This study was performed in accordance with the
Declaration of Helsinki and approved by the Ethics Committee of
Taizhou People’s Hospital, (Taizhou, Jiangsu, China). Written
informed consent was obtained from the patient. A 68-year-old male
patient was admitted to the Department of Respiratory Medicine,
Taizhou People’s Hospital in May 2013, with complaints of shortness
of breath and intermittent wheezing and a cough associated with the
production of small amounts of phlegm that had existed for one and
a half years. The patient was a nonsmoker with no prior history of
lung disease and no exposure to occupational or dust hazards.
Following admission, physical examination revealed a body weight of
62 kg, height of 172 cm, body temperature of 36°C, pulse of 92 bpm,
respiratory rate of 21 bpm and blood pressure of 130/80 mmHg. No
focal findings were observed on examination, particularly of the
skin. In addition, no palpable lymph nodes and no
hepatosplenomegaly was observed. The patient was slightly haggard
in appearance, with no cyanosis of the lips. Bilateral respiratory
movements were identical, vocal fremitus was equal bilaterally and
dry rales were audible in the right lung.
A complete blood test revealed a white cell count of
6.21×109 cells/l and the percentage of large white blood
cells was 54.9%. The hepatic function (including serum lactate
dehydrogenase levels) and renal function of the patient were
unremarkable. The serum levels of carcinoembryonic antigen,
neuron-specific enolase and CYFRA21-1 were also unremarkable. The
results from the blood gas analysis were as follows: pH, 7.41;
PaO2, 81.1 mmHg and PaCO2, 33.5 mmHg (under
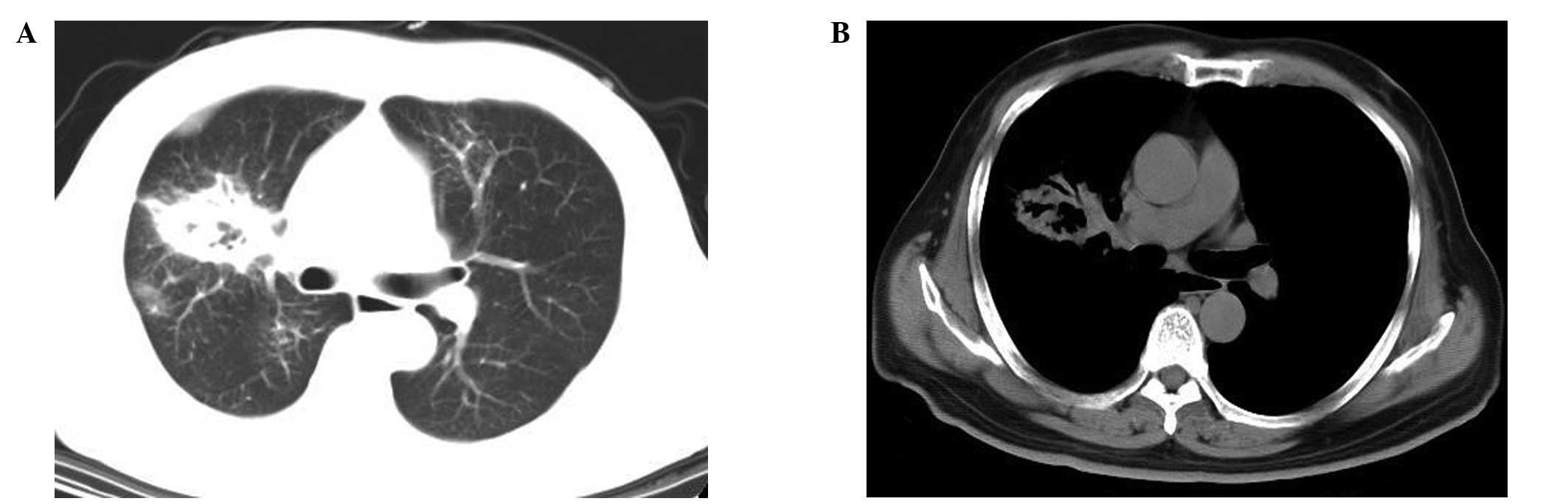
the condition of no oxygen inhalation). Chest CT scans revealed a
mass in the right middle lobe of the lung with ground-glass
opacities at the lesion margins, as well as air bronchograms in the
areas of consolidation (Fig. 1).
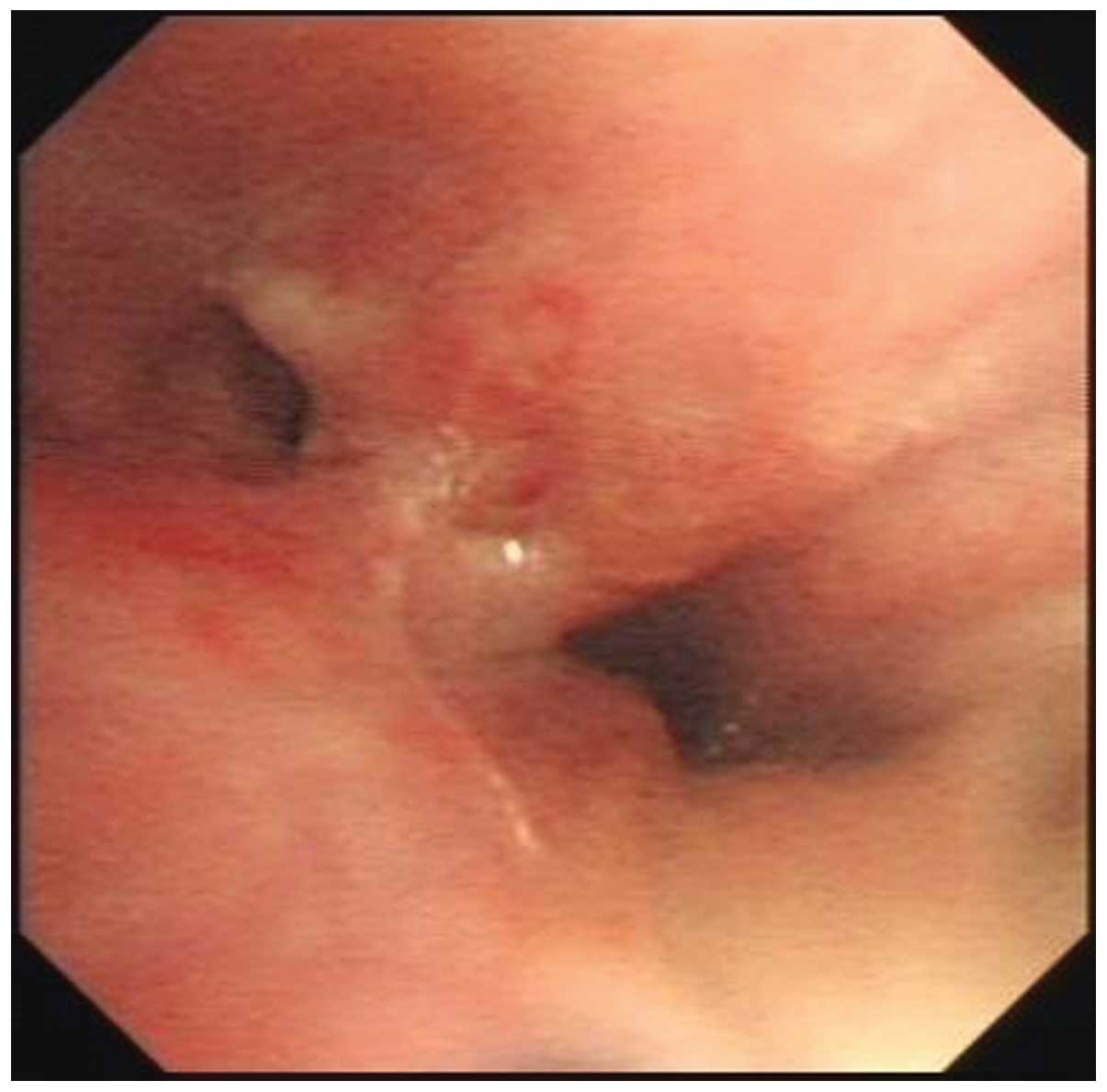
Bronchoscopy was performed and revealed an endobronchial lesion and
partial stenosis in the distal end of the middle segment bronchus
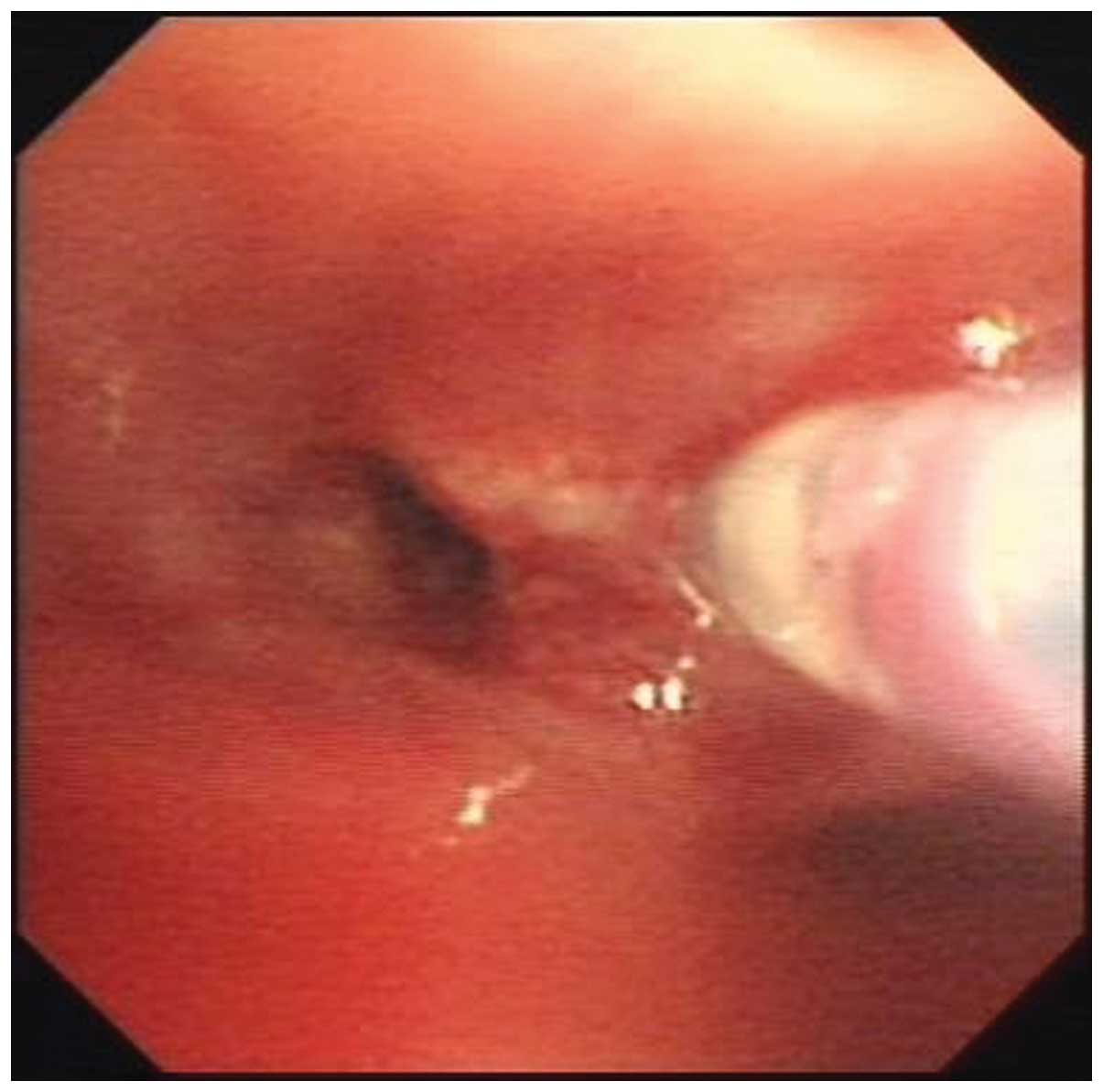
(Fig. 2). TBNA of the right hilar
lymph node, as well as endobronchial biopsy, were performed
(Fig. 3). The initial pathology of
the specimens via endobronchial biopsy revealed infiltrative
atypical lymphoid tissue that was highly suspicious for lymphoma;
however, greater amounts of tissue were required for a definitive
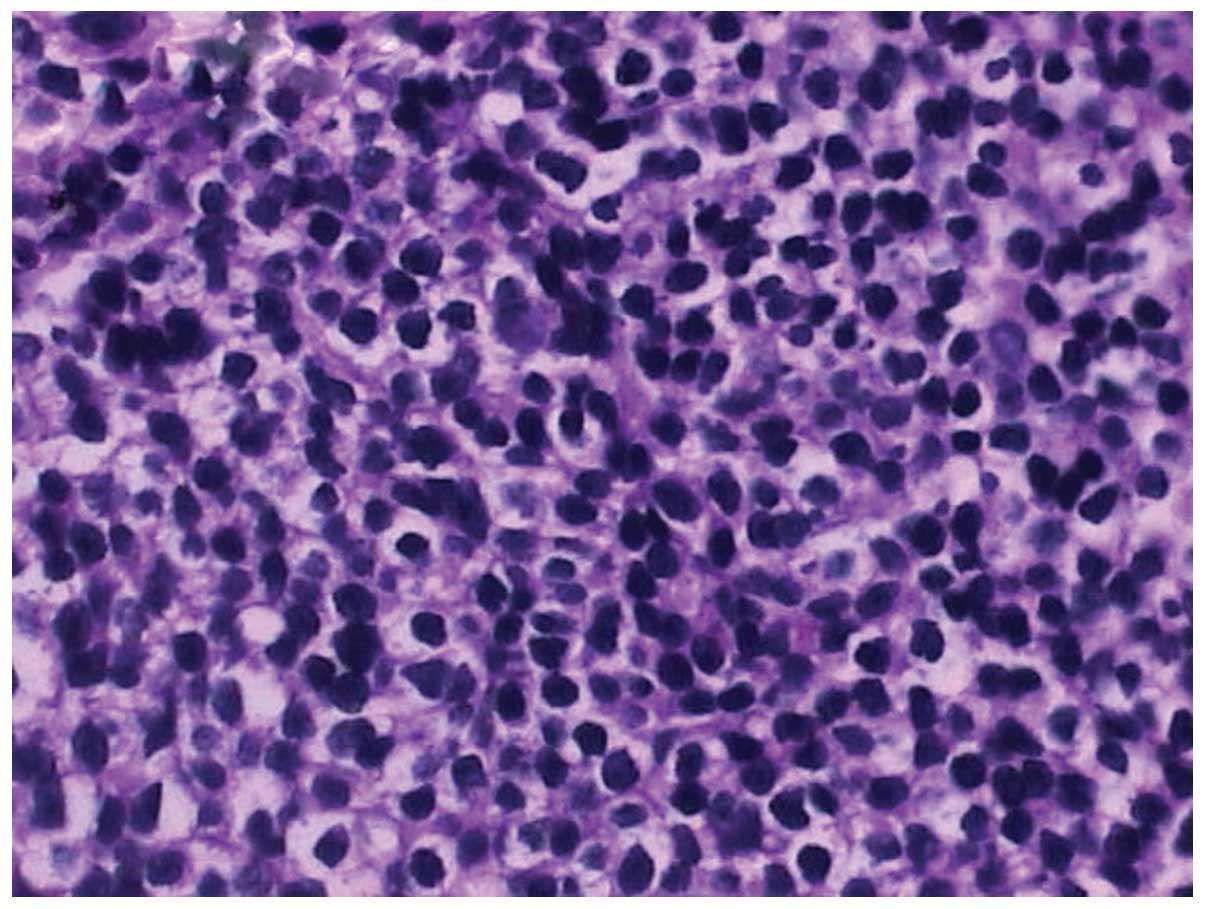
diagnosis. The following histopathological examination of pulmonary
specimens collected via TBNA revealed an aggressive large cell
lymphoma, with scattered large, oddly shaped nuclei resembling
Reed-Sternberg cells (Fig. 4).
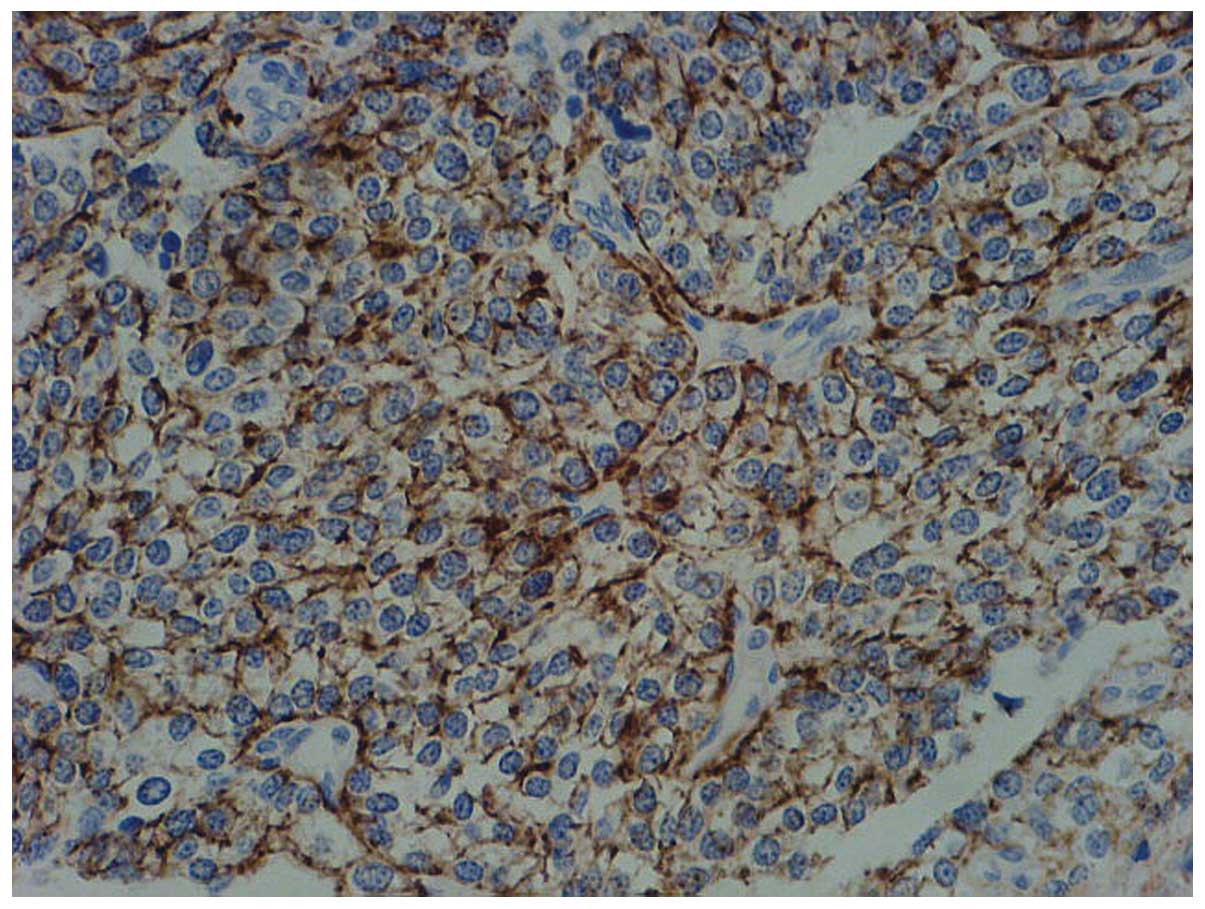
Immunohistochemical staining was performed and revealed high
expression levels of cluster of differentiation 20 (Fig. 5). Therefore, the patient was
diagnosed with primary pulmonary DLBCL.
Following the diagnosis, a bone marrow biopsy was
performed, which revealed normocellular marrow, and no granulomas
or tumor (in particular no evidence of DLBCL) were detected.
Furthermore, no evidence of a B-cell lymphoproliferative disorder
was confirmed by flow cytometric analysis of the bone marrow
aspirate. A complete enhanced CT scan of the abdomen and magnetic
resonance imaging scan of the head showed no abnormalities. The
patient was transferred to the department of hematology for CHOP
chemotherapy treatment [cyclophosphamide 750 mg/m2
intravenously (i.v.) day 1, doxorubicin 50 mg/m2 i.v.
day 1, vincristine 2 mg i.v. day 1 and prednisolone 100 mg orally
days 1–5, which were planned to be repeated every 21 days for six
cycles) following the diagnosis. Chest CT scans were performed
following the administration of four cycles of CHOP chemotherapy,
which showed no signs of disease. In addition, a marked improvement
of the patient’s respiratory symptoms was observed. Complete
remission was confirmed at the 8-month follow-up.
Discussion
PPL is defined as clonal lymphoid proliferation
affecting one or both lungs (parenchyma and/or bronchi) (6). PPL is extremely rare and accounts
3–4% of all Hodgkin’s lymphoma (HL) and <1% of all NHL (7,8 ).
DLBCL is the most common PPL following MALT-type lymphoma, which
occurs only in 10% cases of primary pulmonary NHLs (1,9). The
pathogenesis of primary pulmonary DLBCL has yet to be clearly
elucidated; however, a review of the literature indicates that it
may be associated with long-term treatment using methotrexate
(10) or immunosuppression, as
observed in solid organ (heart/lung) allograft recipients on OKT3
or cyclosporin A, or in HIV infection (6,11).
To the best of our knowledge, patients with primary
pulmonary DLBCL have no overt symptoms during the initial stages;
however, as the disease progresses, they are likely to present with
non-specific symptoms, including dyspnea, cough, chest pain, sputum
and other obstructive and infectious symptoms, as well as fever and
weight loss (6). As a consequence,
the diagnosis of primary pulmonary DLBCL, in particular in a
primary care setting, is challenging and often leads to
misdiagnosis and delayed treatment, increasing medical costs and
clinical risk (4).
With increasing awareness and improved diagnosis of
primary pulmonary DLBCL, the reported incidence of the disease has
increased significantly (1). Chest
CT findings are various in primary pulmonary DLBCL and include
solitary or multiple pulmonary nodules, masses, consolidation,
hilar/mediastinal adenopathy, pleural effusion and, rarely, direct
chest wall invasion (12,13). In particular, primary pulmonary
DLBCL should be suspected in patients when masses or mass-like
areas of consolidation and pulmonary nodules associated with
findings of air bronchograms and a halo of ground-glass shadowing
at lesion margins are detected.
Although the diagnostic procedure of this disease
has not been well defined, open thoracotomy or chest CT-guided
percutaneous biopsy are the preferred methods used in previous
studies (14,15). Although the positive value of open
thoracotomy biopsy is high, it is associated with high medical cost
and clinical risk. It is not possible to perform this procedure in
certain patients due to bad performance status. By contrast, the
diagnostic value of chest CT-guided percutaneous biopsy is has a
low medical cost and less serious complications. However, the
procedure is limited for the diagnosis of masses that are in or
adjacent to the hilum or mediastinum.
Although the use of bronchial endoscopy has been
reported in previous studies, definitive diagnosis via direct
bronchoscopic biopsy is rare, being achieved in only ~10% of
patients reported in the previous studies (3–5). The
diagnostic value of bronchial, and in particular transbronchial,
biopsy is higher when it targets visible endobronchial lesions or
radiographic abnormalities (16).
Bronchoalveolar lavage (BAL) has also been reported; however, its
value for the positive diagnosis of PPL has been inadequately
assessed and requires further validation in larger prospective
studies (6).
Since the introduction of TBNA in flexible
bronchoscopy in 1983, conventional TBNA has been technically well
established and its role has been expanded in the diagnosis and
staging of lung cancer (17). To
the best of our knowledge, the diagnostic value of TBNA in PPL has
not been mentioned in previous studies. In the present case report,
bronchoscopy was performed and an endobronchial lesion and partial
stenosis in the distal end of the middle segment bronchus was
observed. The initial pathology of the specimens via endobronchial
biopsy revealed infiltrative atypical lymphoid tissue that was
highly suspicious for lymphoma; however, a larger amount of tissue
was required for a definitive diagnosis. Primary pulmonary DLBCL
was confirmed following histopathological and immunohistochemical
staining examination of a pulmonary specimen collected via TBNA.
Therefore, TBNA may be an effective method for increasing the
positive diagnosis rate of primary pulmonary DLBCL.
Methods for the treatment of primary pulmonary
DLBCL, including simple monitoring, surgery, chemotherapy and
chemotherapy followed by radiotherapy, are controversial and there
is no uniform treatment strategy (6). However, treatment of primary
pulmonary DLBCL is recommended to be based on biological features,
including stage, histology and performance status (1). Seeram et al (4) reported that the majority of primarily
pulmonary DLBCL is a low-grade malignancy and complete resection
may be curative. However, the patient was not considered a surgical
candidate due to an endobronchial lesion in the middle segment
bronchus, and the patient preferred a conservative approach with
chemotherapy alone. Following the final diagnosis, standard
treatment with CHOP resulted in significant clinical and
radiological response and the patient remained in remission 8
months later. This indicates that anthracycline-based chemotherapy
may be an optimal therapeutic strategy for patients with primary
pulmonary DLBCL (18,19).
In conclusion, primary pulmonary DLBCL is extremely
rare with nonspecific signs and symptoms. TBNA, associated with
endobronchial biopsy, may be an effective method for the diagnosis
of the disease. Furthermore, CHOP chemotherapy may be an effective
treatment for alleviating symptoms of the disease.
References
|
1
|
Zinzani PL, Martelli M, Poletti V, et al;
Italian Society of Hematology; Italian Society of Experimental
Hematology; Italian Group for Bone Marrow Transplantation. Practice
guidelines for the management of extranodal non-Hodgkin’s lymphomas
of adult non-immunodeficient patients. Part I: primary lung and
mediastinal lymphomas A project of the Italian Society of
Hematology, the Italian Society of Experimental Hematology and the
Italian Group for Bone Marrow Transplantation. Haematologica.
93:1364–1371. 2008.
|
|
2
|
Kim JH, Lee SH, Park J, et al: Primary
pulmonary non-Hodgkin’s lymphoma. Jpn J Clin Oncol. 34:510–514.
2004.
|
|
3
|
Ferraro P, Trastek VF, Adlakha H,
Deschamps C, Allen MS and Pairolero PC: Primary non-Hodgkin’s
lymphoma of the lung. Ann Thorac Surg. 69:993–997. 2000.
|
|
4
|
Seeram V, Shujaat A, Jones L and Bajwa A:
An 82-year-old woman with left upper lobe atelectasis. Diffuse
large B-cell lymphoma. Chest. 142:1669–1674. 2012.PubMed/NCBI
|
|
5
|
Liu Y, Hu YX, Tong GQ, et al: Clinical and
radiological features of primary pulmonary non-Hodgkin lymphoma.
Zhonghua Xue Ye Xue Za Zhi. 34:1057–1059. 2013.(In Chinese).
|
|
6
|
Cadranel J, Wislez M and Antoine M:
Primary pulmonary lymphoma. Eur Respir J. 20:750–762. 2002.
View Article : Google Scholar
|
|
7
|
Chilosi M, Zinzani PL and Poletti V:
Lymphoproliferative lung disorders. Semin Respir Crit Care Med.
26:490–501. 2005. View Article : Google Scholar
|
|
8
|
Hu YH, Hsiao LT, Yang CF, et al:
Prognostic factors of Chinese patients with primary pulmonary
non-Hodgkin’s lymphoma: the single-institute experience in Taiwan.
Ann Hematol. 88:839–846. 2009.PubMed/NCBI
|
|
9
|
Wróbel T, Dzietczenia J,
Prochorec-Sobieszek M, Mazur G and Piwkowski P: Primary pulmonary
diffuse large B-cell lymphoma. Am J Hematol. 87:107–108.
2012.PubMed/NCBI
|
|
10
|
Wang H, Wu D, Xiang H, Chen A and Liu J:
Pulmonary non-Hodgkin’s lymphoma developed during long-term
methotrexate therapy for rheumatoid arthritis. Rheumatol Int.
32:3639–3642. 2012.
|
|
11
|
Ferry JA, Sohani AR, Longtine JA, Schwartz
RA and Harris NL: HHV8-positive, EBV-positive Hodgkin lymphoma-like
large B-cell lymphoma and HHV8-positive intravascular large B-cell
lymphoma. Mod Pathol. 22:618–626. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hsu PK, Hsu HS, Li AF, et al:
Non-Hodgkin’s lymphoma presenting as a large chest wall mass. Ann
Thorac Surg. 81:1214–1218. 2006.
|
|
13
|
Peters P, Butler N, Mundy J and Shah P:
Non-Hodgkin’s lymphoma presenting as an isolated soft-tissue chest
wall mass. Eur J Cardiothorac Surg. 37:4872010.
|
|
14
|
Yu H, Chen G, Zhang R and Jin X: Primary
intravascular large B-cell lymphoma of lung: a report of one case
and review. Diagn Pathol. 7:702012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wang Z, Li X, Chen J, et al: Value of
computed tomography-guided core needle biopsy in diagnosis of
primary pulmonary lymphomas. J Vasc Interv Radiol. 24:97–102. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Cordier JF, Chailleux E, Lauque D, et al:
Primary pulmonary lymphomas. A clinical study of 70 cases in
nonimmunocompromised patients. Chest. 103:201–208. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Xia Y and Wang KP: Transbronchial needle
aspiration: where are we now? J Thorac Dis. 5:678–682.
2013.PubMed/NCBI
|
|
18
|
Neri N, Jesús Nambo M and Avilés A:
Diffuse large B-cell lymphoma primary of lung. Hematology.
16:110–112. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Aviles A, Nambo MJ, Huerta-Guzman J, Silva
L and Neri N: Rituximab in the treatment of diffuse large B-cell
lymphoma primary of the lung. Hematology. 18:81–84. 2013.
View Article : Google Scholar : PubMed/NCBI
|