Introduction
Obstructive nephropathy (ON) is one of the most
important causes of chronic kidney disease in children and infants
(1–2). Renal interstitial fibrosis is the
final pathway and the major pathological basis of ON. Thus far, a
number of experimental investigations, both in vitro and
in vivo, support the hypothesis that numerous factors
contribute to the development of renal interstitial fibrosis,
including the cell itself, extra cellular matrix, cytokines, growth
factors, and the interactions between these factors (3–4).
Epithelial-to-mesenchymal transition (EMT) has been hypothesized to
play an important role in this process in recent years (5). From an experimental point of view,
renal obstruction caused by unilateral ureteral obstruction (UUO)
is the most classical model of induced renal fibrosis (6).
Dietary flavonoid quercetin is known to promote
optimal health, partly via its anti-oxidation effect against
reactive oxygen species (7). This
compound decreases oxidative stress to improve antioxidant status,
inhibits liver cell apoptosis in diabetic rats and alleviates renal
fibrosis in western-style diet-fed C57/BL6J mice (8). Recently, quercetin was found to
regulate inflammatory gene expression in high fat diet-fed mice
(9) and to possess therapeutic
effects that aided the recovery of renal morphology following UUO
in neonatal rats (10). Of note,
it has been demonstrated that progressive tubulointerstitial and
glomerular damage persisted in the obstructed and contralateral
kidney, and a decrease in the glomerular filtration rate and an
increase in proteinuria occurred at the end of one year following
the relief of UUO (11).
Consequently, novel therapy approaches are required to prevent the
progression of renal injury along with surgical intervention.
Hyperoside, as a major compound of glycoside
flavanols secreted in natural plants, has been extensively used for
the clinical treatment of anti-oxidation and analgesia; however, it
is not clear as to whether it exhibits an anti-fibrotic effect in
renal scarring. Findings of a previous study conducted in our
laboratory showed that a combination of quercetin and hyperoside
(QH) from a traditional Chinese herb, Abelmoschl Manihot,
showed satisfactory anti-proliferative activities in 786-O human
renal cancer cell lines (12).
However, no evidence exists regarding the therapeutic effects of
the two compounds for renal fibrotic lesions. Therefore, the
present study was carried out to detect the actions of QH on smooth
muscle actin (SMA) and fibronectin (FN) expression, and to evaluate
the effects of concomitant QH administration in rats with
experimentally induced UUO.
Materials and methods
Reagents
Polyphenolics were extracted from a standardized QH
dehydrate supplement (ratio 1:1) in capsule form which was provided
by Suzhong Pharmaceutical Co. (Taizhou, China). Anti-FN, anti-α-SMA
and anti-β-actin antibodies were purchased from Santa Cruz
Biotechnology, Inc. (Santa Cruz, CA, USA). Dulbecco’s modified
Eagle’s medium (DMEM)/F12 medium and fetal bovine serum were
purchased from Gibco-BRL (Grand Island, NY, USA) and Perbio Science
Company (New Zealand), respectively. The bicinchoninic acid protein
kit and all other chemical reagents used in the experiments were
purchased from Sigma-Aldrich (St. Louis, MO, USA).
Animal experiments and western blot
analysis
Male Wistar rats (body weight 230–250 g) were
randomly divided into three groups. The sham group (n=6) did not
receive an operation of UUO. The untreated UUO group (n=12)
received an operation of UUO without treatment of QH. The UUO + QH
group (n=12) received an operation of UUO and treatment of QH. The
UUO groups with and without QH treatment were further divided into
two time points at three and six days. The rats were fed with QH
(0.1 ml/10 g) once 16 h before surgery began and 8 h after surgery,
then once a day at 9:00 am for five days. All the rats were
sacrificed 24 h later. The harvested kidneys of these rats were
established in the laboratory based on the mechanical sieve method
(13). The methods of western blot
analysis have been described previously (14).
Cell culture and indirect
immunofluorescence analysis
The mesangial cells (SV40 MES 13) were cultured in
DMEM/F12 medium at 37°C and 5% CO2, which consisted of
15% fetal calf serum and penicillin/streptomycin (100 μg/l). The
cells were grown in six-well culture plates (Nunc™, Thermo Fisher
Scientific, Inc., Waltham, MA, USA) with 0.2×106
cells/well density. The previous medium was replaced with
serum-free DMEM/F12 medium cultured for 16 h to synchronize the
cells, subsequent to fusion of 80% of the cells. Different
concentrations of QH (0–60 μg/ml) were added to the medium
according to the test requirements at the same time. Experiments
were located with the control group. Indirect immunofluorescence
analysis was performed as previously described (14).
Statistical analysis
Data were analyzed by the SigmaStat statistical
software (Jandel Scientific, San Rafael, CA, USA) and SigmaPlot
(SPSS, Inc., Chicago, IL, USA). P<0.05 was considered
statistically significant.
Results
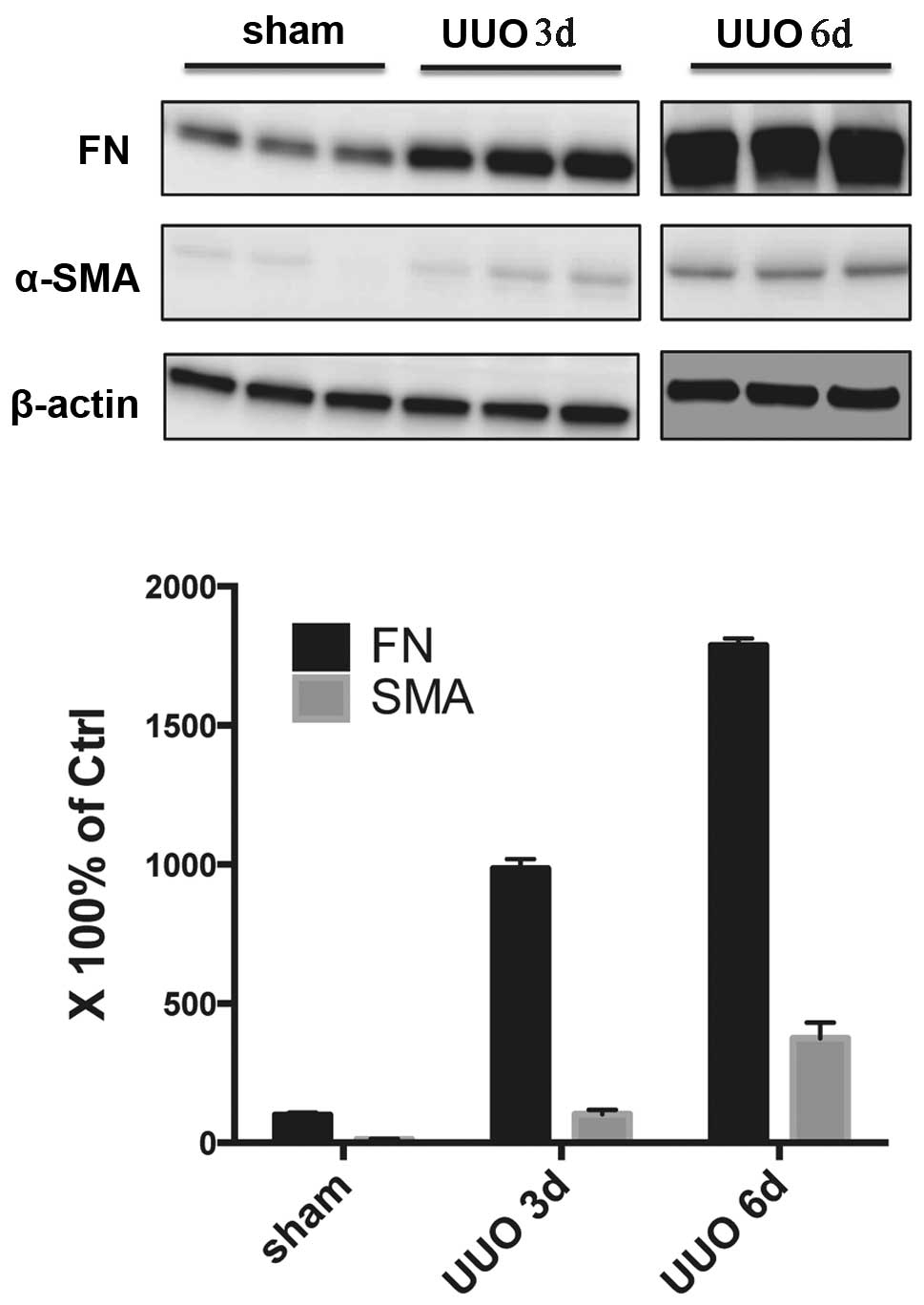
Animal model of renal fibrosis can be
successfully produced by UUO
Negligible levels of α-SMA protein were detected in
the sham group. However in the untreated UUO group, the expression
of α-SMA was significantly increased. The expression of α-SMA was
higher in the six day group compared with the three day group. In
addition, a small amount of FN was expressed in the renal tissue of
rats from the sham group. UUO significantly increased the
expression of FN, and with the extension of obstruction time, FN
expression increased significantly (Fig. 1).
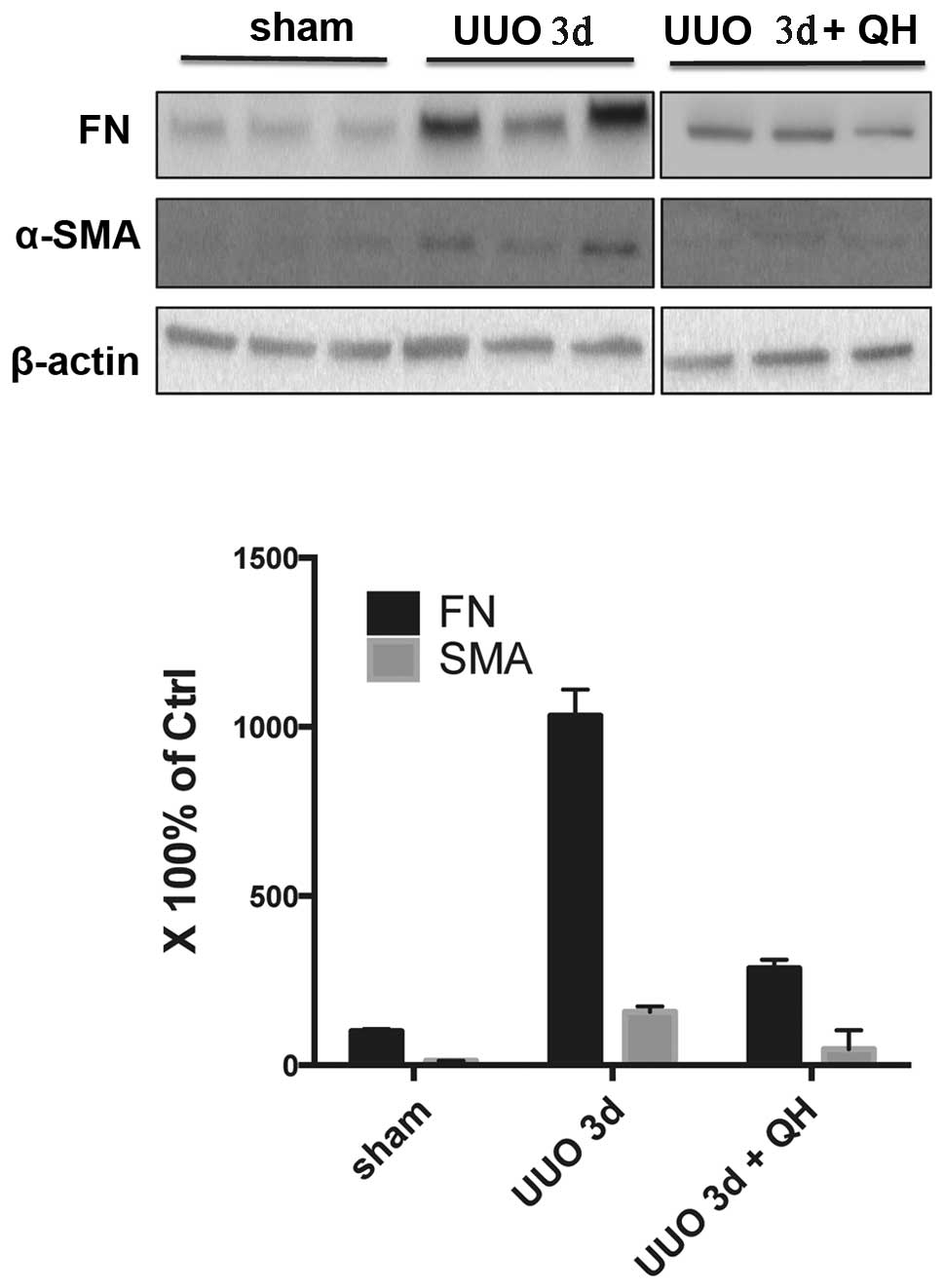
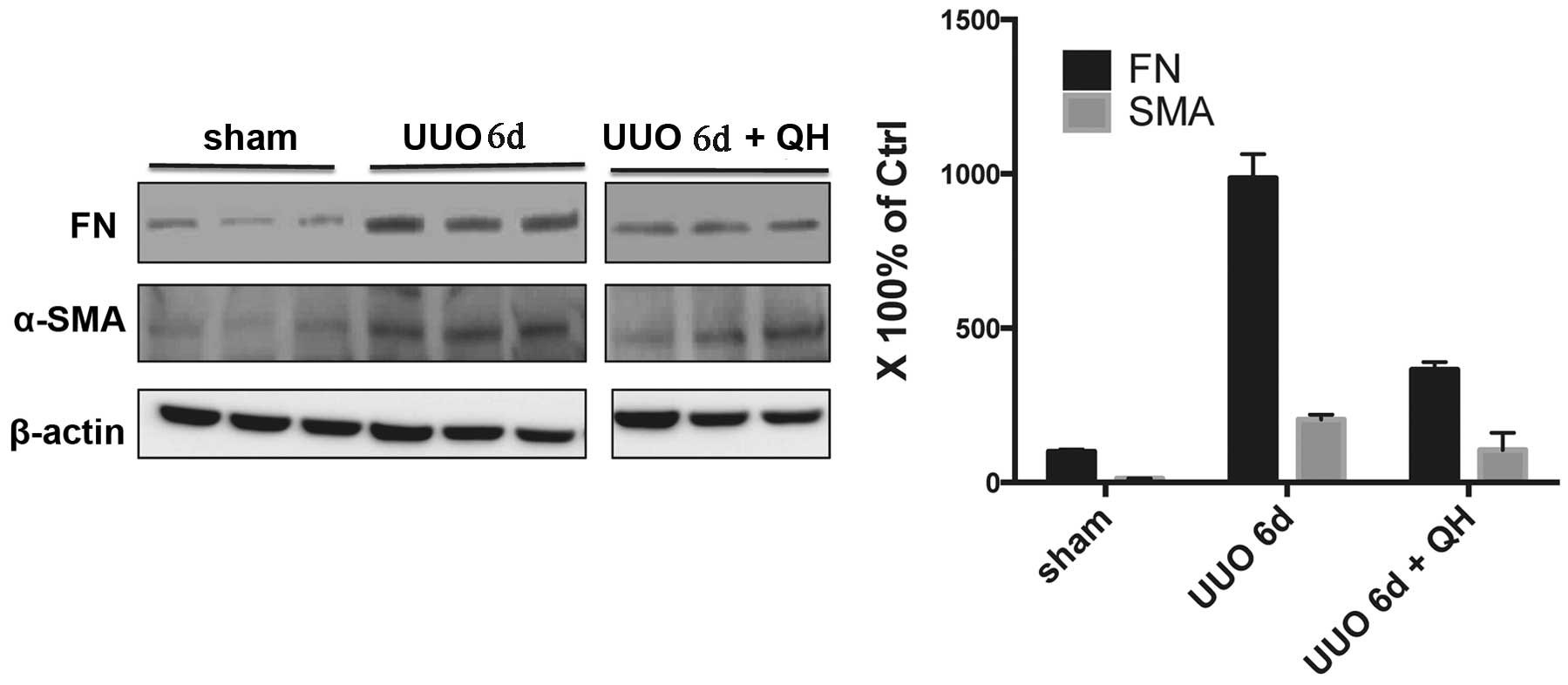
QH reduces the expression of
fibrosis-related proteins in the obstructed kidney
As shown in Fig. 2,
the kidney tissue of the sham group exhibited a negligible
expression of α-SMA. However, the protein expression of α-SMA in
the untreated UUO group at three days was increased, which was
significantly reduced by QH treatment of 0.1 mg/kg/day. Low protein
levels of FN were expressed in the sham group, however, these
increased almost 10-fold following UUO for three days, while the
expression levels of FN decreased significantly when treated with
QH compared with the untreated UUO group. Similar results were also
evident in the six day group (Fig.
3).
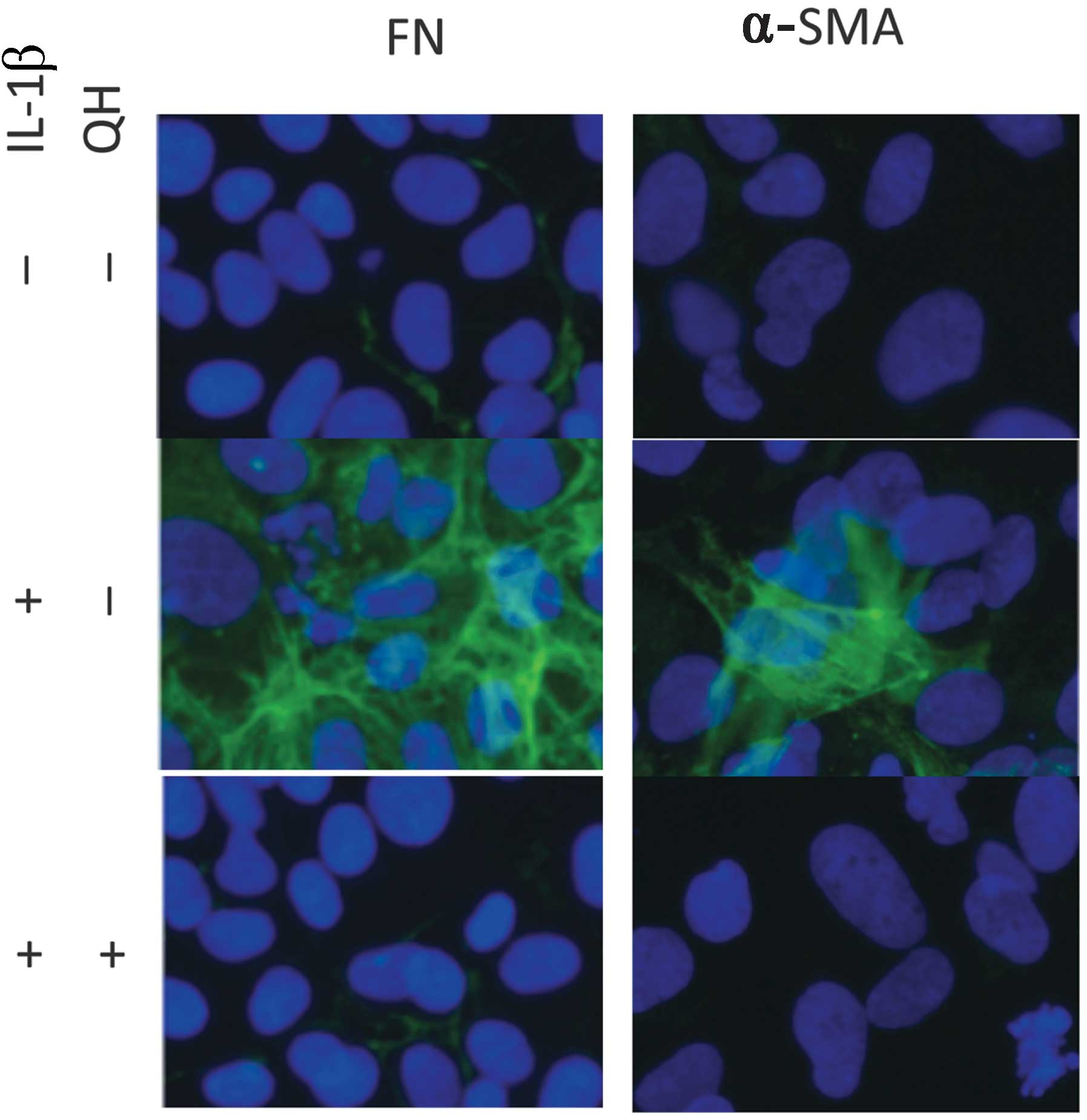
QH reduces the expression of
fibrosis-related protein in mesangial cells in vitro
The potential mechanism of QH in renal fibrosis
therapy was investigated. The results showed that there was a low
expression of FN and α-SMA in mesangial cells. The protein
concentration of mesangial cells stimulated by interleukin-1β
(IL-1β) showed a significant difference compared with the control
group. However, QH was able to reduce the expression of FN and
α-SMA in mesangial cells (Fig.
4).
Discussion
The present study confirms that the combinatorial
treatment of QH has the ability to reduce renal expression of α-SMA
and FN in UUO rats, which are traditional models of renal fibrosis.
Furthermore, the upregulation of kidney mesangial FN and α-SMA
expression secondary to IL-1β induced inflammation was also
suppressed by QH.
Quercetin can prevent lipid peroxidation by blocking
the action of xanthene oxidase, chelating iron, and the directly
scavenging hydroxyl radical (15–17).
Hyperoside can prevent lipid peroxidation through iron chelation,
free radical scavenging and the blockade of tyrosine kinase enzymes
responsible for apoptosis in renal epithelial cells that are
triggered by oxidative stress (18–19).
As reported in the literature, QH could reduce renal
ischemia-reperfusion injury mediated through infiltration of
macrophages by interfering with inducible nitric oxide synthase
activity (20).
According to a previous study, one of the key
strategies for ON should be the prevention and reversal of
interstitial renal fibrosis as well as relief of obstruction
(21). QH provides two of these
agents. To the best of our knowledge, this is the first study
comparing the effects of QH on fibrogenesis in a UUO model. In
addition, the association between α-SMA and FN expression and
hyperoside was revealed for the first time in the present
study.
Recently, a proteasome inhibitor was shown to
abolish transforming growth factor-β-mediated α-SMA and FN
induction, by blocking Ski-related novel protein N degradation,
using human kidney proximal tubular epithelial cells. α-SMA was
vital for the kidney fibrosis process. Fibrosis was associated with
enhanced expression of FN in model of dyslipidemia induced renal
fibrosis (22). Inhibition of FN
expression significantly attenuated the expression of pro-fibrotic
signals, collagen formation and the proliferation of fibroblasts
(23).
Although various factors may be attributable to
renal fibrosis, a definite mechanism for the initiation and
progression of this complex process has not been elucidated. In the
present study, the satisfactory therapeutic effects of QH were
revealed by evidence-based medicine. In the present data,
immunohistochemical analysis demonstrated that the expression of
α-SMA and FN was inhibited by QH intervention, as well as the
marked suppression observed in the in vitro experiment, all
of which provided novel experimental evidence for the treatment of
renal fibrosis. Future studies are required to determine how QH
influences the proliferation of renal resident cells, its
anti-inflammation mechanism, and whether such mechanisms are
operative in vivo and in vitro. This will enable the
development of novel interventions for the protection of humans
from renal scarring for therapeutic purposes.
Acknowledgements
This study was partially supported by grants from
the National Natural Science Foundation of China (nos. 81000311 and
81270831).
References
|
1
|
Seikaly MG, Ho PL, Emmett L, et al:
Chronic renal insufficiency in children: the 2001 Annual Report of
the NAPRTCS. Pediatr Nephrol. 18:796–804. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bek K, Akman S, Bilge I, et al: Chronic
kidney disease in children in Turkey. Pediatr Nephrol. 24:797–806.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Acikgoz Y, Can B, Bek K, et al: The effect
of simvastatin and erythropoietin on renal fibrosis in rats with
unilateral ureteral obstruction. Ren Fail. 36:252–257. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sagar SK, Zhang C, Guo Q, et al: Role of
expression of endothelin-1 and angiotensin-II and hypoxia-inducible
factor-1α in the kidney tissues of patients with diabetic
nephropathy. Saudi J Kidney Dis Transpl. 24:959–964. 2013.
|
|
5
|
Carew RM, Wang B and Kantharidis P: The
role of EMT in renal fibrosis. Cell Tissue Res. 347:103–116. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chevalier RL, Forbes MS and Thornhill BA:
Ureteral obstruction as a model of renal interstitial fibrosis and
obstructive nephropathy. Kidney Int. 75:1145–1152. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Shen Y, Ward NC, Hodgson JM, et al:
Dietary quercetin attenuates oxidant-induced endothelial
dysfunction and atherosclerosis in apolipoprotein E knockout mice
fed a high-fat diet: a critical role for heme oxygenase-1. Free
Radic Biol Med. 65:908–915. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kobori M, Masumoto S, Akimoto Y and Oike
H: Chronic dietary intake of quercetin alleviates hepatic fat
accumulation associated with consumption of a Western-style diet in
C57/BL6J mice. Mol Nutr Food Res. 55:530–540. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Boesch-Saadatmandi C, Wagner AE, Wolffram
S and Rimbach G: Effect of quercetin on inflammatory gene
expression in mice liver in vivo-role of redox factor 1, miRNA-122
and miRNA-125b. Pharmacol Res. 65:523–530. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Choi YJ, Arzuaga X, Kluemper CT, et al:
Quercetin blocks caveolae-dependent pro-inflammatory responses
induced by co-planar PCBs. Environ Int. 36:931–934. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Noorafshan A, Karbalay-Doust S and
Poorshahid M: Stereological survey of the ameliorative effects of
sulforaphane and quercetin on renal tissue in unilateral ureteral
obstruction in rats. Acta Clin Croat. 51:555–562. 2012.PubMed/NCBI
|
|
12
|
Li W, Liu M, Xu YF, et al: Combination of
quercetin and hyperoside has anticancer effects on renal cancer
cells through inhibition of oncogenic microRNA-27a. Oncol Rep.
31:117–124. 2014.PubMed/NCBI
|
|
13
|
Tan R, Zhang J, Tan X, et al:
Downregulation of SnoN expression in obstructive nephropathy is
mediated by an enhanced ubiquitin-dependent degradation. J Am Soc
Nephrol. 17:2781–2791. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Su J, Yin LP, Zhang X, et al: Chronic
allograft nephropathy in rats is improved by the intervention of
rhein. Transplant Proc. 45:2546–2552. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Papiez MA, Cierniak A, Krzysciak W, et al:
The changes of antioxidant defense system caused by quercetin
administration do not lead to DNA damage and apoptosis in the
spleen and bone marrow cells of rats. Food Chem Toxicol.
46:3053–3058. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lu NH, Chen C, He YJ, et al: Effects of
quercetin on hemoglobin-dependent redox reactions: relationship to
iron-overload rat liver injury. J Asian Nat Prod Res. 15:1265–1276.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chidambaram U, Pachamuthu V, Natarajan S,
et al: In vitro evaluation of free radical scavenging activity of
Codariocalyx motorius root extract. Asian Pac J Trop Med.
6:188–194. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lee S, Park HS, Notsu Y, et al: Effects of
hyperin, isoquercitrin and quercetin on lipopolysaccharide-induced
nitrite production in rat peritoneal macrophages. Phytother Res.
22:1552–1556. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kicel A and Wolbiś M: Phenolic content and
DPPH radical scavenging activity of the flowers and leaves of
Trifolium repens. Nat Prod Commun. 8:99–102. 2013.PubMed/NCBI
|
|
20
|
Li ZL, Hu J, Li YL, et al: The effect of
hyperoside on the functional recovery of the ischemic/reperfused
isolated rat heart: potential involvement of the extracellular
signal-regulated kinase 1/2 signaling pathway. Free Radic Biol Med.
57:132–140. 2013. View Article : Google Scholar
|
|
21
|
Ito K, Chen J, El Chaar M, et al: Renal
damage progresses despite improvement of renal function after
relief of unilateral ureteral obstruction in adult rats. Am J
Physiol Renal Physiol. 287:F1283–F1293. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Tan R, He W, Lin X, et al: Smad
ubiquitination regulatory factor-2 in the fibrotic kidney:
regulation, target specificity, and functional implication. Am J
Physiol Renal Physiol. 294:F1076–F1083. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wu T, Zhang B, Ye F and Xiao Z: A
potential role for caveolin-1 in VEGF-induced fibronectin
upregulation in mesangial cells: involvement of VEGFR2 and Src. Am
J Physiol Renal Physiol. 304:F820–F830. 2013. View Article : Google Scholar : PubMed/NCBI
|