Introduction
Alzheimer’s disease (AD) is a progressive
neurodegenerative disease of the central nervous system, with
memory loss as the primary clinical manifestation. As a result of
the aging population, the number of patients with AD is predicted
to be >100 million in 2050 worldwide (1). However, at present, there are no
effective drugs that delay the progression of AD.
Previous studies have demonstrated that β-amyloid
(Aβ) peptides have an important role in AD. Aggregation of Aβ may
cause neurofibrillary tangles, inflammation and neuronal loss,
resulting in the development of AD (2). It is thought that reduced generation
or accelerated clearance of Aβ may delay the pathological
progression of AD (3,4). Autophagy is considered to be an
important regulatory factor in Aβ clearance. During autophagy, Aβ
is taken up by autophagic vacuoles, which then fuse to the lysosome
and are degraded (5,6). Therefore, autophagy enhances Aβ
clearance. It has been previously demonstrated that mammalian
target of rapamycin (mTOR) kinase is an important energy
sensitivity factor. mTOR is inhibited when there is lack of energy,
and inhibition of mTOR results in the activation of autophagy to
produce additional energy (7,8).
Therefore, inhibition of mTOR may enhance autophagy, and as a
result increase the clearance of intracellular Aβ, thereby delaying
the pathological progression of AD (9).
Panax ginseng is a traditional Chinese
medicine, which has been used as a medicine for thousands of years.
Studies have shown that ginseng has a number of biological
activities, including as an antioxidant, anti-aging agent,
inhibitor of cell apoptosis and cognition enhancer (10,11).
Ginsenosides are active compounds extracted from ginseng, and it
has been demonstrated that ginsenoside Rg1 is able to improve
memory and has anti-dementia effects (12). Ginsenoside compound K is a
metabolite of panaxadiol (a saponin) that is generated by the
metabolic action of intestinal flora in humans. It is considered
that numerous ginsenosides are metabolized into compound K prior to
becoming active in vivo. Compound K is hypothesized to be
the active compound in vivo, while ginsenosides may be
prodrugs that have no effect (13). Therefore, the pharmacological
mechanisms of compound K require further investigation. In present
study, the Aβ-scavenging effect of compound K and the underlying
mechanisms were investigated in primary astrocytes.
Materials and methods
Primary culturing of mouse
astrocytes
C57 mice were purchased from the Experimental Animal
Center of Xinxiang Medical University (Xinxiang, China) and at 18
days of pregnancy were anaesthetized using pentobarbital and then
paunched. The fetal mice were removed and placed into pre-cooling
D-Hank’s buffer. The brains of the fetal mice were removed and the
meninges were discarded. The cerebral cortex was separated from the
brain tissues and then digested for 15 min at 37°C in 8 ml 0.125%
trypsin (2.5% trypsin diluted 20-fold with D-Hank’s and DNase, at a
final concentration of 0.2 mg/ml). Digestion was terminated by the
addition of 8 ml Dulbecco’s modified Eagle’s medium (Gibco, Grand
Island, NY, USA) containing 10% horse serum (Gibco) for 5 min. The
upper suspension was transferred to a 50-ml tube and an additional
8 ml complete growth medium was added to the deposit and the
previous step was repeated. The cells in the suspension were placed
at a density of 2×105 cell/cm2 in a
75-cm2 flask that was pre-coated with poly-D-lysine. The
cells were incubated at 37°C and 5% CO2 for 9 days, and
the medium was replaced every three days. The flask was then placed
on a ZHWY-110X shaker table (Zhicheng Co., Shanghai, China) at 200
rpm at 37°C overnight. On the second day, the flask was rinsed
twice with D-Hank’s at 37°C, and the remaining adherent cells were
collected with trypsin and subcultured. Following attachment, the
cells were treated with Ara-C for 96 h (medium renewal once) at a
final concentration of 10 μM, and the cells obtained were
astrocytes. This study was performed in strict accordance with the
recommendations in the Guide for the Care and Use of Laboratory
Animals of the National Institutes of Health (8th edition,
Bethesda, MD, USA, 2010). The animal use protocol was reviewed and
approved by the Institutional Animal Care and Use Committee of the
First Affiliated Hospital of Xinxiang Medical University (Weihui,,
China).
Detection of aggregated LC3
A viral expression vector containing a red
fluorescent protein-LC3 construct (Jikai Biotechnology Co. Ltd.,
Shanghai, China) was transfected into the primary astrocytes.
Transfection was performed using a Sunma-sofast gene transfection
kit according to the manufacturer’s instructions (Sunma
Biotechnology Co., Ltd., Xiamen, China). LC3 proteins were then
confirmed to be stably expressed in the cells. After two days, the
cells were treated with 50 μM compound K (Fangcheng Biotechnology
Co. Ltd., Beijing, China) for 2 h, and the aggregation state of the
LC3 proteins was detected by confocal laser scanning microscopy
(TCS-SP5, Leica, Bensheim, Germany).
Western blot analysis
Cells treated with different concentration of
compound K were treated with radio-immunoprecipitation assay lysis
buffer at 75°C for 10 min. The lysates were incubated at 99°C for
15 min, and then centrifuged at 10,000 × g for 5 min at 4°C. The
supernatant was separated using SDS-PAGE and then transferred to a
polyvinylidene difluoride membrane under semi-dry electrotransfer
conditions. The membrane was incubated in blocking buffer [1X
Tris-buffered saline (TBS), 0.5% Tween 20 and 5% skimmed milk
powder] for 30 min, followed by incubation with the primary
antibody (targeting P70S6K, P70S6K-P, ULK1, ULK1-P, mTOR, mTOR-P,
P62 or GAPDH; Cell Signaling Technology Inc., Beverly, MA, USA) at
4°C overnight. The membranes were then rinsed 3 times with TBS and
Tween 20 (TBST) for 15 min, and then incubated with the
corresponding monoclonal, anti-rabbit secondary antibody
(Santa-Cruz Biotechnology Inc., Santa Cruz, CA, USA) for 2 h, prior
to being rinsed again with TBST 3 times. The membrane was then
incubated with SuperSignal West Dura Chemiluminescent Substrate
(Pierce, Thermo Scientific, Rockford, IL, USA) for 5 min and images
were captured by X-ray film exposure.
Aβ clearance assay
The Aβ clearance assay in primary astrocytes was
performed as previously described by Cramer et al (14). Briefly, the cells were incubated
with different concentrations of compound K (0, 1, 10, 20 and 50
μM) for 18 h, meanwhile, chloroquine (an inhibitor of autophagy)
was used as a control and then exogenous Aβ (Invitrogen Life
Technologies, Carlsbad, CA, USA) was added to a final concentration
of 2 μg/ml. The cells were then incubated for a further 3 h. The
cells were washed 3 times with phosphate-buffered saline, and then
treated with lysis buffer (50 mM Tris and 1% SDS) at 37°C for 15
min. The lysates were centrifuged at 12,000 × g for 15 min, and the
supernatant was collected. Aβ was then quantified using an
enzyme-linked immunosorbent assay (ELISA kit for Aβ detection).
ELISA
An ELISA for Aβ detection was conducted in
accordance with the manufacturer’s instructions (Invitrogen Life
Technologies). The diluted samples were incubated with Aβ antibody
in a 96-well plate that was pre-coated with Aβ antibody. After 3 h,
the plate was rinsed with cleaning solution (Biyuntian Co.,
Shanghai, China) four times, and then incubated with the secondary
antibody for 30 min and rinsed five times. The chromogenic
substrate was then added and the plates were incubated for a
further 30 min. Finally the reaction was terminated using stop
solution. The intensity of color developed was measured using
microplate reader (Bio-Rad 680, Bio-Rad, Hercules, CA, USA) at 570
nm. In order to eliminate the interference of the cell density, the
cells were lysed (50 mm Tris-HCl, 0.15 M sodium chloride, 1% P40
and 0.1% SDS) and the protein content was measured using the
bicinchoninic acid assay method. The measured density was adjusted
according to the total protein content.
Statistical analysis
The data are expressed as the mean ± standard
deviation and were analyzed using SPSS software, version 16.0
(SPSS, Inc., Chicago, IL, USA). One-way analysis of variance was
used to compare the scores of different groups. P<0.05 was
considered to indicate a statistically significant difference.
Results
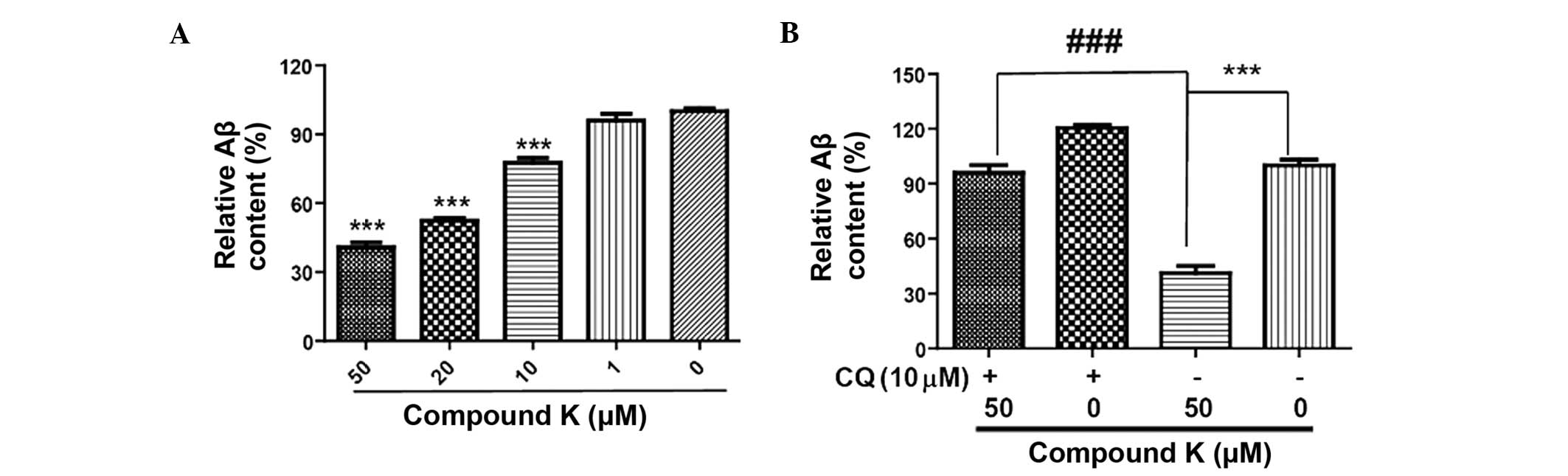
Compound K promotes clearance of Aβ in
primary astrocytes
The levels of Aβ in astrocytes treated with compound
K were significantly lower compared with those in untreated
astrocytes. The differences were significantly different for the
10, 20 and 50 μM concentrations of compound K (P<0.001; Fig. 1). These results indicate that
compound K enhances Aβ clearance in primary astrocytes. In order to
investigate the association between compound K and autophagy,
chloroquine, an inhibitor of autophagy, was used as a control. The
results demonstrated that chloroquine markedly attenuated the
effect of compound K on the enhancement of Aβ clearance. This
indicates that compound K promotes Aβ clearance through the
enhancement of autophagy in primary astrocytes.
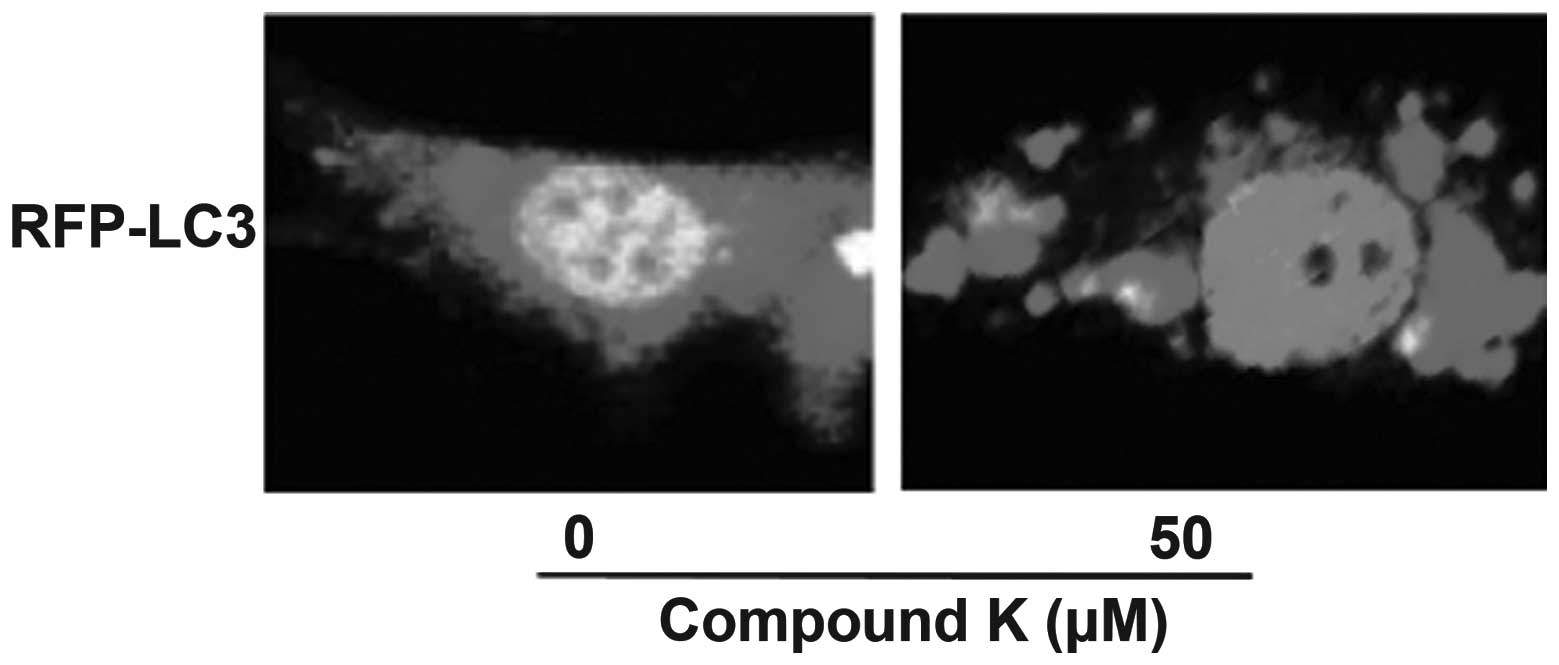
Compound K enhances autophagy in primary
astrocytes
Autophagy has an important role in Aβ clearance. In
order to further clarify the association between autophagy and Aβ
clearance, the effect of compound K on autophagy was investigated.
LC3 is an important marker of autophagosome formation. When
autophagy is enhanced, LC3 proteins that are dispersed throughout
the cell gather to the membrane of autophagosome, which can then be
detected using laser scanning confocal microscopy. Following
transfection with LC3, the primary astrocytes were found to stably
express LC3 protein. It was observed that LC3 proteins aggregated
following incubation with 50 μM compound K for 2 h. This indicates
that compound K enhances autophagy in astrocytes, which may
contribute to increased clearance of Aβ (Fig. 2).
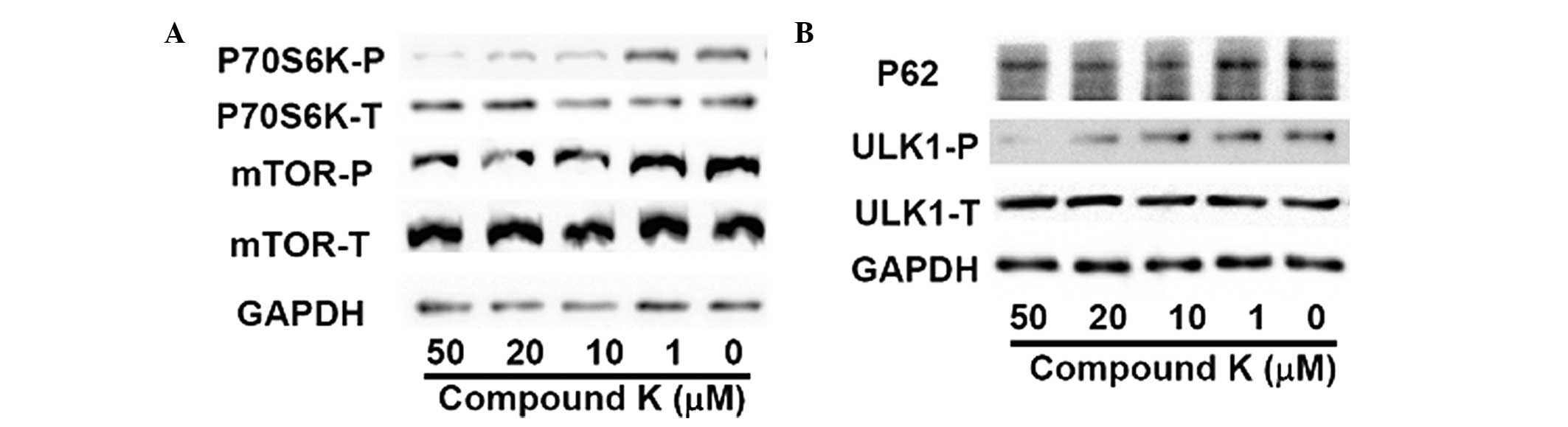
Compound K enhances autophagy by
inhibiting the mTOR signal pathway
As an important factor of energy sensitivity, mTOR
kinase is inhibited when there is an energy shortage, leading to an
enhancement of autophagy. Therefore, the phosphorylation levels of
mTOR and P70S6K, the substrate of mTOR, in primary astrocytes
treated with compound K were investigated by western blot analysis.
As shown in Fig. 3A, the
phosphorylation levels of mTOR and P70S6K were reduced by compound
K, indicating that compound K enhances autophagy by inhibiting the
activity of mTOR. To further investigate this, the phosphorylation
of ULK1, the major initiator protein of autophagy, and the levels
of P62, the marker of the autophagy, were then investigated
(15). As shown in Fig. 3B, compound K significantly reduced
the expression level of P62 and the phosphorylation of ULK1,
indicating that compound K activates and enhances autophagy in
astrocytes.
These results suggest that compound K may have an
inhibitory effect on the phosphorylation of mTOR and subsequently
enhance autophagy, which may contribute to increased scavenging of
Aβ in primary astrocytes.
Discussion
Aβ is considered to be one of the key proteins in AD
progression. In vivo, Aβ is in a dynamic equilibrium between
generation and scavenging, and excess generation or weak scavenging
of Aβ is the main pathogeny of AD. Drugs that reduce Aβ production
or increase the clearance of Aβ may slow AD progression. Autophagy
is the most important pathway for the clearance of abnormal
molecules, cell subunits and abnormal aggregates of proteins.
Autophagy levels have been found to be significantly decreased in
various neurodegenerative diseases, including AD and Parkinson’s
disease (16,17). Therefore, enhanced autophagy may
increase the clearance of Aβ, which may delay the pathological
process of AD. In a previous study it was reported that resveratrol
and its derivatives from grape seeds enhance autophagy through AMPK
activation and subsequently the inhibition of mTOR, resulting in
the scavenging of Aβ, which may have a role in the treatment of AD
(18). In addition, latrepirdine,
a type of antihistamine that is already on the market, has been
demonstrated to increase the clearance of Aβ by enhancing
autophagy, and has a role in the treatment of AD (19). Therefore, compounds that are able
to enhance the scavenging of Aβ by promoting autophagy may be
effective in AD therapy, and the screening of this type of compound
is valuable for the treatment of AD.
Previous studies have shown that Panax
ginseng has a number of biological activities, including the
ability to improve memory. Compound K is the metabolite of numerous
ginsenosides in the intestine, and is the main active entity of
Panax ginseng in vivo. It has previously been shown that
ginsenosides are able to upregulate the expression of brain-derived
neurotrophic factor and inhibit the phosphorylation of Tau, which
results in slowing of the formation of neurofibrillary tangles and
should improve learning ability and memory. In addition, it has
been shown to have an anti-AD role in a rat model of AD (20).
The effect of compound K on Aβ scavenging has yet to
be fully elucidated. In the present study, the effect of compound K
on Aβ scavenging and the mechanisms involved was investigated using
the Aβ clearance assay in vitro. The results demonstrated
that compound K significantly enhanced the clearance of Aβ in
primary astrocytes. Furthermore, it was shown that the
phosphorylation of mTOR was inhibited by compound K, which may
contribute to enhanced autophagy, and thereby increase the
scavenging of Aβ. In conclusion, these results indicate that
compound K may increase the clearance of Aβ from astrocytes and
then slow the pathological progression of AD. This study provides
an important basis for the use of Panax ginseng as a
traditional Chinese medicine for improving memory, and provides a
novel interpretation of the mechanism of compound K in AD
therapy.
References
|
1
|
Brookmeyer R, Johnson E, Ziegler-Graham K
and Arrighi HM: Forecasting the global burden of Alzheimer’s
disease. Alzheimers Dement. 3:186–191. 2007.
|
|
2
|
Younkin SG: The role of A beta 42 in
Alzheimer’s disease. J Physiol Paris. 92:289–292. 1998.
|
|
3
|
Ohno M, Sametsky EA, Younkin LH, et al:
BACE1 deficiency rescues memory deficits and cholinergic
dysfunction in a mouse model of Alzheimer’s disease. Neuron.
41:27–33. 2004.PubMed/NCBI
|
|
4
|
Jarvis CI, Goncalves MB, Clarke E, et al:
Retinoic acid receptor-α signalling antagonizes both intracellular
and extracellular amyloid-β production and prevents neuronal cell
death caused by amyloid-β. Eur J Neurosci. 32:1246–1255. 2010.
|
|
5
|
Bachmeier C, Beaulieu-Abdelahad D, Mullan
M and Paris D: Selective dihydropyiridine compounds facilitate the
clearance of β-amyloid across the blood-brain barrier. Eur J
Pharmacol. 659:124–129. 2011.PubMed/NCBI
|
|
6
|
Kennelly SP, Abdullah L, Paris D, et al:
Demonstration of safety in Alzheimer’s patients for intervention
with an anti-hypertensive drug Nilvadipine: results from a 6-week
open label study. Int J Geriatr Psychiatry. 26:1038–1045. 2011.
|
|
7
|
Klionsky DJ and Emr SD: Autophagy as a
regulated pathway of cellular degradation. Science. 290:1717–1721.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhu Z, Yan J, Jiang W, et al: Arctigenin
effectively ameliorates memory impairment in Alzheimer’s disease
model mice targeting both β-amyloid production and clearance. J
Neurosci. 33:13138–13149. 2013.PubMed/NCBI
|
|
9
|
Spilman P, Podlutskaya N, Hart MJ, et al:
Inhibition of mTOR by rapamycin abolishes cognitive deficits and
reduces amyloid-beta levels in a mouse model of Alzheimer’s
disease. PLoS One. 5:e99792010.PubMed/NCBI
|
|
10
|
Cho CW, Kim YC, Kang JH, et al:
Characteristic study on the chemical components of Korean curved
ginseng products. J Ginseng Res. 37:349–354. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Seo SK, Hong Y, Yun BH, et al:
Antioxidative effects of Korean red ginseng in postmenopausal
women: A double-blind randomized controlled trial. J
Ethnopharmacol. 154:753–757. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhang X, Wang J, Xing Y, et al: Effects of
ginsenoside Rg1 or 17β-estradiol on a cognitively impaired,
ovariectomized rat model of Alzheimer’s disease. Neuroscience.
220:191–200. 2012.
|
|
13
|
Wakabayashi C, Murakami K, Hasegawa H,
Murata J and Saiki I: An intestinal bacterial metabolite of ginseng
protopanaxadiol saponins has the ability to induce apoptosis in
tumor cells. Biochem Biophys Res Commun. 246:725–730. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Cramer PE, Cirrito JR, Wesson DW, et al:
ApoE-directed therapeutics rapidly clear beta-amyloid and reverse
deficits in AD mouse models. Science. 335:1503–1506. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Mizushima N: The role of the Atg1/ULK1
complex in autophagy regulation. Curr Opin Cell Biol. 22:132–139.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hara T, Nakamura K, Matsui M, et al:
Suppression of basal autophagy in neural cells causes
neurodegenerative disease in mice. Nature. 441:885–889. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Nixon RA: The role of autophagy in
neurodegenerative disease. Nat Med. 19:983–997. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Vingtdeux V, Chandakkar P, Zhao H, et al:
Novel synthetic small-molecule activators of AMPK as enhancers of
autophagy and amyloid-β peptide degradation. FASEB J. 25:219–231.
2011.PubMed/NCBI
|
|
19
|
Steele JW and Gandy S: Latrepirdine
(Dimebon®), a potential Alzheimer therapeutic, regulates
autophagy and neuropathology in an Alzheimer mouse model.
Autophagy. 9:617–618. 2013.
|
|
20
|
Gao C, Liu Y, Jiang Y, Ding J and Li L:
Geniposide ameliorates learning memory deficits, reduces tau
phosphorylation and decreases apoptosis via GSK3β pathway in
streptozotocin-induced alzheimer rat model. Brain Pathol.
24:261–269. 2014.PubMed/NCBI
|