Introduction
Elderly individuals are the fastest growing segment
of the population in Western countries. Over half of elderly
individuals have dementia; however, the etiology of dementia has
yet to be elucidated (1). A number
of causes of dementia have been identified, and vascular risk
factors (VRF) are associated with a higher incidence of dementia
(2). Patients with vascular
dementia (VaD) are often isolated and withdrawn from society due to
negative symptoms and functional disabilities (3), which are characterized by
functionally impairing deterioration of memory, language,
personality or visuospatial skills (4).
The pathogenesis of dementia remains unclear and
effective drugs for the treatment of this disease are lacking.
Therefore, the development of animal models of VaD is important for
use in studies to understand the pathophysiology of dementia, as
well as to determine the efficacy of novel therapeutic drugs.
Currently, rats are usually used as VaD animal models; however,
establishing rat models of VaD is expensive and challenging.
Therefore, in the present study, repeated ischemia-reperfusion (IR)
of the total bilateral carotid artery, combined with blood pressure
reduction, was used to establish a VaD model in mice. The mice were
subjected to behavioral tests to identify whether they had
significant postoperative learning and memory dysfunction, impaired
spatial orientation constancy and an impaired spatial recognition
ability. In addition, the number of hippocampal CA1 neurons was
investigated. The suitability of this animal model of VaD for use
in the study of the pathogenesis and prevention of VaD was thus
assessed.
Materials and methods
Animals
A total of 40 Kunming mice (clean grade; 20 male and
20 female), weighing between 20 and 22 g, were provided by the
Experimental Animal Center of the Chinese Academy of Medical
Sciences (Beijing, China). The present study was performed in
accordance with the recommendations in the Guide for the Care and
Use of Laboratory Animals (National Institutes of Health, 8th
Edition, Bethesda, MD, USA). The animal protocol was reviewed and
approved by the Institutional Animal Care and Use Committee (IACUC)
at the China-Japan Friendship Hospital (Beijing, China).
Establishment of the animal model
The mice were anesthetized via intraperitoneal
injection with 480 mg/kg 10% chloral hydrate and then the anterior
cervical skin was disinfected with 75% alcohol. An anterior neck
middle incision was made, and the bilateral carotid arteries were
separated, and then a thread was passed below each carotid artery
for closure. Sodium nitroprusside (Beijing Double-Crane
Pharmaceutical Co., Ltd., Beijing, China) was intraperitoneally
injected (3.5 mg/kg) to cause hypotension. The bilateral carotid
arteries were closed by ligation for 10 min, and then loosened for
10 min, and this was repeated 3 times. The threading was then
withdrawn and the incision sutured. The mice were placed in cages
for rearing.
Grouping
The mice were randomly divided into two groups: a
sham group and a model group (each with 10 mice in a 10 days group
and 10 mice in a 30 days group. The mice in the sham group were
subjected only to peeling of the bilateral common carotid arteries;
the blood vessels were not closed and the blood pressure was not
lowered. The mice in the model group were subjected to the
aforementioned modeling method.
Step-down avoidance test
A passive avoidance reaction tank, 60×12×33 cm in
size (Institute of Materia Medica, Chinese Academy of Medical
Sciences, Beijing, China) was used as the jumping test device. It
was separated into two equal parts by a black plastic sheet, and an
energized copper grid was placed on the bottom. An insulated rubber
mat with diameter and height of 4.5 cm was placed on the right of
the device, which was used as the safe platform for the mouse to
avoid an electric shock. A voltage regulator was used to regulate
the voltage.
The mice were placed in the jumping device to adapt
for 3 min and then a 40 v alternating current was turned on to
shock the mice. The mice jumped to the safe platform to avoid the
electric shock. The time from shock occurrence to jumping was
record as the response period. The frequency of electric shock
(number of errors) and the electric shock time within 5 min were
also observed. The response period, number of errors and electric
shock time were considered as their learning performance. After 24
h, the test was repeated. The mice were placed on the safe platform
and an electric current was applied. The time of mice jumping from
the safe platform to the copper grid within 5 min was observed as
the incubation period, and the number of electric shocks was taken
as the number of errors. The incubation period, number of errors
and the electric shock time were considered as the memory
performance.
Water maze test
The water maze was provided by the Institute of
Materia Medica, Chinese Academy of Medical Sciences, and was made
from black plastic sheeting (water depth, 10 cm; water temperature,
23±1°C). It was divided into four zones, specifically A, B and C
zones and an end zone that contained a footboard for the mice to
climb out of water. The swim from the A, B and C zones to the end
zone was used as a phase of training, and was repeated three times.
Subsequently, the time for mice to swim from zone A to the
footboard of the end zone (entire swim time) and the frequency of
entering the blind side (number of errors) within 5 min were
recorded and taken as the learning performance. This was repeated
after 24 h, and the data obtained was considered as the memory
performance. If the mice did not swim to the end within 5 min, 5
min was used as the whole swim time.
Morphological observations
After 10 and 30 days following the surgery, mice in
each group were selected and were decapitated after anesthesia. The
brain was quickly removed, followed by coronal incision. The
hippocampus was obtained and was fixed in 20% formalin. Following
dehydration with ethanol and embedding with paraffin, 5 μm sections
were made, followed by hematoxylin and eosin staining. The
hippocampal CA1 region was then observed under an optical
microscope (CKX41, Olympus, Tokyo, Japan).
Statistical analysis
Data are expressed as the mean ± standard deviation.
Statistical analysis was performed using SPSS statistical software,
version 13.0 (SPSS, Inc., Chicago, IL, USA). A t-test was used to
analyze the differences between different groups, and P<0.05 was
considered to indicate a statistically significant difference.
Results
Behavioral experiments
The response period of the model group 10 days
following surgery was longer than that of the sham group, and was
markedly more prolonged 30 days following surgery (P<0.001). The
incubation period 10 days and 30 days following surgery was
significantly shorter than that in the sham group (P<0.01). The
number of errors and electric shock time 10 days and 30 days
following surgery in the learning and memory periods were
significantly increased compared with those in the sham group
(P<0.001). This indicates that the learning and memory function
of the mice in model group was damaged, and this damage increased
with time following the surgery (Tables I and II).
 | Table ILearning performance in the step-down
avoidance test. |
Table I
Learning performance in the step-down
avoidance test.
| Group | n | Response period
(sec) | Number of errors | Electric shock time
(sec) |
|---|
| Sham 10 days | 10 | 5.20±4.39 | 0.76±0.42 | 0.90±0.64 |
| Sham 30 days | 10 | 4.70±3.30 | 0.80±0.79 | 0.65±0.31 |
| Model 10 days | 10 | 19.00±5.62a | 2.60±0.70b | 14.00±4.27b |
| Model 30 days | 10 | 50.60±44.33b,c | 3.00±1.05b | 49.00±44.59b,c |
 | Table IIMemory performance in the step-down
avoidance test. |
Table II
Memory performance in the step-down
avoidance test.
| Group | n | Incubation period
(sec) | Number of errors | Electric shock time
(sec) |
|---|
| Sham 10 days | 10 | 230.31±98.20 | 0.38±0.23 | 0.35±0.47 |
| Sham 30 days | 10 | 227.10±105.20 | 0.40±0.52 | 0.29±0.33 |
| Model 10 days | 10 | 41.7±33.27a | 2.90±0.88a | 6.20±3.77a |
| Model 30 days | 10 | 15.10±16.74a,b | 3.20±0.79a | 9.60±2.99a |
Water maze test
The learning time and number of errors in the
learning and memory periods in the model group 10 days following
surgery were significantly increased compared with those in the
sham group (P<0.01), and the increases were more pronounced 30
days following surgery (P<0.001). A comparison of the results
for the model group 10 days and 30 days following surgery showed
that the number of errors in the learning period, and the entire
swim time and number of errors in the memory period increased
markedly at 30 days (P<0.05), and the entire swim time in the
learning period was significantly higher 30 days after surgery
(P<0.01). This indicates that the mice in the model group
developed a severe spatial resolution disorder, and the extent of
the disorder increased with time following the surgery (Tables III and IV).
 | Table IIILearning performance in the water maze
test. |
Table III
Learning performance in the water maze
test.
| Group | n | Entire swim time
(sec) | Number of errors |
|---|
| Sham 10 days | 10 | 93.20±42.80 | 5.88±2.18 |
| Sham 30 days | 10 | 90.12±34.69 | 6.10±3.21 |
| Model 10 days | 10 | 187.40±76.80a | 20.90±8.86a |
| Model 30 days | 10 | 270.70±38.79b,d | 29.40±5.44b,c |
 | Table IVMemory performance in the water maze
test. |
Table IV
Memory performance in the water maze
test.
| Group | n | Entire swim time
(sec) | Number of errors |
|---|
| Sham 10 days | 10 | 93.00±42.35 | 10.50±6.85 |
| Sham 30 days | 10 | 92.54±52.60 | 6.50±3.18 |
| Model 10 days | 10 | 228.80±65.61a | 22.50±6.57a |
| Model 30 days | 10 | 269.80±28.26b,c | 33.10±6.77b,c |
Morphological observations
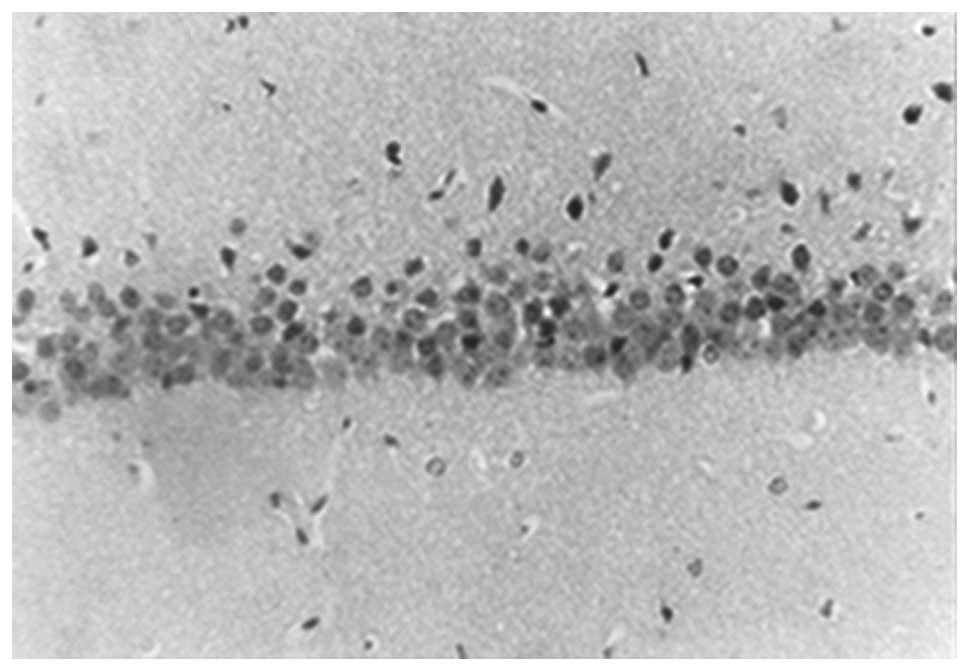
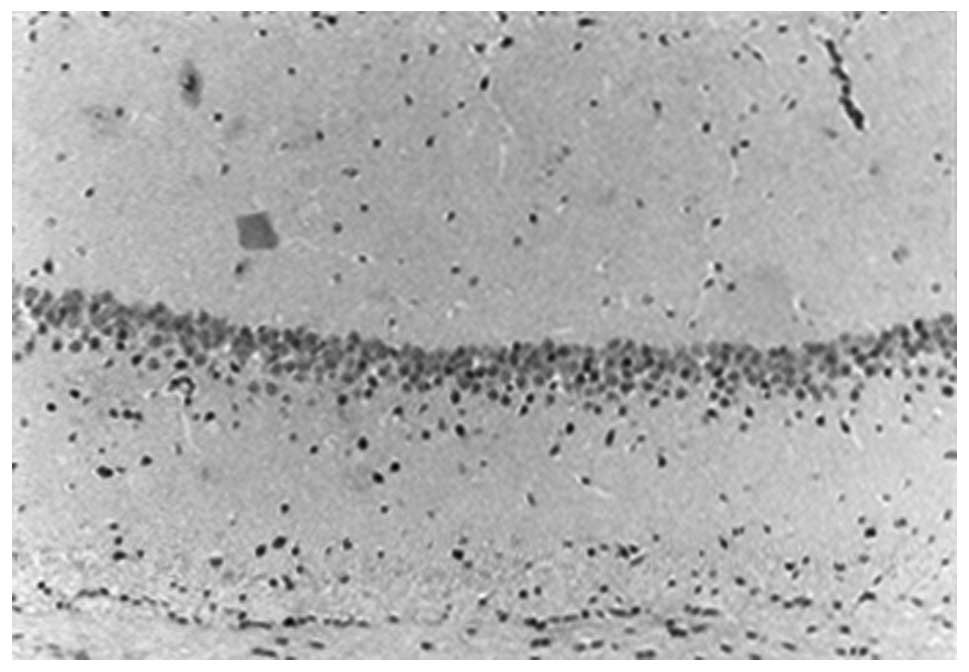
In the sham group, large numbers of hippocampal CA1
neurons were observed, which were tightly and neatly packed, with
rounded nuclei, prominent nucleoli and uniform chromatin. The
number of neurons 10 days and 30 days following surgery showed no
morphological difference (Figs. 1
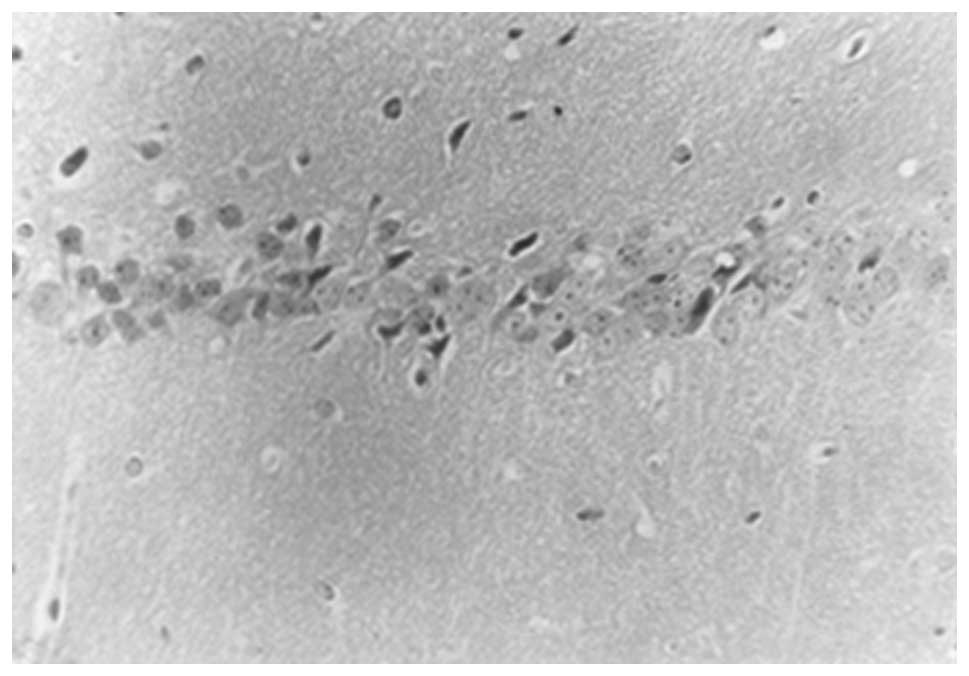
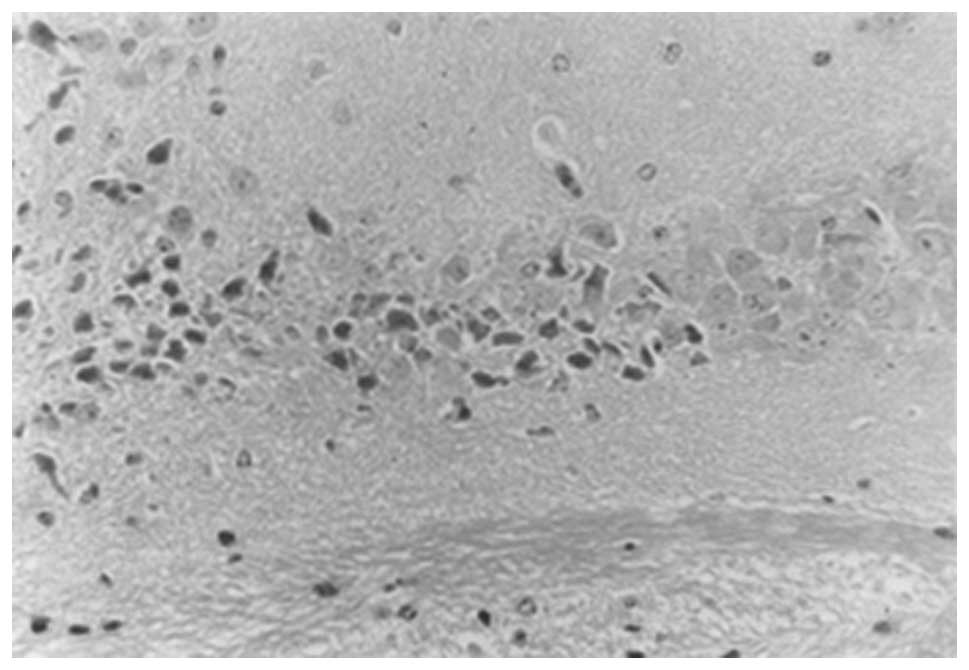
and 2). However, in the model
group, 10 days following surgery, the hippocampal CA1 neurons were
reduced in number and showed a disorganized arrangement. The nerve
nuclei were deeply stained and pyknotic, with large irregularly
shaped glial cells. The number of hippocampal CA1 neurons was
significantly reduced after 30 days compared with the number at 10
days, with an irregular arrangement. In addition, an increase in
the number of glial cells was observed, which were strongly stained
and irregular in shape. Only a small number of normal neurons were
observed (Figs. 3 and 4).
Discussion
With life expectancy increasing every decade,
dementia is a growing problem. Dementia has been a major cause of
morbidity worldwide, and the total number of patients with dementia
is 50 million, with 10 million in China; however, 35 million are
thought to have not yet been diagnosed (5). Pharmacological treatments to delay
the progression of cognitive impairments are modestly successful
and novel therapies are required to improve the cognitive deficits
associated with dementia (6).
Memory and cognitive dysfunction, including VaD, are
usually caused by ischemic and hypoxic damage to the brain.
Previous studies have found that the pathogenesis of VaD is due to
delayed neuronal death in the hippocampal CA1 area, and repeated
cerebral ischemia is an important cause of the development of VaD.
In the present study, repeated cerebral IR combined with blood
pressure reduction was performed to establish the mental
retardation model of behavioral changes in mice. The learning and
memory function of these model animals was severely impaired, which
was demonstrated by the results from the step-down avoidance and
water maze behavioral tests. In addition, the number of neurons in
the hippocampal CA1 area of these mice was observed to be
significantly reduced by light microscopy, indicating that the
vascular dementia animal model was established.
At present, rat models are commonly used to
investigate vascular dementia; however, there is a lack of unified
and recognized methods for the establishment of rat models. A
number of different methods have been reported to establish VaD
cerebral ischemia models. i) Bilateral carotid artery ligation
model (7): Rats subjected to
bilateral common carotid artery occlusion or 2-vessel occlusion
have been used as animal models of subcortical ischemic vascular
dementia (8). The therapeutic
potential of certain drugs has also been evaluated following their
administration to rats with experimental VaD, established by
permanent bilateral common carotid artery occlusion (BCCAO)
(9). ii) In another study, 6-month
old rats were exposed to a diet rich in saturated fats and sucrose,
and chronic BCCAO or sham surgery was performed (6). iii) Chronically hypertensive (for
example, stroke-prone spontaneously hypertensive rats) exhibit
certain features of VaD (10). iv)
Numerous studies regarding ischemic brain damage in gerbils have
been reported; however, studies on neuronal damage associated with
various durations of IR are limited (11). In one study, a VaD model was
established in gerbils that had been subjected to a transient
cerebral ischemia, caused by 5 or 10 min occlusion of the bilateral
carotid arteries, and the changes in the electrophysiological
properties of hippocampal CA1 neurons seen 10 days following the
10-min cerebral ischemia were found to contribute to the impairment
of spatial learning of the gerbils observed at this time (12). Furthermore, it has been identified
that AMPK is transiently phosphorylated following forebrain
ischemia in this model (13). v)
Cerebral artery occlusion model (14): The animals undergo transorbital
occlusion of the middle cerebral artery. vi) In another study,
intercellular astrocytic Ca2+ waves were established by
photochemistry in a mouse model of familial Alzheimer’s disease
(15). Animal models of multiple
ischemic lesions due to intra-vascular emboli (in rodents, rabbits
or primates) have been established for dementia (16). Rat models of dementia established
by intracerebroventricular injection of streptozotocin, and
inhibition of brain mitochondrial cytochrome oxidase by sodium
azide (17). These models have a
number of disadvantages, including transient reversible learning
and memory dysfunction (18), a
craniotomy, high trauma, high animal mortality and being difficult
to carry out (19).
To overcome the shortcomings of the aforementioned
methods for the establishment of an animal model of VaD, including
technical complexity, surgical trauma, high mortality and high
production costs, in the present study a mouse model of VaD was
established. This mouse model of cerebral ischemia may be used in
behavioral observations, studies for the evaluation of drug
efficacy, and screening experiments, and provides a valuable basis
for the investigation of VaD. The model was successfully
established in the present study. This mouse model has a number of
advantages, including a low cost, adequate sources that are easy to
rear, high survival rates, a simple modeling process, high success
rate, low mortality, good reproducibility and marked pathological
changes. Therefore, this model is worthy of application, and
provides a foundation for further studies of the pathogenesis and
drug and clinical treatment of VaD.
The mouse genome is only 80% homologous to that of
humans, and dementia is closely associated with the age of humans.
Although this study was limited to VaD in scope, the blood pressure
of the mice was not detected after modeling. The pathology results
confirmed the presence of dementia-like symptoms; however, this
does not guarantee that all the model forms of VaD were consistent
and comparable. Therefore, further studies are required in order to
improve the repeatability of the model.
References
|
1
|
Kravitz E, Schmeidler J and Beeri MS:
Cognitive decline and dementia in the oldest-old. Rambam Maimonides
Med J. 3:e00262012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Blom K, Emmelot-Vonk MH and Koek HD: The
influence of vascular risk factors on cognitive decline in patients
with dementia: a systematic review. Maturitas. 76:113–117. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Honda Y, Meguro K, Meguro M and Akanuma K:
Social withdrawal of persons with vascular dementia associated with
disturbance of basic daily activities, apathy, and impaired social
judgment. Care Manag J. 14:108–113. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Micanovic C and Pal S: The diagnostic
utility of EEG in early-onset dementia: a systematic review of the
literature with narrative analysis. J Neural Transm. 121:59–69.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Du J, Ma M, Zhao Q, et al: Mitochondrial
bioenergetic deficits in the hippocampi of rats with chronic
ischemia-induced vascular dementia. Neuroscience. 231:345–352.
2013. View Article : Google Scholar
|
|
6
|
Langdon KD, Granter-Button S, Harley CW,
Moody-Corbett F, Peeling J and Corbett D: Cognitive rehabilitation
reduces cognitive impairment and normalizes hippocampal CA1
architecture in a rat model of vascular dementia. J Cereb Blood
Flow Metab. 33:872–879. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kirino T: Delayed neuronal death in the
gerbil hippocampus following ischemia. Brain Res. 239:57–69. 1982.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kitamura A, Fujita Y, Oishi N, et al:
Selective white matter abnormalities in a novel rat model of
vascular dementia. Neurobiol Aging. 33:e25–e35. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Stasiak A, Mussur M, Unzeta M, Samadi A,
Marco-Contelles JL and Fogel WA: Effects of novel monoamine
oxidases and cholinesterases targeting compounds on brain
neurotransmitters and behavior in rat model of vascular dementia.
Curr Pharm Des. 20:161–171. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hainsworth AH, Brittain JF and Khatun H:
Pre-clinical models of human cerebral small vessel disease: utility
for clinical application. J Neurol Sci. 322:237–240. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lee JC, Ahn JH, Lee DH, et al: Neuronal
damage and gliosis in the somatosensory cortex induced by various
durations of transient cerebral ischemia in gerbils. Brain Res.
1510:78–88. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Li J, Sasaki H, Fujiwara H, Kato H, Kaneko
K, Yamazaki Y and Fujii S: Synaptic plasticity in hippocampal CA1
neurons and learning behavior in transient ischemia-loaded gerbils.
Biomed Res. 34:75–85. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Nam HG, Kim W, Yoo DY, et al:
Chronological changes and effects of AMP-activated kinase in the
hippocampal CA1 region after transient forebrain ischemia in
gerbils. Neurol Res. 35:395–405. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Steinberg GR, Gelb AW, Lam AM, et al:
Correlation between somatosensory evoked potentials and neuronal
ischemic changes following middle cerebral artery occlusion.
Stroke. 17:1193–1197. 1986. View Article : Google Scholar
|
|
15
|
Crowe SE, Kantevari S and Ellis-Davies GC:
Photochemically initiated intracellular astrocytic calcium waves in
living mice using two-photon uncaging of IP(3). ACS Chem Neurosci.
1:575–585. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Jiwa NS, Garrard P and Hainsworth AH:
Experimental models of vascular dementia and vascular cognitive
impairment: a systematic review. J Neurochem. 115:814–828. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Weinstock M and Shoham S: Rat models of
dementia based on reductions in regional glucose metabolism,
cerebral blood flow and cytochrome oxidase activity. J Neural
Transm. 111:347–366. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Breunig JJ, Guillot-Sestier MV and Town T:
Brain injury, neuroinflammation and Alzheimer’s disease. Front
Aging Neurosci. 5:262013.
|
|
19
|
Li WZ, Wu WY, Huang H, Wu YY and Yin YY:
Protective effect of bilobalide on learning and memory impairment
in rats with vascular dementia. Mol Med Rep. 8:935–941.
2013.PubMed/NCBI
|