Introduction
Oxidative stress is the cause of numerous diseases.
It has previously been shown that oxidative stress has an important
role in the pathogenesis of acute pancreatitis (1). Furthermore, it has been demonstrated
that chronic inflammation may produce excessive reactive oxygen
species and a large number of free radicals, which induce oxidative
damage to cells (2).
Growing cell lines in three dimensional culture
reduces the complexity of the in vivo state and enables the
manipulation of culture conditions and functions. The functions of
the cultured cells depend on the cytoskeleton, and integrins
provide a structural link between extracellular matrix proteins and
the actin cytoskeleton. In the classical two-dimensional culture
systems, cell culture is a type of plate culture. Cultured cells
with extracellular matrix constitute the overall environment;
therefore, there are big differences in the biological
characteristics from in vivo cells. The morphological
features are also changed. Application of a three-dimensional
culture system provides the basis for the spatial structure and
growth of cells. In the present culture system, Matrigel™ was used
as the culture medium. A three-dimensional culture system plays an
important role in regulating cell growth, differentiation and
migration.
In the present study, a three-dimensional model of
cultured pancreatic epithelial cells was constructed, and the cells
were stimulated with H2O2. Morphological
changes in the pancreatic epithelial cells in the two- and
three-dimensional culture systems following
H2O2 treatment were observed. Using the
three-dimensional culture model, it was investigated whether
pancreatic epithelial cells exhibited cytoskeleton reorganization
in response to H2O2.
Materials and methods
Cell culture
The AR42J pancreatic epithelial cell line and Panco2
pancreatic cancer cell line were obtained from the China Center for
Type Culture Collection (Wuhan, China). Cells were maintained at
37°C in complete Dulbecco’s minimum essential medium (DMEM) in an
atmosphere of 5% CO2.
Three-dimensional cell culture model
Matrigel was used to construct a three-dimensional
culture system as follows: 2 ml Matrigel was placed into a culture
dish, and 0.3 ml 10X DMEM and 0.25 ml calf serum with AR42J or
Panco2 cells were then added to the culture dish, and the cell
density was maintained at ~2.5×105. All procedures were
performed on ice. The culture dish was placed in an incubator once
the gel had formed.
Study design
H2O2 was diluted to 1 μmol/l
or 200 μmol/l respectively. When cells reached confluence,
H2O2 was added. The cultured cells were
incubated with 1 or 200 μmol/l H2O2 for 15
min, 1 h and 4 h. Stimulated cells were compared with unstimulated
controls at each time-point. Following incubation, β-actin and
α-tubulin cytoskeletal changes and nuclear factor
κ-light-chain-enhancer of activated B cells (NF-κB) expression
levels were assessed. The cytoskeletal changes were detected using
laser scanning confocal microscopy (LSCM) (LSM 510 META; Carl Zeiss
AG, Oberkochen, Germany).
Fluorescence microscopy
Panco2 and AR42J cells were grown on cover slips.
Following fixation, the cells were blocked in phosphate-buffered
saline and 0.1% Triton X-100 supplemented with 10% fetal calf serum
for 1 h. Monoclonal mouse anti-human antibodies against β-actin
(300 μl, 1:250; Santa Cruz Biotechnology, Inc., Santa Cruz, CA,
USA) or α-tubulin (300 μl, 1:20; Santa Cruz Biotechnology, Inc.)
were added and then incubated in a humid chamber for 16 h. The
cells were subsequently observed using LSCM.
Analysis of NF-κB expression
Cytosolic and nuclear fractions of the AR42J
pancreatic epithelial and Panco2 pancreatic cancer cell lines were
isolated. The protein content of these cell fractions was analyzed
using the Bradford method. The cell fractions were added to a
96-well plate containing a consensus-binding site of
oligonucleotides for NF-κB. The NF-κB expression was detected by
ELISA, in which NF-κB was captured by a double-stranded
oligonucleotide probe containing the consensus-binding sequence for
NF-κB. The binding of NF-κB to its consensus sequence was detected
using a primary anti-NF-κB antibody (Santa Cruz Biotechnology,
Inc.), followed by a secondary antibody conjugated to horseradish
peroxidase.
Statistical analysis
Data were analyzed using SPSS version 12.0 (SPSS,
Inc., Chicago, IL, USA). Each treatment group had a sample size of
six. Differences between treatment groups were estimated using
Dunnett’s multiple range test. P<0.05 was considered to indicate
a statistically significant difference.
Results
Two-dimensional culture
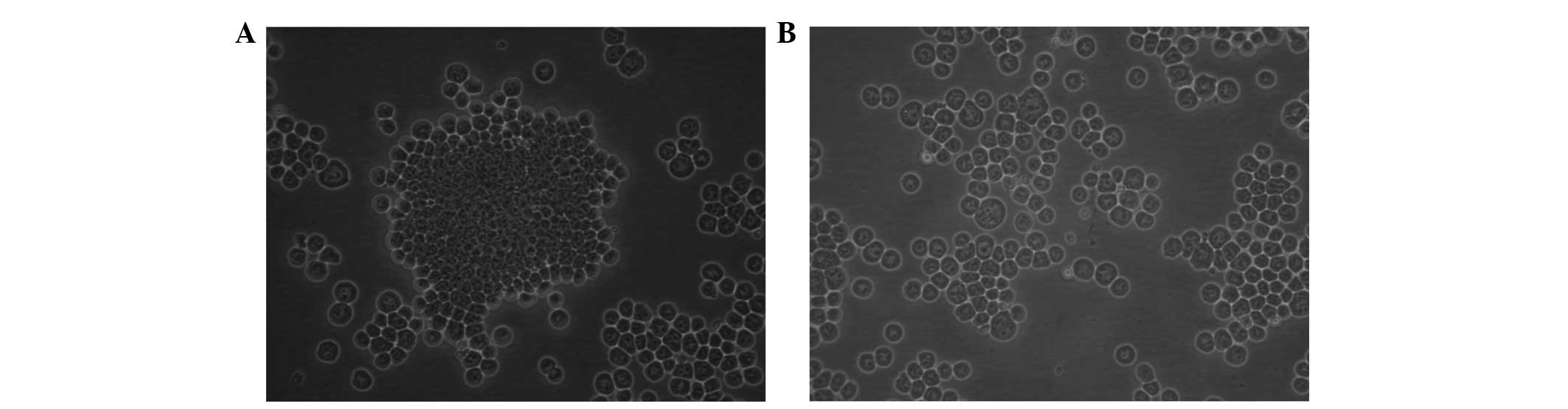
The morphological changes in the normal AR42J
pancreatic epithelial cells cultured in the two-dimensional system
and treated with 200 μmol/l H2O2 for 15 min
were observed. It was found that cells grew to confluent
monolayers; however, following H2O2 treatment
the cells were dispersed and reduced in size (Fig. 1).
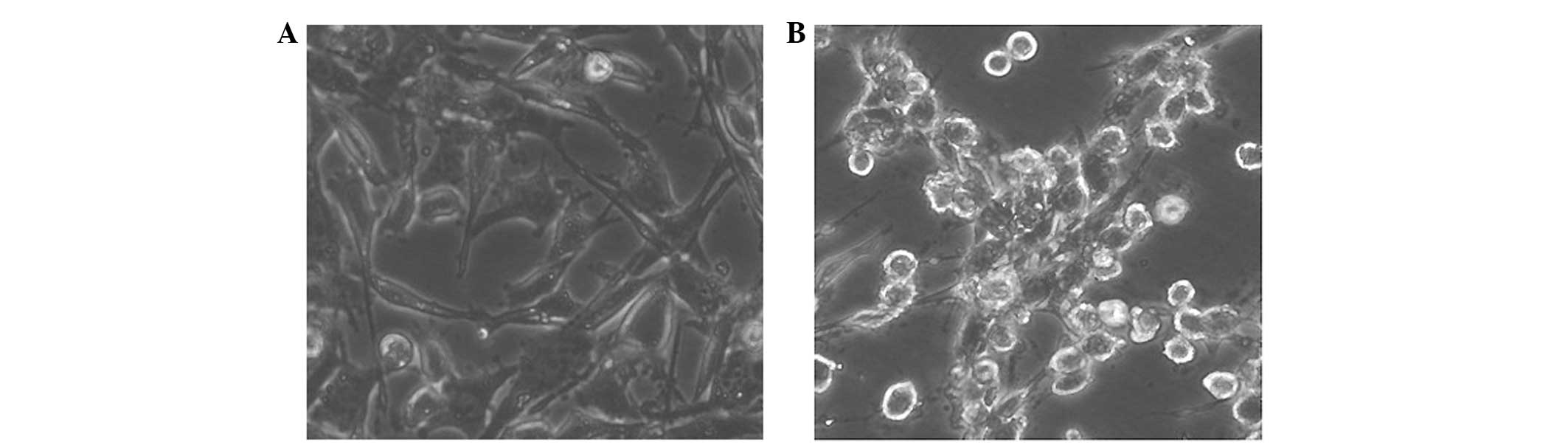
In addition, the cytoskeletal changes in the normal
AR42J pancreatic epithelial cells cultured in the two-dimensional
system and treated with H2O2 were analyzed.
It was observed that H2O2 induced the
rearrangement of the cytoskeleton. Following 15 min incubation with
200 μmol/l H2O2, the cultured cells
contracted, and cell atypia was observed in all cultures after 1 h.
However, 1 μmol/l H2O2 did not induce cell
atypia (Fig. 2).
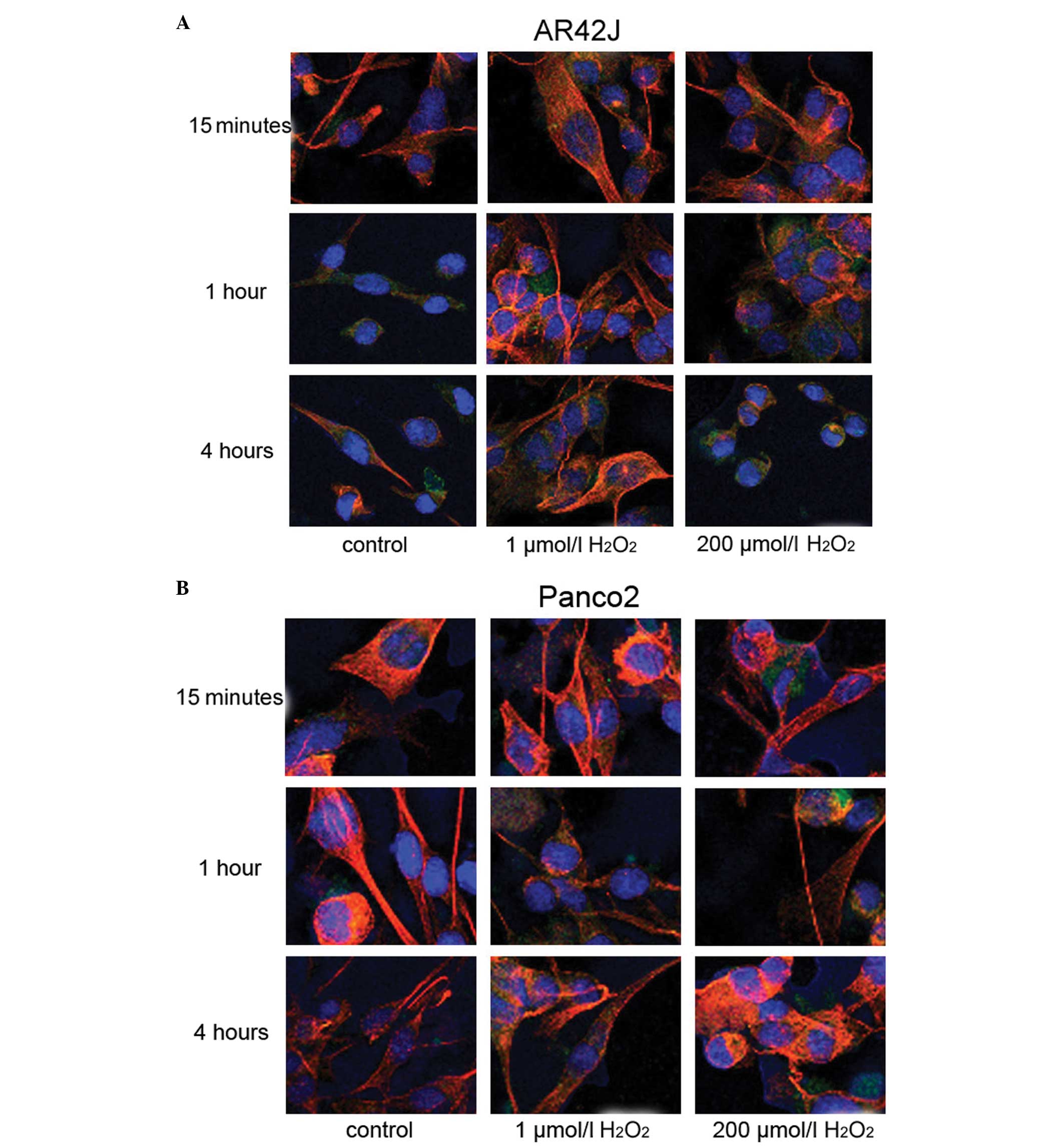
Three-dimensional culture
Cytoskeletal changes in the pancreatic epithelial
cells cultured in the three-dimensional system were observed. In
the untreated cells, the actin cytoskeleton (green) appeared as a
dense network of irregular filaments randomly oriented through the
cytoplasm. Tubulin filaments (red) appeared thinner and better
defined compared with actin, running primarily along the cellular
long axis. Cells treated with 1 μmol/l H2O2
did not differ in morphology from those in the control cultures,
regardless of incubation time. Similar observations were noted for
the normal pancreatic epithelial and cancer cells.
When the cells were treated with 200 μmol/l
H2O2 for 15 min, the actin cytoskeleton
showed significant reorganization, as shown in Fig. 3. Additionally, the filamentous
structure of actin and tubulin disappeared in these cells. When the
cells were treated for 1 h, the actin and tubulin filaments
normalized a little. Four hours after H2O2
treatment, the cell cytoskeleton structure became thicker and
coarser than that in the control group. However, 1 μmol/l
H2O2 treatment had no marked effect on the
cytoskeleton.
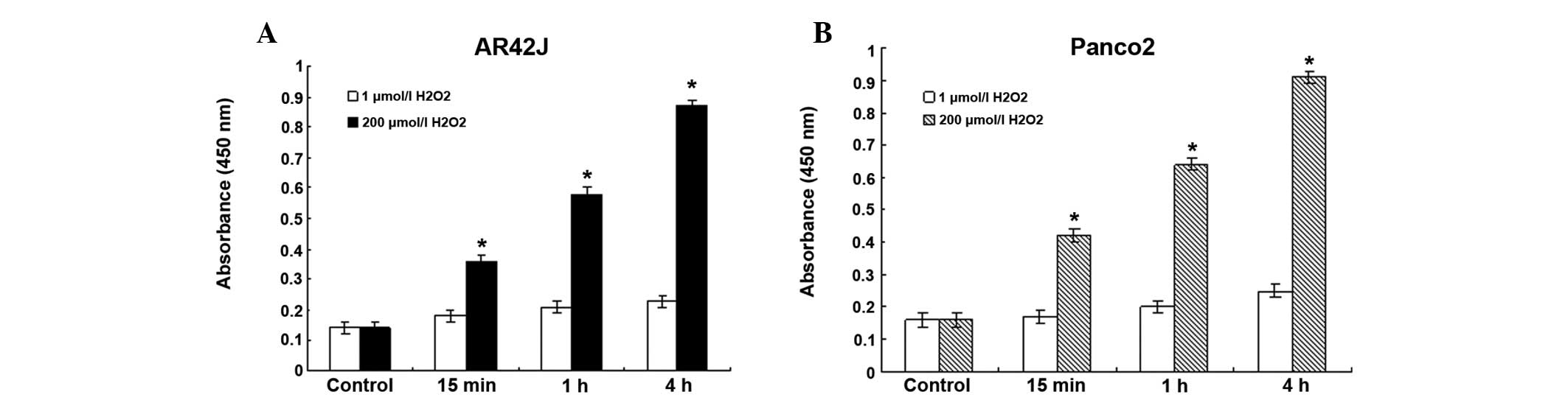
NF-κB expression
As shown in Fig. 4,
when the cells were treated with 1 μmol/l
H2O2, the expression level of NF-κB did not
change significantly. However, treatment with 200 μmol/l
H2O2 significantly increased the level of
NF-κB in pancreatic epithelial cells (P<0.05). When the cells
were treated with 200 μmol/l H2O2, the
expression of NF-κB began to increase 15 min after treatment. After
4 h, the expression level of NF-κB was almost two-fold that of the
level at the 15-min point in the same group.
Discussion
Oxidative stress is the cause of numerous diseases.
It is caused directly or indirectly by the generation of reactive
oxygen species in the body and the imbalance between the pro- and
antioxidants (3). It has been
shown that chronic inflammation may produce excessive reactive
oxygen radicals, which induce oxidative damage to cells (4). Chronic pancreatitis (CP) is a common
disease worldwide. However, the pathogenesis of this disease is not
clear. At present, there is not a specific diagnostic method and
effective treatment for CP. Therefore, the pathogenesis of CP, and
its clinical and pathological diagnosis are of particular
importance.
Oxidative stress may cause epithelial cell
dysfunction or death (5).
H2O2 has been found to affect epithelial cell
function by activating certain redox-sensitive transcription
factors (6) or protein kinase C
translocation (7). Low doses of
H2O2 can induce actin rearrangement. However,
the effects of H2O2 on other components of
the cytoskeleton have yet to be elucidated (8). In the present study, the effect of
different exposure times and concentrations of
H2O2 on the cytoskeleton and morphology of
pancreatic epithelial cells was investigated. Cultured AR42J
pancreatic epithelial and Panco2 pancreatic cancer cells were fixed
at different time-points (15 min, 1 h and 4 h) after stimulation
with H2O2, and stained with anti-tubulin and
anti-actin antibodies.
Pancreatic carcinoma is characterized by its
aggressive local invasion of adjacent structures. At the time of
first diagnosis, only 10–20% of cases are eligible for the
potentially curative Whipple’s procedure. Furthermore, pancreatic
cancer is relatively resistant to chemotherapy and radiotherapy.
Therefore, an enhanced understanding of the biological role of the
genotypic changes that occur during pancreatic carcinogenesis may
provide novel ideas for the development of strategies to prevent
and treat this disease. It is already known that persistent
inflammation may favor the malignant transformation of pancreatic
ductal cells, leading to dysplasia and, ultimately, cancer. In
patients with CP, the acinar injury may recur and lead to repeated
inflammation with the continuous infiltration of inflammatory
cells, eventually leading to atrophy and fibrosis (9). The risk of developing pancreatic
cancer in patients with hereditary pancreatitis is 53-fold that of
the risk in unaffected individuals, which is higher than the risk
noted with numerous other inflammatory diseases (10). Therefore, the aim of the present
study was to determine the impact of oxidative stress on pancreatic
epithelial and pancreatic cancer cells, and to investigate the
association between oxidative stress and pancreatic cancer.
The establishment and maintenance of epithelial cell
polarity is important for the normal function of certain organs
(11). The formation and
maintenance of cell polarity depends on cell-cell and cell-matrix
contacts, such as junction complexes, focal adhesions and the
cytoskeleton (12). The
cytoskeleton is important for cell polarity. A number of
cytoskeletal factors affect cell shape and polarity.
In the classical two-dimensional culture system,
cells are grown in a flask with a flat bottom. The extracellular
matrix is different from that of the in vivo environment,
which results in different biological characteristics. Therefore,
this type of culture method does not reflect the in vivo
status. The biological and morphological characteristics of these
cultured cells are changed. The three-dimensional culture system
uses Matrigel to constitute the three-dimensional extracellular
matrix. This extracellular matrix provides space and mechanical
structure for cell growth, and has an important role in regulating
cell migration and differentiation (13).
In the present study, Matrigel was used to construct
a three-dimensional culture model of pancreatic epithelial cells.
H2O2 was used to mimic in vivo
oxidative stress. The effect of reactive oxygen species on the
cytoskeletal and morphological changes in pancreatic epithelial
cells cultured in the three-dimensional system were observed. The
results demonstrated that oxidative stress induces the early onset
of reversible cell contraction and cytoskeleton depolarization in
pancreatic epithelial cells, together with increased activity of
NF-κB.
The cytoskeleton consists of microfilaments,
microtubules and intermediate filaments, all of which are important
in the maintenance of cell shape. Changes in these filaments allow
rapid changes in the cell three-dimensional structure (14). Microfilaments are the contractile
protein actin in monomeric and filamentous form (15). Microtubules are essential for cell
division and intracellular transport. They are composed of tubulin
subunits together with ancillary microtubule-associated proteins
(16). NF-κB is a ubiquitous
transcriptional factor, which regulates the expression of
cytokines, growth factors and cell adhesion molecules. NF-κB can be
activated by cytokines, free radicals, inhaled particles,
ultraviolet radiation and the products of certain bacteria and
viruses. The abnormal activation of NF-κB is correlated with a
number of autoimmune inflammatory diseases, including arthritis,
pulmonary fibrosis and tumors (17). In the present study it was found
that oxidative stress in pancreatic epithelial cells increased the
expression of NF-κB, and the expression levels were consistent with
the changes in the cytoskeleton, suggesting that there is a link
between NF-κB and the cytoskeleton.
In an experimental pancreatic cancer model,
N-nitrosobis(2-oxopropyl)amine has been shown to induce an increase
in lipoperoxides and a reduction in antioxidants in the pancreas.
This indicates that there is a close association between oxidative
stress and pancreatic cancer (18). The present study suggests that
reactive oxygen species may induce the destruction of the
cytoskeleton in epithelial cells, followed by the activation of
NF-κB, which is important for the progression of pancreatic cancer.
These results may provide evidence to clarify the association
between oxidative stress and pancreatic cancer.
Acknowledgements
This study was supported by a grant from the China
Postdoctoral Science Foundation (no. 20070420151) and the Natural
Science Foundation of Hubei Province (no. 2012FFB04419).
References
|
1
|
Schulz HU, Niederau C, Klonowski-Stumpe H,
et al: Oxidative stress in acute pancreatitis.
Hepatogastroenterology. 46:2736–2750. 1999.PubMed/NCBI
|
|
2
|
Verlaan M, Roelofs HM, van-Schaik A, et
al: Assessment of oxidative stress in chronic pancreatitis
patients. World J Gastroenterol. 12:5705–5710. 2006.PubMed/NCBI
|
|
3
|
Li J, Lee JM and Johnson JA: Microarray
analysis reveals an antioxidant responsive element-driven gene set
involved in conferring protection from an oxidative stress-induced
apoptosis in IMR-32 cells. J Biol Chem. 277:388–394. 2002.
View Article : Google Scholar
|
|
4
|
Dandrea T, Hellmold H, Jonsson C, et al:
The transcriptosomal response of human A549 lung cells to a
hydrogen peroxide-generating system: relationship to DNA damage,
cell cycle arrest, and caspase activation. Free Radic Biol Med.
36:881–896. 2004. View Article : Google Scholar
|
|
5
|
Katsube T, Tsuji H and Onoda M: Nitric
oxide attenuates hydrogen peroxide-induced barrier disruption and
protein tyrosine phosphorylation in monolayers of intestinal
epithelial cell. Biochim Biophys Acta. 1773:794–803. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tudek B, Winczura A, Janik J, et al:
Involvement of oxidatively damaged DNA and repair in cancer
development and aging. Am J Transl Res. 2:254–284. 2010.PubMed/NCBI
|
|
7
|
Jałoszyński P, Jaruga P, Oliński R, et al:
Oxidative DNA base modifications and polycyclic aromatic
hydrocarbon DNA adducts in squamous cell carcinoma of larynx. Free
Radic Res. 37:231–240. 2003.PubMed/NCBI
|
|
8
|
Lim JW, Song JY, Seo JY, et al: Role of
pancreatitis-associated protein 1 on oxidative stress-induced cell
death of pancreatic acinar cells. Ann NY Acad Sci. 1171:545–548.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Weber H, Hühns S, Jonas L, et al: Hydrogen
peroxide-induced activation of defense mechanisms against oxidative
stress in rat pancreatic acinar AR42J. Free Radic Biol Med.
42:830–841. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Etemad B and Whitcomb DC: Chronic
pancreatitis: diagnosis, classification, and new genetic
developments. Gastroenterology. 120:682–707. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Weber H, Hühns S, Lüthen F and Joanas L:
Calpain-mediated breakdown of cytoskeletal proteins contributes to
cholecystokinin-induced damage of rat pancreatic acini. Int J Exp
Pathol. 90:387–399. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Banan A, Choudhary S, Zhang Y, et al:
Oxidant-induced intestinal barrier disruption and its prevention by
growth factors in a human colonic cell line: role of the
microtubule cytoskeleton. Free Radic Biol Med. 28:727–738. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Fatokun AA, Stone TW and Smith RA:
Hydrogen peroxide-induced oxidative stress in MC3T3-E1 cells: The
effects of glutamate and protection by purines. Bone. 39:542–551.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Valen G, Sondén A, Vaage J, et al:
Hydrogen peroxide induces endothelial cell atypia and cytoskeleton
depolymerization. Free Radic Biol Med. 26:1480–1488. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bijlsma MF, Borensztajn KS, Roelink H, et
al: Sonic hedgehog induces transcription-independent cytoskeletal
rearrangement and migration regulated by arachidonate metabolites.
Cell Signal. 19:2596–2604. 2007. View Article : Google Scholar
|
|
16
|
Banan A, Keshavarzian A, Zhang L, et al:
NF-kappaB activation as a key mechanism in ethanol-induced
disruption of the F-actin cytoskeleton and monolayer barrier
integrity in intestinal epithelium. Alcohol. 41:447–460. 2007.
View Article : Google Scholar
|
|
17
|
Nakashima H, Nakamura M, Yamaguchi H, et
al: Nuclear factor-kappaB contributes to hedgehog signaling pathway
activation through sonic hedgehog induction in pancreatic cancer.
Cancer Res. 66:7041–7049. 2006. View Article : Google Scholar
|
|
18
|
Arjona-Sánchez A, Ruiz-Rabelo J, Perea MD,
et al: Effects of capecitabine and celecoxib in experimental
pancreatic cancer. Pancreatology. 10:641–647. 2010.PubMed/NCBI
|