Introduction
Vaginal childbirth can cause pelvic injury, which
can lead to pelvic-floor dysfunction (PFD) (1). During vaginal delivery, the fetal
head pressure on the pelvic floor results in direct trauma to the
pelvic organs (2). These injuries
can lead to the development of pelvic-floor disorders, including
stress urinary incontinence (SUI) and fecal incontinence (3). The available therapeutic options for
PFD include a variety of methods, such as pelvic-floor muscle
exercise, electrical stimulation, pessaries and surgical therapy.
Although several treatments exist, no current therapy can fully
repair the underlying pathophysiology (4–5).
Recently, cell-based therapy has gained attention as
a potential treatment for PFD, particularly for SUI. Numerous
animal and clinical studies (6–8) have
demonstrated the potential of such therapies to bring about
functional and anatomical improvements. Adipose-derived mesenchymal
stem cells (ASCs) have the same capacity for self-renewal and
differentiation as bone marrow mesenchymal stem cells (BMSCs).
Furthermore, they can be easily harvested by a simple, minimally
invasive method and they are more abundant than BMSCs (9). ASCs possess the ability to facilitate
the synthesis of new collagen and the potential to differentiate
into smooth muscle cells, which constitute the pelvic floor
(10). Based on this, ASCs are
considered as ideal adult stem cells to treat PFD (11); however, the length of their
survival and their distribution in living animals remains
unknown.
The aim of this study was to apply advanced imaging
systems to aid in the development of cell therapy for PFD. The fate
and distribution of ASCs in nude mice were tracked in vivo
in real time following vaginal balloon dilation, synergizing with
non-invasive bioluminescent optical imaging.
Materials and methods
Isolation and culture of rabbit ASCs
All animal procedures were performed under
guidelines approved by the Institutional Animal Care and Use
Committee (Shanghai Jiao Tong University Affiliated Sixth People’s
Hospital, Shanghai, China). ASCs were obtained from adipose tissue
in the inguinal fat pads of a New Zealand white male rabbit (The
Animal Institute, School of Medicine, Shanghai Jiao Tong
University, Shanghai, China). The fat pads were washed three times
in phosphate-buffered saline containing 1% penicillin/streptomycin
(P/S; Gibco Life Technologies, Beijing, China) and then digested
with 5 ml collagenase I (1 mg/ml; Gibco). After 60 min of digestion
in a shaking incubator (BS-4G, Changzhou Feipu Experimental
Instrument Factory, Beijing, China) at 200 rpm and 37°C, 8 ml
high-glucose Dulbecco’s Modified Eagle Medium (DMEM; Gibco), 10%
fetal bovine serum (FBS; Sigma, St Louis, MO, USA) and 1% P/S were
added to terminate digestion. The cells were sieved through a 70-μm
cell strainer (Shanghai Jun Sheng Biological Technology Co., Ltd.,
Shanghai, China) followed by centrifugation at 400 × g for 15 min.
The cell-containing pellets were seeded into a 100-mm2
cell culture dish in the presence of complete media (DMEM + 10% FBS
+ 1% P/S) at 37°C in a 5% CO2 incubator (Thermo Electron
Corporation, Waltham, MA, USA). Media were changed every three days
to remove nonadherent cells and tissue debris.
Lentiviral infection
The lentiviral vectors (SunBio Biotech, Beijing,
China) contained the enhanced green fluorescent protein (eGFP) and
Luciferase (Luc) genes. For infection, the ASCs at passage 3 were
seeded in a six-well cell culture cluster, infected with
concentrated lentivirus particle stock (1×107
transduction U/ml; multiplicity of infection, 50) when 70–80%
confluence was achieved, and then incubated in medium with 10 μg/ml
polybrene (Sigma) for 24 h. The ASCs infected with recombinant
lentivirus were selected and purified by puromycin (1 μg/ml; SunBio
Biotech) and the transduction efficiency of the ASCs was evaluated
by flow cytometry (FACS Calibur™ flow cytometer, BD Biosciences,
Franklin Lanes, NJ, USA).
Cell proliferation assay
Cell viability subsequent to infection was measured
by cell-counting kit (CCK)-8 assays. At the desired time-points,
the (eGFP + Luc)-ASCs at passage 6 were incubated in CCK-8 solution
(Dojindo, Kumamoto, Japan) at 37°C in a 5% CO2 incubator
for 3 h. The absorbance of the supernatants was measured at a
wavelength of 450 nm.
Luc assays
To determine Luc activity in vitro, different
quantities of (eGFP + Luc)-ASCs (10, 100, 500, 1,000, 5,000 and
10,000)/100 μl at passage 6 were seeded onto black, clear-bottom
96-well plates in 100 μl medium (DMEM + 10% FBS + 1% P/S),
respectively. A row of wells was seeded with unlabeled cells as
controls. To every 100 μl culture medium (in a 96-well plate) was
added 15 μl D-luciferin (Sciencelight, Shanghai, China) solution
(150 μl/ml). A highly sensitive, cooled charge-coupled device (CCD)
camera (Xenogen IVIS; Xenogen, Hopkinton, MA, USA) was used to
detect the bioluminescent signals. Quantification of the signals
was performed by the acquisition and analysis software Living Image
(Xenogen).
To establish the correlation between the number of
transplanted cells and the light produced in vivo, serial
dilution of (eGFP + Luc)-ASCs (1×106/100 μl,
1×105/100 μl, 1×104/100 μl,
1×103/100 μl and 1×102/100 μl) were grafted
in the subcutaneous space in the dorsum of each nude mouse. The
animals were intraperitoneally injected with D-luciferin (150
mg/kg) and anesthetized with isoflurane, prior to being directly
imaged by CCD. Quantification of the signals was performed by the
software Living Image (Xenogen).
Vaginal distention (VD) and cell
inoculation
Twenty female BALB/c nude mice (The Animal
Institute, School of Medicine) at five weeks old (16±1.0 g) were
used in this study in accordance with the National Research Council
Guide for the Care and Use of Laboratory Animals. Mice were
anesthetized by intraperitoneal injection of sodium pentobarbital
(30 mg/kg; Shanghai Yuanye Bio-Technology Co., Ltd, Shanghai,
China), prior to undergoing a simulated childbirth injury by VD. A
modified 8-Fr Foley catheter was inserted into the vagina and the
balloon was inflated to 2.5 ml for 4 h. One hour after injury,
1×106 labeled ASCs at passage 9 resuspended in 50 μl
medium were injected into the vaginal submucosa of 10 mice. To act
as a control, a further 10 mice were injected in the same way with
cell-free media.
In vivo non-invasive bioluminescence
imaging (BLI) and image quantification
At 1 h and one, two, three, four, six and eight
weeks after VD, mice were anesthetized (1–3% isoflurane; Science
and Technology Development Co., Ltd., Shenzhen City Rui Bolong,
Guangdong, China), and given the substrate D-luciferin by
intraperitoneal injection at 150 mg/kg. The mice were then placed
in a light-tight camera box with continuous exposure to 1–3%
isoflurane. BLI signals were detected by the IVIS camera system,
integrated, digitized and displayed. Quantification of the signals
in vivo was performed by the software Living Image
(Xenogen).
Histology
To confirm the survival of engrafted ASCs, the
entire vagina, heart, lung and liver were harvested as frozen
sections for histological analysis at 8 weeks after VD. Sections
(4-μm thick) were stained by DAPI, followed by detection of
eGFP-positive cells by fluorescence microscopy (Leica AF6000,
Leica, Wetzlar, Germany).
Statistical analyses
Quantitative values are expressed as the mean ±
standard error of the mean. Regression plots were used to describe
the association between bioluminescence and cell number.
R2 values were reported to assess the quality of the
regression model. P<0.05 was considered to indicate a
statistically significant difference.
Results
Isolation, culture and eGFP expression
efficiency of ASCs
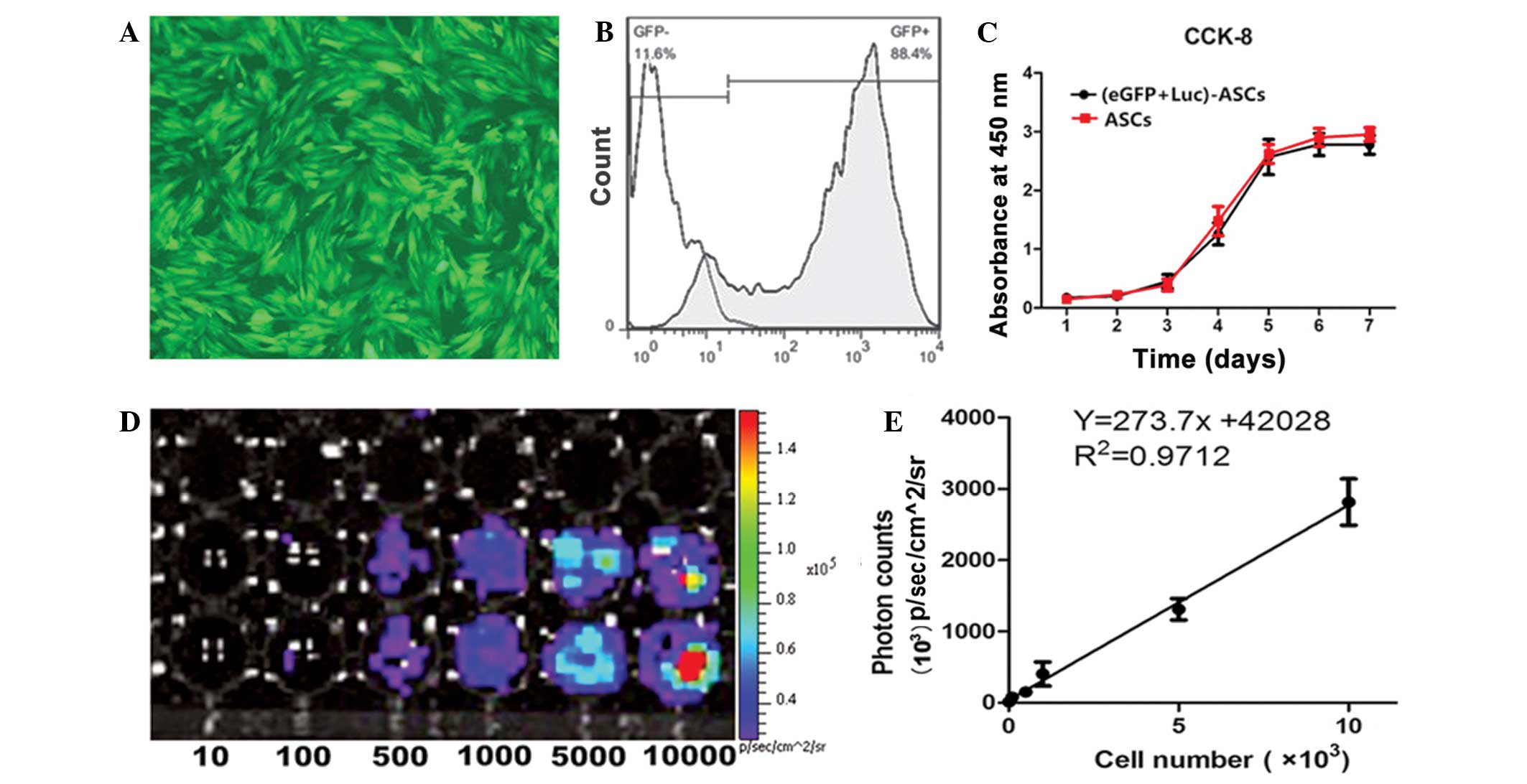
ASCs showed high proliferation rates and adherence
to plastic surfaces. The spindle-shaped adherent cells grew and
reached confluence in 5–7 days. ASCs labeled with eGFP and Luc
genes had a high eGFP expression level and were selected and
purified by puromycin for use in the subsequent experiments
(Fig. 1A). The transduction
efficiency of the ASCs at passage 6 was up to 88.4%, as shown by
flow cytometry (Fig. 1B). The
CCK-8 assay was used to evaluate the viability of the ASCs
following transduction. No significant differences were observed
between the unlabeled and eGFP + Luc-labeled ASCs (Fig. 1C).
Light production capacity of (eGFP +
Luc)-ASCs at passage 6 in vitro
The light production capacity of (eGFP + Luc)-ASCs
at passage 6 in vitro was measured in lysates from
predetermined numbers (10, 100, 500, 1,000, 5,000 and 10,000) of
(eGFP + Luc)-ASCs. A minimum of 100 (eGFP + Luc)-ASCs were required
for cell detection with the imaging system (Fig. 1D). Luc was expressed in
vitro steadily. The slopes of the linear regression plots
showed standard plots of light production versus cell number
(R2=0.9712). The slope of the linear regression plot was
273.7±14.91 for the ASCs (Fig. 1E)
(slope of regression plot = number of relative light units/cell).
The number of (eGFP + Luc)-ASCs was linearly correlated with light
production, indicating that the BLI signals could be used to
quantitatively track labeled ASCs.
In vivo non-invasive imaging for
implanted (eGFP + Luc)-ASCs
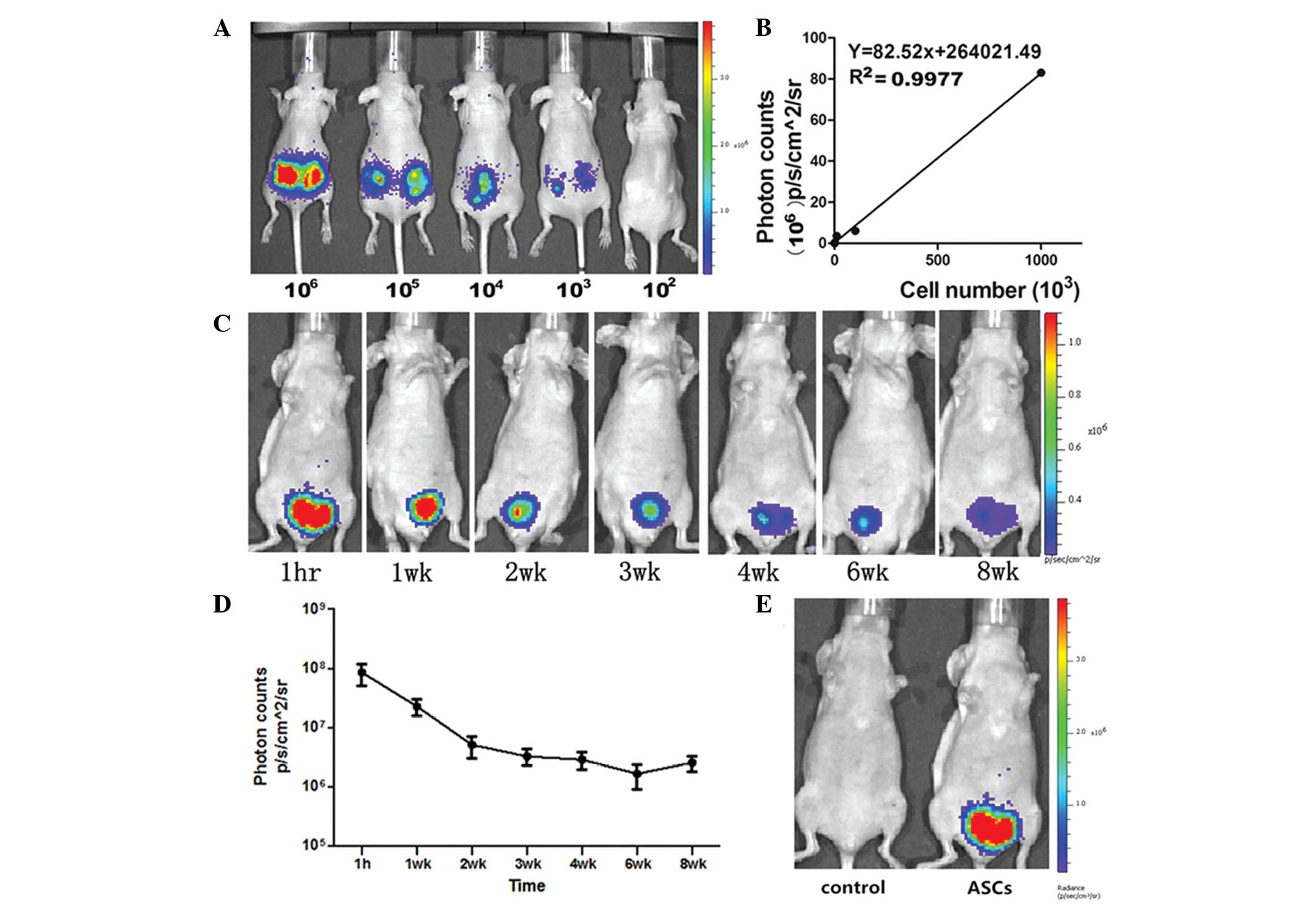
To quantify the implanted (eGFP + Luc)-ASCs
in vivo, five mice were implanted with predetermined numbers
of (eGFP + Luc)-ASCs (1×102, 1×103,
1×104, 1×105 and 1×106) at the
same time to establish the correlation between the number of
transplanted cells and the light produced (Fig. 2A). The slope of the linear
regression plots was 82.52 (R2=0.9977) (Fig. 2B).
Following cell inoculation in the VD mice, the mice
were imaged longitudinally (1 h, one, two, three, four, six and
eight weeks after inoculation) to analyze their light-producing
behavior. The average BLI signals in mice were as follows: 1 h
post-inoculation, (8.49±6.01)x107
p/sec/cm2/sr; 1 week post-inoculation,
(2.26±1.20)x107 p/sec/cm2/sr; 2 weeks
post-inoculation, (5.07±3.51)x106
p/sec/cm2/sr; 3 weeks post-inoculation,
(3.24±1.74)x106 p/sec/cm2/sr; 4 weeks
post-inoculation, (2.85±1.65)x106
p/sec/cm2/sr; 6 weeks post-inoculation,
(1.64±1.28)x106 p/sec/cm2/sr; and 8 weeks
post-inoculation, (2.54±1.33)x106
p/sec/cm2/sr. The BLI signals of the ASCs predominantly
existed at the inoculation site of the pelvic organ, and no visible
signals were observed in other organs such as the lung, heart and
liver. During the first three weeks, BLI signals markedly
decreased. The signals were then observed to stabilize during the
remaining weeks of the experiment. A total of 97% of transplanted
cells were lost at 12 weeks after transplantation, and ~27,621 of
the implanted cells survived according to the linear regression
plot. Visible signals were still observed in mice injected with
(eGFP + Luc)-ASCs by the end of the experiment (Fig. 2C and D). No visible signal was
observed in the control group (Fig.
2E).
Histology
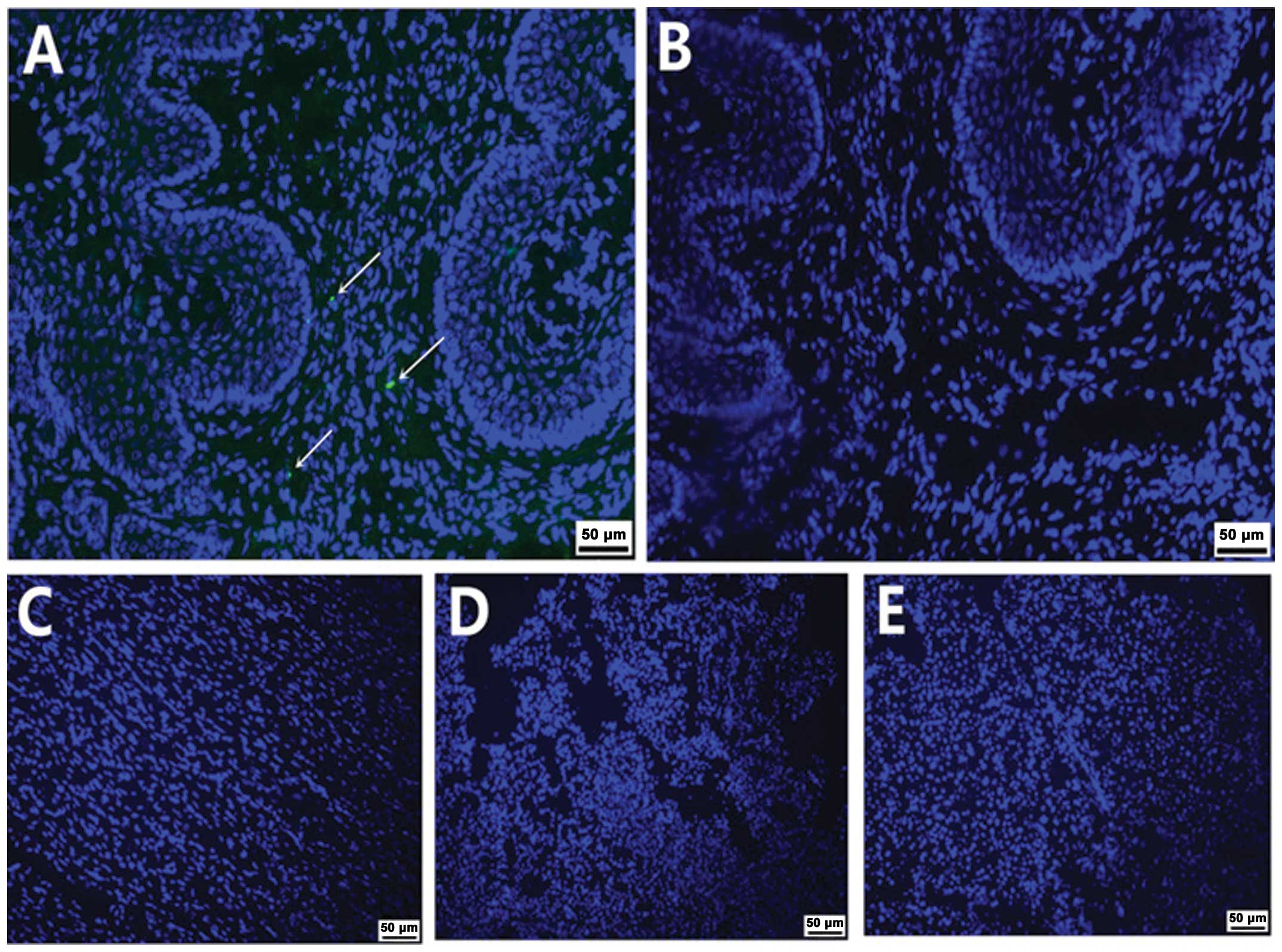
To identify if the implanted cells had survived, the
tissues of the vagina and other organs in the transplantation group
were harvested for DAPI staining at eight weeks after
transplantation. eGFP-positive ASCs were detected at the submucous
layer of the vagina (Fig. 3A). No
eGFP-positive cells were detected in the control group (Fig. 3B) or other organs, such as the
heart, lung and liver (Fig. 3C, D and
E).
Discussion
Cell-based therapy has shown the potential to
improve function and anatomy in PFD; however, no study has been
conducted regarding the survival and distribution of ASCs in
pelvic-floor injury in vivo in real time. In the present
study, a non-invasive BLI-based approach was used to analyze in
vivo the behavior of eGFP + Luc-expressing ASCs in model nude
mice with pelvic-floor injury.
The ability to detect ASCs early on in live animals
and in real time is ideal for accurately monitoring the behavior of
ASCs. There are different methods to track the stem cells in
vivo. Previous studies tracked the transplanted ASCs in
pelvic-floor injury models using bromodeoxyuridine and
CellTracker™CM-Dil (12–14). Although this method is relatively
straightforward, a significant weakness is that the fluorescence
intensity decreases during cellular proliferation in vivo.
Furthermore, the technique cannot monitor the inoculated cells in
real time. The present study monitored the labeled cells implanted
in mice for >8 weeks in vivo in real time by a
non-invasive BLI-based approach. This period was longer than that
in a previous study (8), which may
be attributable to i) the high and stable transduction efficiency
and ii) BLI. Firstly, lentiviral vectors could label both dividing
and non-dividing cells, and the transduction efficiency of the ASCs
was up to 88.4%. Vectors are a high-performance tool for labeling
ASCs in order to detect the majority of the transplanted cells.
Secondly, based on the generation of visible light photons by Luc
reporters introduced into live cells, BLI techniques provide
excellent signal-to-noise ratios due to the low intrinsic
bioluminescence of mammalian tissues. BLI can be used to assess the
viability of stem cells in vivo in real time, facilitating
the development of stem cell therapy.
The transplanted cells encountered mass death within
three weeks after transplantation. This phenomenon was consistent
with a previous report (15) and
may have resulted from i) limited survival space in the
pelvic-floor tissues following injection; ii) cell necrosis; and
iii) inflammation and immune reactions following transplantation,
leading to cell loss. The repair functions of stem cells rely on
the population of stem cells (16). Consequently, it is of great
importance to augment the number of implanted ASCs and diminish
cell loss. Although 97% of transplanted cells were lost, ~27,621
implanted cells survived in the pelvic floor. A previous study also
indicated that the transplantation of ASCs could improve urethral
function (13). It remains unknown
how the transplanted ASCs function, although we propose that the
local injection of ASCs could promote recovery by an atrophic
factor mechanism that modifies cellular and extracellular elements
of pelvic organs.
The BLI signals of ASCs predominantly existed at the
inoculation site of the pelvic organ, and no visible signals were
observed in other organs, such as the lung, heart and liver, which
suggested that the optimal location of ASCs via local
administration is at the injury site. Implanted cells had little or
no tendency to migrate to other organs. The reasons may be that i)
local administration augments the number of transplanted cells at
the injury site; ii) the injury site releases chemokines that can
attract ASCs via a cytokine gradient (17–19);
iii) the long-term survival of stem cells could facilitate
differentiation into vessel cells to maintain cell nutrition and
reduce the cell loss at the inoculation site. Although intravenous
injection is less invasive, a limited number of implanted cells
home to the injury site, and the majority of the cells are held in
the lungs (15). It is therefore
more effective to use local administration than intravenous
administration. Future studies are likely to be focused on the
optimal delivery of the cells.
One potential limitation is that the molecular
mechanisms of the recovery of ASCs have not yet been clearly
elucidated. Further research is required to determine the mechanism
of accelerated recovery of cell-based therapies. Experiments to
solve the growth crisis should be a focus in our future
studies.
In conclusion, bioluminescence imaging could be used
to monitor ASCs in vivo in real time, and the local
administration of ASCs following simulated childbirth injury could
allow the long-term survival of the cells at the inoculating site
despite mass cell death, providing evidence of the potential for
cell-based therapy to treat pelvic-floor injury.
Acknowledgements
The Research Fund of Science and Technology
Commission of Shanghai Municipality (no. 13DZ1941404).
References
|
1
|
Dannecker C and Anthuber C: The effects of
childbirth on the pelvic-floor. J Perinat Med. 28:175–184. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Dietz HP and Wilson PD: Childbirth and
pelvic floor trauma. Best Pract Res Clin Obstet Gynaecol.
19:913–924. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chaliha C: Postpartum pelvic floor trauma.
Curr Opin Obstet Gynecol. 21:474–479. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wilson D, Dornan J, Milsom I, Freeman R,
et al: UR-CHOICE: can we provide mothers-to-be with information
about the risk of future pelvic floor dysfunction? Int Urogynecol
J. 11:1449–1452. 2014. View Article : Google Scholar
|
|
5
|
Hagen S and Stark S: Conservative
prevention and management of pelvic organ prolapse in women.
Cochrane Database Syst Rev. Dec 7–2011.(Epub ahead of print).
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Carr LK, Robert M, Kultgen PL, et al:
Autologous muscle derived cell therapy for stress urinary
incontinence: a prospective, dose ranging study. J Urol.
189:595–601. 2013. View Article : Google Scholar
|
|
7
|
Lin CS and Lue TF: Stem cell therapy for
stress urinary incontinence: a critical review. Stem Cells Dev.
21:834–843. 2012. View Article : Google Scholar :
|
|
8
|
Cruz M, Dissaranan C, Cotleur A, et al:
Pelvic organ distribution of mesenchymal stem cells injected
intravenously after simulated childbirth injury in female rats.
Obstet Gynecol Int. 2012:6129462012. View Article : Google Scholar
|
|
9
|
Sen A, Lea-Currie YR, Sujkowska D, et al:
Adipogenic potential of human adipose derived stromal cells from
multiple donors is heterogeneous. J Cell Biochem. 81:312–319. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Park BS, Jang KA, Sung JH, et al:
Adipose-derived stem cells and their secretory factors as a
promising therapy for skin aging. Dermatol Surg. 34:1323–1326.
2008.PubMed/NCBI
|
|
11
|
Tobita M, Orbay H and Mizuno H:
Adipose-derived stem cells: current findings and future
perspectives. Discov Med. 11:160–170. 2011.PubMed/NCBI
|
|
12
|
Edalatmanesh MA, Bahrami AR, Hosseini E,
Hosseini M and Khatamsaz S: Neuroprotective effects of mesenchymal
stem cell transplantation in animal model of cerebellar
degeneration. Neurol Res. 33:913–920. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lin G, Wang G, Banie L, et al: Treatment
of stress urinary incontinence with adipose tissue-derived stem
cells. Cytotherapy. 12:88–95. 2010. View Article : Google Scholar :
|
|
14
|
Kinebuchi Y, Aizawa N, Imamura T, Ishizuka
O, Igawa Y and Nishizawa O: Autologous bone-marrow-derived
mesenchymal stem cell transplantation into injured rat urethral
sphincter. Int J Urol. 17:359–368. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Vilalta M, Dégano IR, Bagó J, et al:
Biodistribution, long-term survival, and safety of human adipose
tissue-derived mesenchymal stem cells transplanted in nude mice by
high sensitivity non-invasive bioluminescence imaging. Stem Cells
Dev. 17:993–1003. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Mitterberger M, Pinggera GM, Marksteiner
R, et al: Adult stem cell therapy of female stress urinary
incontinence. Eur Urol. 53:169–175. 2008. View Article : Google Scholar
|
|
17
|
Woo LL, Hijaz A, Kuang M, Penn MS, Damaser
MS and Rackley RR: Over expression of stem cell homing cytokines in
urogenital organs following vaginal distention. J Urol.
177:1568–1572. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lenis AT, Kuang M, Woo LL, et al: Impact
of parturition on chemokine homing factor expression in the vaginal
distention model of stress urinary incontinence. J Urol.
189:1588–1594. 2013. View Article : Google Scholar
|
|
19
|
Bernardo ME, Pagliara D and Locatelli F:
Mesenchymal stromal cell therapy: a revolution in Regenerative
Medicine? Bone Marrow Transplant. 47:164–171. 2012. View Article : Google Scholar
|