Introduction
Characterized by gingival inflammation and
periodontal tissue impairment, periodontitis is a serious threat to
the oral health of human beings. In general, patients with
periodontitis suffer from loose teeth due to the bone resorption
without experiencing any pain (1,2).
Over the past decades, a number of therapeutic approaches have been
actively developed to prevent and treat periodontitis, and one of
these potential treatment strategies is to facilitate bone
regeneration via the usage of synthetic scaffolds. With their
desirable osteoconductivity, bioactivity, interconnected porosity
and biocompatibility, several well-prepared scaffolds have been
generated and applied to maintain, induce and restore biological
functions (3,4). As a consequence, studies into the
development of novel scaffolds and associated investigations into
the bio-effects of these scaffolds on disease models are
intensively pursued in the biomedical field.
As a crucial structure in periodontal tissue, the
cementum connects with the alveolar bone via fibers, retains the
position of the teeth and shares a similar composition to bone;
however, under conditions of impairment, the cementum typically
undergoes no or little remodeling due to the high content of
inorganic salt in its structure (5,6). To
regenerate periodontal tissue, cementogenesis has been considered
to be an undividable route that is regulated via various signals
(7). According to previous
studies, wnt signaling may participate in the formation of the root
cementum and is involved in the differentiation and proliferation
of cementoblasts (8–11).
By inhibiting the β-catenin inhibitor glycogen
synthase kinase 3β, lithium ions can activate wnt signals in
vitro and in vivo (3).
Upon a suitable incubation concentration, lithium ions can enhance
the proliferation of human mesenchymal stem cells, resulting in the
formation of osteogenic and adipogenic lineages (12,13).
Furthermore, a suitable combination of calcium phosphate core and
lithium coating can promote the proliferation of MG63 cells and
enhance the protein expression of osteogenic transcription factors
(14,15). In addition to the above in
vitro evidence, the high osteoconductivity of lithium ions has
been equally documented in vivo, and lithium ions have been
demonstrated to enhance bone repair and facilitate bone
regeneration in a murine model (16–18).
Lithium ions have been considered as a possible approach to improve
dental implant osseointegration by facilitating osteoblast
differentiation (19,20). More notably, lithium-doped bioglass
has been shown to significantly enhance the proliferation and
differentiation of periodontal ligament cells upon the activation
of wnt signals (20). In
combination, these findings suggest the potential of lithium-based
materials as agents for bone-related disease, including certain
diseases in the field of dentistry; however, to the best of our
knowledge, the aforementioned studies were only carried out in
natural physical conditions rather than in inflammatory
environments. It is therefore necessary to explore the effect of
lithium-based materials on a disease model, such as periodontitis.
As such, the aim of this study was to investigate the effect of
lithium chloride (LiCl) on cementoblast function, particularly in
the presence of lipopolysaccharide (LPS).
Materials and methods
Preparation of LPS
LPS was purified from Prophyromonas
gingivalis (ATCC 33277; American Type Culture Collection,
Manassas, VA, USA) using the cold MgCl2/ethanol
procedure. The prepared LPS was suspended in LPS-free water at
various experimental concentrations for further use.
Cell culture
An immortalized murine cementoblast cell line
(OCCM-30), which was a gift from Professor Somerman at the
University of Washington (Seattle, WA, USA), was cultured in
accordance with previously described methods (21). The OCCM-30 cells were maintained in
Dulbecco’s Modified Eagle’s Medium/F12 (Invitrogen Life
Technologies, Carlsbad, CA, USA), supplemented with 10% fetal
bovine serum containing 100 U/ml penicillin and 100 μg/ml
streptomycin in a humidified atmosphere of 5% CO2 at
37°C.
Cytotoxicity studies of LPS and LiCl
MTT assays were carried out to quantify the
cytotoxicity of LPS and LiCl (Sigma-Aldrich Trading Co., Ltd.,
Shanghai, China). In a typical procedure, OCCM-30 cells were
cultured in 96-well plates at a density of 5×103 per
well for 12 h to allow the attachment of the cells. Serial
dilutions of LPS and LiCl were then added to separate culture
media. At the end of various incubation periods, the media
containing LPS and LiCl were removed, and the cells were treated
with MTT for a further 4 h. Following MTT treatment, the
supernatant was removed and the formazan crystals were dissolved by
the addition of dimethylsulfoxide (DMSO; MP Biomedicals, LLC, Santa
Ana, CA, USA). The absorbance at 490 nm was measured via a
micro-ELISA reader (iMark 680; Bio-Rad Laboratories, Inc.,
Hercules, CA, USA). Six replicates were performed for each group
and the percentage viability was normalized to the viability of
untreated cells.
Cytotoxicity studies of LiCl in a
periodontitis model
The periodontitis model was prepared using LPS as
the inducing agent, according to a previous route (22). An MTT assay was carried out to
quantify the cytotoxicity of LiCl in the presence of LPS (25
ng/ml). OCCM-30 cells were cultured in 96-well plates at a density
of 5×103 per well for 12 h to allow the attachment of
the cells. A total of 50 μg/ml LiCl was then added to the culture
medium. At the end of the incubation period, the medium containing
LiCl was removed, and the cells were treated with MTT for further 4
h. The supernatant was then removed and the formazan crystals were
dissolved by the addition of DMSO. The absorbance at 490 nm was
measured via a micro-ELISA reader. Six replicates were performed
for each group and the percentage viability was normalized to the
viability of untreated cells.
Examination of alkaline phosphatase (ALP)
activity
OCCM-30 cells at a density of 2×104 were
cultured overnight in a 24-well plate to allow the attachment of
the cells. Following culture, the cells were washed twice with
phosphate-buffered saline (PBS) for 10 sec and then divided into
four treatment groups, as follows: i) Blank group (no additional
agent); ii) LiCl group (treatment with 50 μg/ml LiCl); iii) LPS
group (treatment with 25 ng/ml LPS); and iv) LiCl + LPS group
(treatment with 50 μg/ml LiCl and 25 ng/ml LPS). The three
experimental groups were treated for one week when ALP expression
was prominent. After seven days of incubation, 0.9% NaCl solution
containing 1% Triton X-100 (Roche Diagnostics, Basel, Swirzerland)
was selected to dissolve out the cellular proteins. Centrifugation
was performed at 150 × g for 4 min at room temperature and the
supernatants were further assayed for ALP activity using an ALP
assay kit (Sigma-Aldrich, St. Louis, MO, USA). All results were
normalized to the total protein levels in the OCCM-30 cells.
Mineralization assay with alizarin red
staining
OCCM-30 cells at a density of 1×105 were
cultured in a 24-well plate. Two days later, the medium was removed
and the cells were washed twice with PBS for 10 sec. The cells
(control/LiCl/LPS/LiCl+LPS) were then cultured in osteogenic medium
[5 nM dexamethasone, 250 μM L-ascorbic acid 2-phosphate, 10 mM
β-glycerophosphate (Sigam-Aldrich) in DMEM/F12] at 37°C. After 10
days of incubation, the cells were washed with deionized water
several times and fixed in ice-cold 95% ethanol at room temperature
for 30 min. The cells were then stained with alizarin red (2%, pH
4.2) for 15 min and observed under an Olympus BX-51 optical system
microscope (Olympus Corp., Tokyo, Japan) under white light. To
obtain quantified analysis, cetylpyridinium chloride (1%, 1 ml;
Shenggong Co., Ltd., Shanghai, China) was added to the plates, and
the optical density (OD) values were measured at 540 nm using a
micro ELISA reader (iMark 680; Bio-Rad Laboratories, Inc.).
RNA isolation and reverse
transcription-polymerase chain reaction (RT-PCR)
To determine the levels of mRNA expression, OCCM-30
cells at a density of 5×104 were cultured in a six-well
plate. After 12 h, the three experimental groups (50 μg/ml LiCl, 25
ng/ml LPS and 50 μg/ml LiCl + 25 ng/ml LPS) and the blank control
group were established. Following incubation for two days, total
RNA was extracted using TRIzol™ reagent (Invitrogen Life
Technologies). cDNA was synthesized using a ReverTra
Dash® RT-PCR kit (Toyobo, Osaka, Japan). Aliquots of
total cDNA were amplified in a PC701 thermal cycler (Astec Co.,
Ltd., Fukuoka Japan). The reaction mixture was denatured at 94°C
for 5 min, then subjected to 30 cycles of 94°C for 30 sec, 52°C for
50 sec and 72°C for 50 sec, followed by a final extension step at
72°C for 10 min. The amplification reaction products were resolved
on 1.5% agarose/Tris-acetate-EDTA gels, electrophoresed at 100 mV
and visualized via ethidium bromide staining. The gene expression
of receptor activator of nuclear factor-κB ligand (RANKL) and
osteoprotegerin (OPG) was quantified via normalization to the
standard GAPDH (a routinely used reference gene). The primers used
for the RT-PCR were as follows: RANKL upstream,
5′-TATGATGGAAGGCTCATGGT-3′ and downstream,
5′-TGTCCTGAACTTTGAAAGCC-3′; OPG upstream,
5′-AAAGCACCCTGTAGAAAACA-3′ and downstream, 5′-CCGTTTTATCCTCTCTA-3′;
GAPDH upstream, 5′-TCCACTCACGGCAAATTCAACG-3′ and downstream,
5′-TAGACTCCACGACATACTCAGC-3′ (Invitrogen Life Technologies).
ELISA
OCCM-30 cells at a density of 2×105 were
cultured in a six-well plate overnight. Following culture, the
cells were washed twice with PBS for 10 sec and the three
experimental groups (50 μg/ml LiCl, 25 ng/ml LPS and 50 μg/ml LiCl
+ 25 ng/ml LPS) and the blank control group were established. The
experimental groups were treated for 48 h. Supernatants from the
cell culture were harvested via centrifugation (150 × g, 4 min,
room temperature) and stored at -20°C. The OPG levels in the
supernatants and RANKL levels in the cell lysate were measured
using mouse ELISA kits (R&D Systems Inc., Minneapolis, MN, USA)
in accordance with the manufacturer’s instructions.
Statistical analysis
Data are expressed as the mean ± standard deviation.
The statistical analysis was performed using Origin 8.0 software
(OriginLab Corp., Northampton, MA, USA).
Results
Cell viability analysis of
cementoblasts
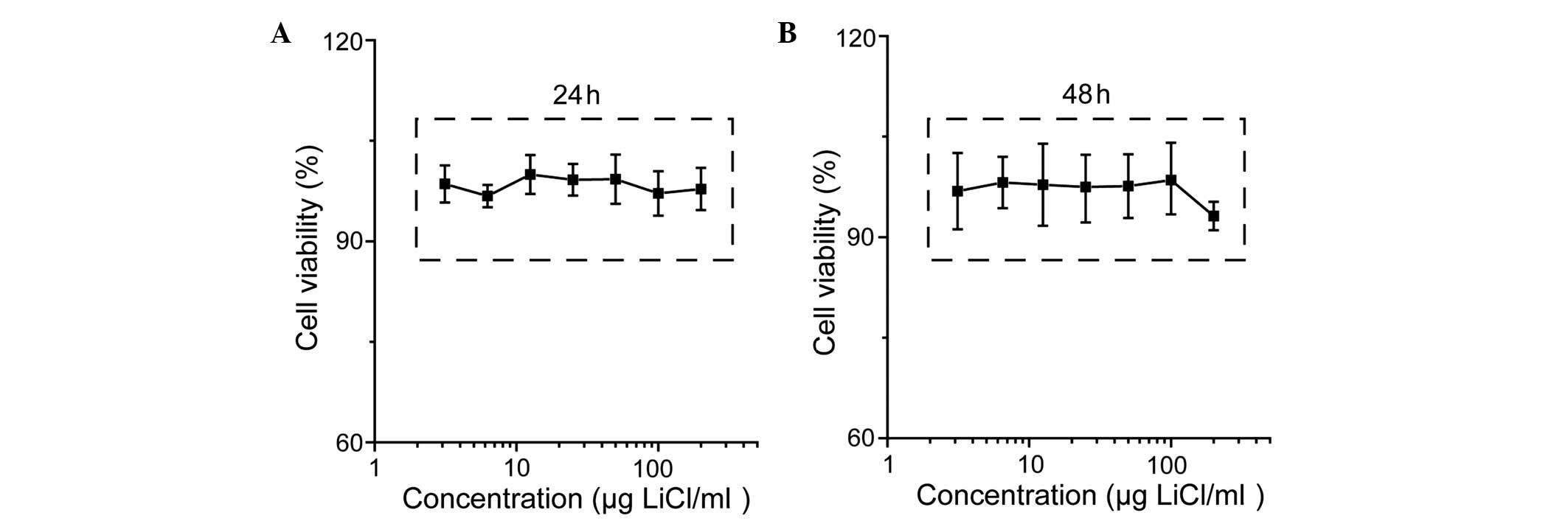
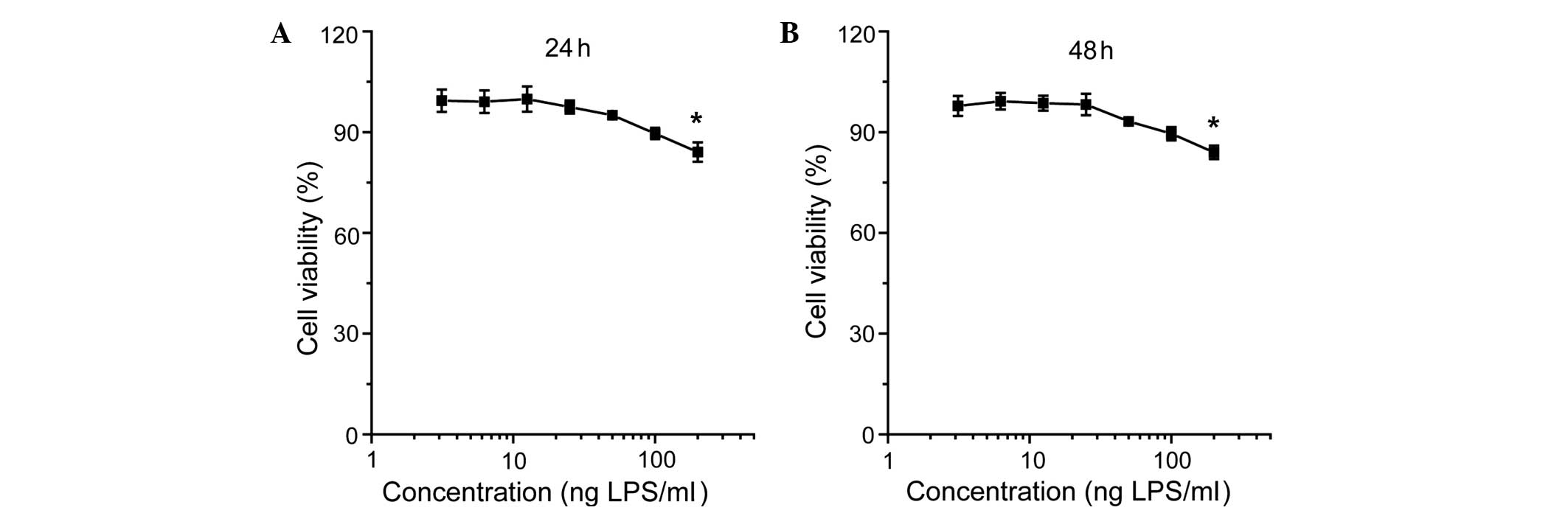
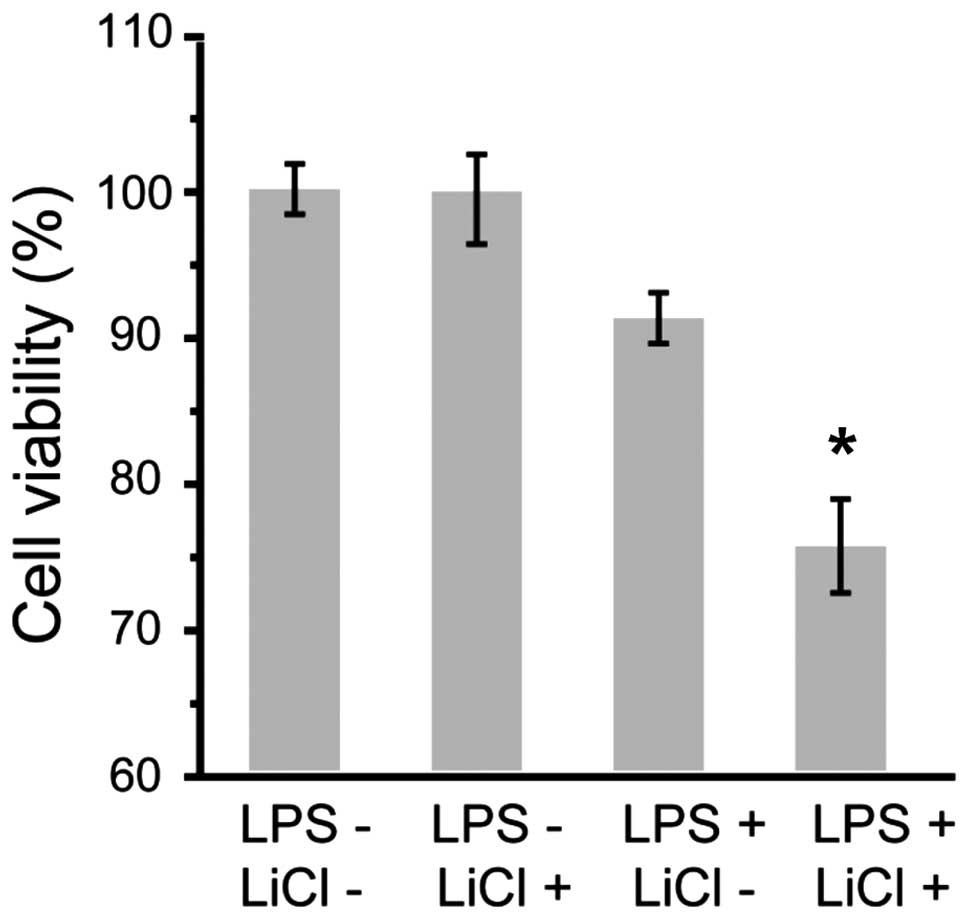
As shown in Fig. 1,
the viability of the cementoblasts was not significantly affected
by LiCl; however, incubation with LPS reduced cell viability in a
concentration-dependent manner (Fig.
2). The effect of inhibition was more evident for incubation
with LiCl and LPS at the indicated concentrations (Fig. 3).
Effect of LiCl on differentiation in the
presence of LPS
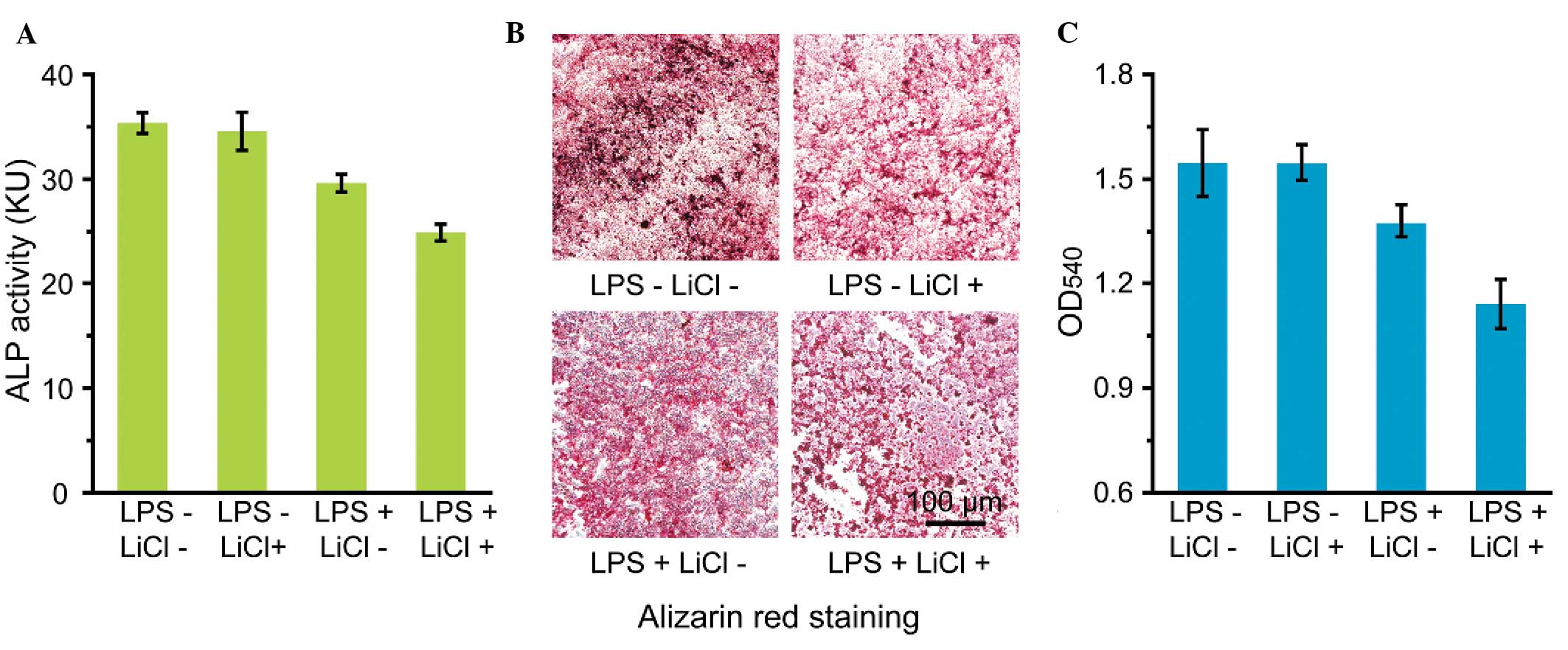
As Fig. 4A shows,
co-incubation with LiCl and LPS decreased the expression of ALP in
the cementoblasts. Similarly, the mineralization of the
cementoblasts under the co-incubation condition was markedly
reduced compared with that of the control group (Fig. 4B and C).
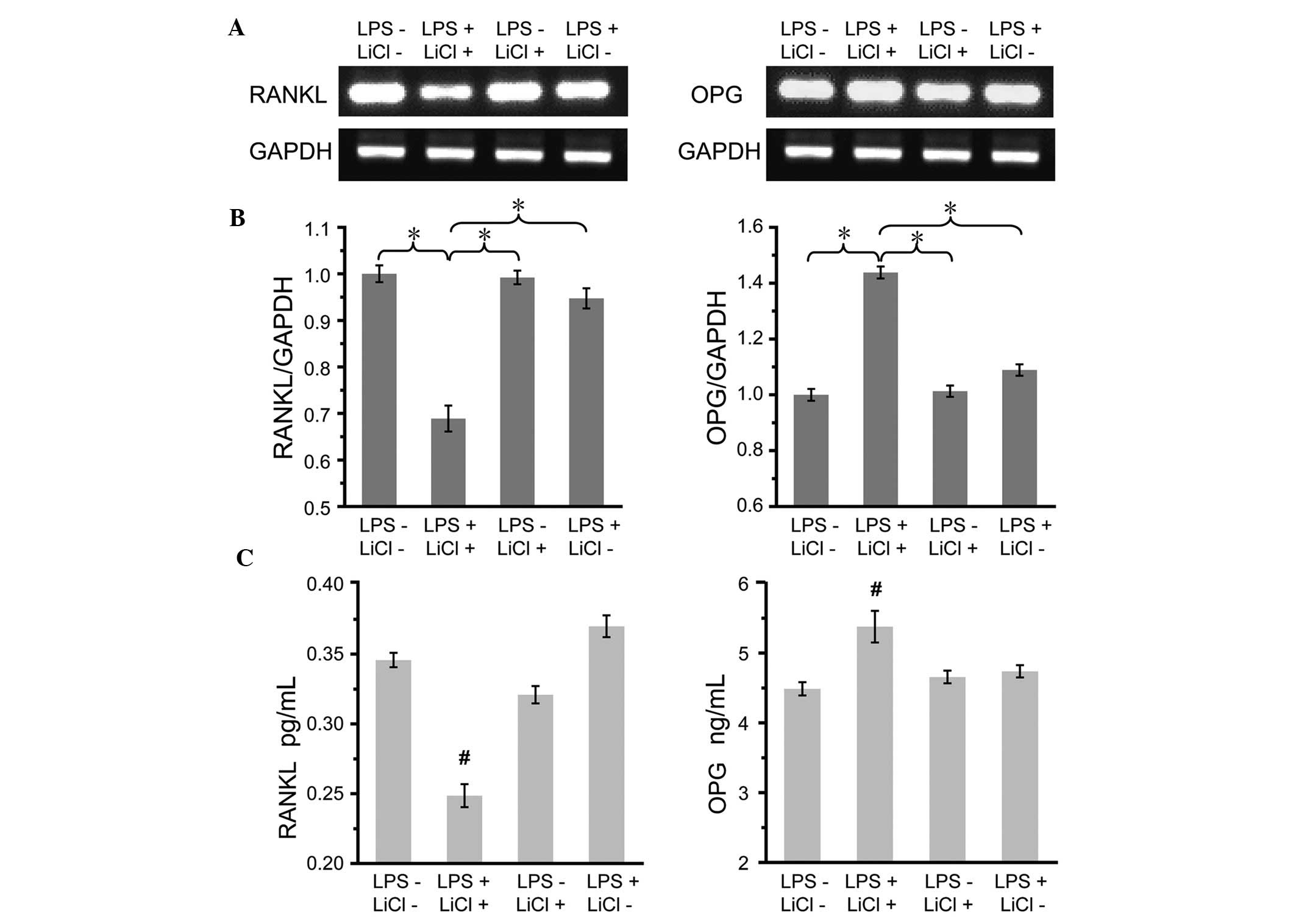
Analysis of OPG and RANKL expression
The mRNA expression of OPG and RANKL was firstly
assessed under the condition of co-incubation. As shown in Fig. 5A and B, RANKL mRNA was expressed at
low levels while OPG mRNA expression was markedly enhanced compared
with that in the control group. Similar trends were observed in the
RANKL and OPG protein expression, as determined using ELISA. In the
co-incubation group, decreased RANKL and increased OPG protein
expression was observed compared with the control group (Fig. 5C).
Discussion
Periodontal tissue loss can be a frequent obstacle
preventing successful treatment in patients with periodontitis.
With the development of tissue engineering, a variety of scaffolds
modified by different biological agents have been developed. Among
these agents, lithium ions have been demonstrated to facilitate
bone formation and have been used in the scaffold design process
(3). In a previous study, we have
shown that lithium ions can affect the metabolism of the cementum
(23); however, in that study,
cementum resorption was evaluated under normal conditions but not
under an inflammatory state. The aim of the present study,
therefore, was to establish a periodontitis model in vitro
and evaluate the effect of lithium ions in the presence of LPS.
To the best of our knowledge, this study has been
the first to evaluate the effect of lithium ions on a periodontitis
model. Secreted by Gram-negative bacteria, LPS has been reported to
trigger inflammatory responses and cause the destruction of
periodontal tissues. In addition, LPS can stimulate osteoblasts to
produce cytokines and receptor activators, inhibit osteoblast
differentiation and affect gene expression (24–27).
Based on this evidence, LPS was selected as the agent used to
establish the periodontitis model in the present study. MTT assay
revealed that incubation of cementoblasts with LiCl had little
effect on the cementoblast survival (Fig. 1); however, LPS reduced cell
viability in a concentration-dependent manner, demonstrating the
negative effect of LPS on cell proliferation (Fig. 2). In accordance with the MTT
results and the findings of previous studies (8,12,19),
50 μg/ml LiCl and 25 ng/ml LPS were selected as the incubation
concentrations for the other experiments. As shown in Fig. 3, the inhibitory effect of LPS on
cementoblast proliferation was significantly aggravated in the
co-incubation condition. It has been well accepted that
proliferation is an essential process for cementogenesis,
particularly in its early stage. The effect of lithium ions on a
periodontitis model could, therefore, provide novel insight into
cementogenesis.
As an early marker of cementoblast differentiation,
ALP plays an important role in transferring phosphate groups from
the cells to the matrix. The incubation of cementoblasts with LiCl
alone showed nearly no effect on the ALP activity of the cells,
which was in accordance with the results of a previous report
(Fig. 4A) (8). The activity of ALP was slightly
reduced by LPS due to the negative effect of LPS on cell
differentiation. By contrast, the ALP activity decreased markedly
under the co-incubation condition, which was consistent with the
results of the MTT assays. Alizarin red staining (Fig. 4B) and quantitative OD value
analysis (Fig. 4C) further
demonstrated the formation of red-stained mineralized nodules and
the evident decrease in mineralization in the cementoblasts under
the co-incubation condition, indicating that incubation with LiCl
and LPS could reduce the ALP activity and further aggravate the
negative regulatory effect on cementoblast differentiation.
Wnt/β-catenin signaling is involved in increases in
bone mass via numerous mechanisms, including the induction of
osteogenesis and stem cell renewal, which indicates that the
activation of wnt signaling may facilitate the regeneration of bone
and associated periodontal tissues (28). Previous findings have shown that
LiCl can activate wnt signaling to suppress cementoblast functions
(8). Performing an important role
in osteoclast differentiation and bone resorption, RANKL can bind
with its cognate RANK receptor on the surface of pre-osteoclasts
and trigger differentiation towards mature osteoclasts. OPG can
block the action of RANKL and protect bone from resorption.
Generally, decreased RANKL or increased OPG expression can promote
cementum formation and provide an osteoprotective condition
(21); therefore, the effect of
LiCl on the expression of OPG and RANKL in the presence of LPS was
evaluated in the present study. Fig.
5A and B demonstrated that LiCl had the potential to alter
osteoclastogenesis by regulating the OPG/RANKL ratio. In previous
studies, exposure to LPS alone led to a slight enhancement in OPG
and RANKL mRNA expression due to a self-protection reaction
(22,29,30).
In previous studies using osteoblasts (31,32),
incubation with LiCl alone could upregulate OPG and downregulate
RANKL mRNA expression. When the effect of LiCl was evaluated in the
present periodontitis model, reduced RANKL mRNA and elevated OPG
mRNA expression was observed. Additional investigations into the
protein expression levels of OPG and RANKL revealed similar trends
(Fig. 5C). The results in
combination have demonstrated that LiCl may be beneficial to
cementum protection via alleviating osteoclastogenesis.
The present study is the first, to the best of our
knowledge, to evaluate the effect of lithium ions on a
periodontitis model established using LPS. Negative effects were
observed for cementoblast proliferation, while positive effects
were noted for the inhibition of osteoclastogenesis. Detailed
investigations, including MTT assays, microscopic imaging and the
quantitative analysis of mRNA and protein expression, were
performed, and the results indicated that bacterial infection
should be controlled prior to the application of lithium ions for
treatment. The present methodology could be extended to other
disease-treatment systems by changing the disease model and
materials, which would enable further applications associated with
treatment and therapy in the clinic. In conclusion, lithium ions
can be applied to facilitate cementum repair in scaffold design;
however, its potential toxicity due to the presence of LPS warrant
additional consideration.
Acknowledgements
This study was supported by the National Natural
Science Foundation of China no. NSFC 81170999); Special Industrial
Research supported by the Development and Reform Commission of
Jilin Province (no. 2013C025-2); and the Specialized Research Fund
for the Doctoral Program of Higher Education (no. SRFDP
20110061110072). The authors would like to thank Professor Somerman
at the University of Washington.
References
|
1
|
Hodgdon A: Dental and related infections.
Emerg Med Clin North Am. 31:465–480. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Gulati M, Anand V, Jain N, et al:
Essentials of periodontal medicine in preventive medicine. Int J
Prev Med. 4:988–994. 2013.PubMed/NCBI
|
|
3
|
Billström GH, Blom AW, Larsson S and
Beswick AD: Application of scaffolds for bone regeneration
strategies: current trends and future directions. Injury. 44(Suppl
1): S28–S33. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wagoner Johnson AJ and Herschler BA: A
review of the mechanical behavior of CaP and CaP/polymer composites
for applications in bone replacement and repair. Acta Biomater.
7:16–30. 2011. View Article : Google Scholar
|
|
5
|
Kao DW and Fiorellini JP: Regenerative
periodontal therapy. Front Oral Biol. 15:149–159. 2012. View Article : Google Scholar
|
|
6
|
Mudda JA and Bajaj M: Stem cell therapy: a
challenge to periodontist. Indian J Dent Res. 22:132–139. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ripamonti U, Petit JC and Teare J:
Cementogenesis and the induction of periodontal tissue regeneration
by the osteogenic proteins of the transforming growth factor-beta
superfamily. J Periodontol Res. 44:141–152. 2009. View Article : Google Scholar
|
|
8
|
Nemoto E, Koshikawa Y, Kanaya S, et al:
Wnt signaling inhibits cementoblast differentiation and promotes
proliferation. Bone. 44:805–812. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kim TH, Lee JY, Baek JA, et al:
Constitutive stabilization of β-catenin in the dental mesenchyme
leads to excessive dentin and cementum formation. Biochem Biophys
Res Commun. 412:549–555. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bae CH, Lee JY, Kim TH, et al: Excessive
Wnt/β-catenin signaling disturbs tooth-root formation. J
Periodontal Res. 48:405–410. 2013. View Article : Google Scholar
|
|
11
|
Du Y, Ling J, Wei X, et al: Wnt/β-catenin
signaling participates in cementoblast/osteoblast differentiation
of dental follicle cells. Connect Tissue Res. 53:390–397. 2012.
View Article : Google Scholar
|
|
12
|
de Boer J, Wang HJ and Van Blitterswijk C:
Effects of Wnt signaling on proliferation and differentiation of
human mesenchymal stem cells. Tissue Eng. 10:393–401. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
de Boer J, Siddappa R, Gaspar C, van
Apeldoorn A, Fodde R and van Blitterswijk C: Wnt signaling inhibits
osteogenic differentiation of human mesenchymal stem cells. Bone.
34:818–826. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wang J, de Groot K, van Blitterswijk C and
de Boer J: Electrolytic deposition of lithium into calcium
phosphate coatings. Dent Mater. 25:353–359. 2009. View Article : Google Scholar
|
|
15
|
Heo JS, Lee SY and Lee JC: Wnt/β-catenin
signaling enhances osteoblastogenic differentiation from human
periodontal ligament fibroblasts. Mol Cells. 30:449–454. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Clément-Lacroix P, Ai M, Morvan F, et al:
Lrp5-independent activation of Wnt signaling by lithium chloride
increases bone formation and bone mass in mice. Proc Natl Acad Sci
USA. 102:17406–17411. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zeng YT, Fu B, Tang GH, Zhang L and Qian
YF: Effects of lithium on extraction socket healing in rats
assessed with micro-computed tomography. Acta Odontol Scand.
71:1335–1340. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Tang GH, Xu J, Chen RJ, Qian YF and Shen
G: Lithium delivery enhances bone growth during midpalatal
expansion. J Dent Res. 90:336–340. 2011. View Article : Google Scholar
|
|
19
|
Galli C, Piemontese M, Lumetti S, Manfredi
E, Macaluso GM and Passeri G: GSK3b-inhibitor lithium chloride
enhances activation of Wnt canonical signaling and osteoblast
differentiation on hydrophilic titanium surfaces. Clin Oral
Implants Res. 24:921–927. 2013. View Article : Google Scholar
|
|
20
|
Han P, Wu C, Chang J and Xiao Y: The
cementogenic differentiation of periodontal ligament cells via the
activation of Wnt/β-catenin signalling pathway by Li+
ions released from bioactive scaffolds. Biomaterials. 33:6370–6379.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Mada Y, Miyauchi M, Oka H, et al: Effects
of endogenous and exogenous prostaglandin E2 on the proliferation
and differentiation of a mouse cementoblast cell line (OCCM-30). J
Periodontol. 77:2051–2058. 2006. View Article : Google Scholar
|
|
22
|
Nociti FH Jr, Foster BL, Barros SP,
Darveau RP and Somerman MJ: Cementoblast gene expression is
regulated by Porphyromonas gingivalis lipopolysaccharide partially
via toll-like receptor-4/MD-2. J Dent Res. 83:602–607. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wang Y, Gao S, Jiang H, et al: Lithium
chloride attenuates root resorption during orthodontic tooth
movement in rats. Exp Ther Med. 7:468–472. 2014.PubMed/NCBI
|
|
24
|
Keeting PE, Rifas L, Harris SA, et al:
Evidence for interleukin-1 beta production by cultured normal human
osteoblast-like cells. J Bone Miner Res. 6:827–833. 1991.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sakuma Y, Tanaka K, Suda M, et al:
Impaired bone resorption by lipopolysaccharide in vivo in mice
deficient in the prostaglandin E receptor EP4 subtype. Infect
Immun. 68:6819–6825. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ishimi Y, Miyaura C, Jin CH, et al: IL-6
is produced by osteoblasts and induces bone resorption. J Immunol.
145:3297–3303. 1990.PubMed/NCBI
|
|
27
|
Bandow K, Maeda A, Kakimoto K, et al:
Molecular mechanisms of the inhibitory effect of lipopolysaccharide
(LPS) on osteoblast differentiation. Biochem Biophys Res Commun.
402:755–761. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Liu W, Konnermann A, Guo T, et al:
Canonical Wnt signaling differently modulates osteogenic
differentiation of mesenchymal stem cells derived from bone marrow
and from periodontal ligament under inflammatory conditions.
Biochim Biophys Acta. 1840:1125–1134. 2014. View Article : Google Scholar
|
|
29
|
Nemoto E, Darveau RP, Foster BL,
Nogueira-Filho GR and Somerman MJ: Regulation of cementoblast
function by P. gingivalis lipopolysaccharide via TLR2. J Dent Res.
85:733–738. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Spencer GJ, Utting JC, Etheridge SL,
Arnett TR and Genever PG: Wnt signalling in osteoblasts regulates
expression of the receptor activator of NFkappaB ligand and
inhibits osteoclastogenesis in vitro. J Cell Sci. 119:1283–1296.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Glass DA II, Bialek P, Ahn JD, et al:
Canonical Wnt signaling in differentiated osteoblasts controls
osteoclast differentiation. Dev Cell. 8:751–764. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Arioka M, Takahashi-Yanaga F, Sasaki M, et
al: Acceleration of bone regeneration by local application of
lithium: Wnt signal-mediated osteoblastogenesis and Wnt
signal-independent suppression of osteoclastogenesis. Biochem
Pharmacol. 90:397–405. 2014. View Article : Google Scholar : PubMed/NCBI
|