Introduction
An increasing number of studies have indicated that
the follicular microenvironment of a human oocyte is a crucial
factor for its developmental competence (1). Follicular fluid (FF) is the medium that
ensures oocyte maturation, fertilization and transport, and
facilitates in vitro sperm capacitation (2,3). Thus,
FF serves a function in early embryo development and implantation
(4). Immune cells, including
leukocytes and cytokines, are involved in the physiology of
follicular development, ovulation and corpus luteum formation
(5–7). As modulators of the immune system,
cytokines influence the physiology and pathology of the female
reproductive system.
To date, associations between particular cytokines
and the outcome of in vitro fertilization (IVF) have been
extensively studied (8–11). Thus, ovulation is a well-recognized
inflammatory process that is putatively modulated by cytokines.
Numerous studies have demonstrated that a variety of
proinflammatory cytokines, including interleukin (IL)-6, IL-8,
IL-11, tumor necrosis factor (TNF)-α, leukemia inhibiting factor
and the IL-1 system, are crucially involved in folliculogenesis
(12), implantation (13) and follicular maturation (14–16).
High-mobility group box 1 (HMGB1; also known as
amphoterin or HMG1) is a highly conserved nuclear protein, which
exhibits DNA-binding properties and participates in DNA
transcription, replication and repair (17). Certain studies have demonstrated that
HMGB1 is a necessary and sufficient mediator of lethal inflammation
and functions as a novel proinflammatory cytokine (18–20).
Various cell types, such as activated macrophages/monocytes,
natural killer cells, mature dendritic cells, pituicytes and
erythroleukemic cells, are known to secrete HMGB1 into the
extracellular milieu. Following release from necrotic cells and
macrophages, HMGB1 functions as an inflammatory stimulator to
upregulate the expression levels of IL-1, IL-6, TNF-α, C-reactive
protein and macrophage inflammatory proteins-1α and −1β (21,22).
Since HMGB1 is a crucial cytokine that mediates the
response to infection and inflammation (23), the FF levels of HMGB1 protein were
hypothesized to correlate with follicular development and IVF/
intracytoplasmic sperm injection (ICSI) outcome. To date, there
have been a limited number of studies examining the correlation
between HMGB1 protein and IVF/ICSI outcome. Thus, the aim of the
present study was to investigate the presence of HMGB1 protein in
human FF. In addition, the study investigated the possible
association between FF concentrations of HMGB1 protein and
reproductive outcomes in normal ovarian responders, patients with
an antral follicle count between 5–15, undergoing IVF/ICSI.
Materials and methods
Ethical approval and patient
consent
The study was approved by the Ethical Committee of
Wuhan University (Wuhan, China) and was conducted in accordance
with the institutional guidelines. Informed consent was obtained
from each patient.
Subjects
In total, 143 cases of infertile couples who had
undergone IVF/ICSI at Renmin Hospital of Wuhan University were
included in the study. The study protocol was approved by the
Institutional Review Board. Infertile couples with male and female
etiologies were included, such as sperm abnormalities and tubal
factors, respectively. Patients with endometriosis, ovulatory
disorders, polycystic ovary syndrome or a history of ovarian
surgery and a poor response to IVF were excluded from the study.
The 143 cases of modified natural IVF/ICSI cycles that were
examined were all first attempts. Informed consent was provided by
each of the couples.
Ovarian stimulation, FF sampling and
oocyte collection
Patients underwent a long treatment protocol with
gonadotropin-releasing hormone agonist administration in the
midluteal phase, which was followed by ovarian stimulation with
recombinant follicle-stimulating hormone (FSH; Gonal-f®, Merck
Serono, Geneva, Switzerland; or Puregon®; Schering-Plough
Corporation, Kenilworth, NJ, USA) or human menopausal gonadotropin
(Livzon Pharmaceutical Group Inc., Zhuhai, China). Following
confirmation of ovarian suppression and when at least three
follicles had reached a mean diameter of 18 mm under transvaginal
ultrasound examination (GE Logiq 400 Pro, GE Healthcare Life
Sciences, Shanghai, China), 10,000 IU human chorionic gonadotropin
(hCG; Livzon Pharmaceutical Group Inc.) was administered
subcutaneously. After 34–36 h, oocytes were retrieved using an
Aloka ProSound SSD-3500 ultrasound-guided transvaginal puncture
(Hitachi-Aloka Medical, Ltd., Guangzhou, China). An individual
aspiration was used to collect the oocytes and each follicle was
recovered in a separate tube. FF was collected from ovarian
follicles that were ≥14 mm, and was pooled for each patient. FF
samples were centrifuged at 2,000 × g for 10 min, and the
supernatants were stored at −80°C for further analysis.
Assessment of oocyte morphology and
maturation
Oocytes isolated from FF samples were evaluated. The
cumulus oophorus and corona radiata were removed from the oocytes
by mechanical pipetting in SynVitro Flush containing 300 IU/ml
hyaluronidase (Sigma-Aldrich) for up to 1–2 min depending on the
extent of cumulus investment. Nuclear maturation of the oocytes was
determined by the identification of the first polar body. On day 2
or 3, the oocytes were sorted into four categories, based on their
morphologic appearance, zonal thickness, cytoplasmic fragmentation
and blastomere size. The categories were as follows: Grade I (high
quality, embryos with equal blastomeres and no observed cytoplasmic
fragmentation; grade II (good quality), embryos with equal
blastomeres and <20% fragmentation of the cytoplasm; grade III
(fair quality), embryos with unequal blastomeres and 20–50%
fragmentation of the cytoplasm; and grade IV (poor quality),
embryos with unequal blastomeres and >50% fragmentation of the
cytoplasm (24).
Assessment of fertilization, cleavage
and embryo quality
Fertilization results were assessed 18 h following
the ICSI treatment for the appearance of two distinct pronuclei and
two polar bodies. Cleavage was evaluated 24 h after fertilization.
Embryo quality was assessed on the second day following
insemination and was graded using the aforementioned protocol
(24).
Determination of HMGB1 protein levels
in the FF
HMGB1 protein levels in the FF were determined using
a commercially available ELISA kit (HMGB1 ELISA kit II; Shino-Test
Corporation, Tokyo, Japan), according to the manufacturer's
instructions. Sensitivity was <1.0 ng/ml, while the intra-assay
and inter-assay coefficients of variation were <10.0%.
Determination of estradiol (E2),
progesterone (P) and luteinizing hormone (LH) concentrations in the
FF
Serum levels of E2, P, LH and FSH were measured
using an Immulite® 2500 immunoassay analyzer (Siemens, Munich,
Germany). Prior to each test, the Immulite® 2500 was calibrated
with three control samples containing low, medium and high
concentrations of the appropriate hormones. Dilutions were
performed prior to the measurement of E2 (1:1,000) and P (1:500),
depending on the calibration range.
Statistical analysis
SPSS software, version 13.0 (SPSS, Inc., Chicago,
IL, USA) was used for statistical analysis. Results are expressed
as the mean ± standard deviation. Between-group differences were
evaluated using the Students t-test, while Spearman's correlation
was applied for correlation analysis between the concentrations of
HMGB1 protein in the FF and other variables. Receiver operating
characteristic (ROC) curve analysis was used to determine the
respective performances and sensitivity/specificity values. The
following thresholds were used to classify the area under the ROC
curve (AUCROC) data: 0.9-1, Perfect separation; 0.8–0.9, excellent
discrimination; 0.7–0.8, acceptable discrimination; 0.6–0.7, poor
discrimination; and 0.5–0.6, no discrimination. P<0.05 was
considered to indicate a statistically significant difference.
Results
Clinical characteristics of the
patients
Following the first round of IVF/ICSI treatment, 64
patients were pregnant and 70 patients remained non-gravid.
Standard IVF was used for 101 oocytes and ICSI was applied for 41
oocytes. In eight cases, an oocyte was collected but unsuccessfully
fertilized (one ICSI and seven IVF), and in one case no oocyte was
retrieved. Infertility was due to sperm abnormalities in 32/143
cases and tubal abnormalities in 111/143 cases. Table I details the characteristics of the
patients in the three groups, namely the pregnant, non-pregnant and
oocyte unfertilized groups. Statistically significant differences
were observed between the pregnant and non-pregnant groups with
regard to the FF levels of HMGB1 protein, the LH levels on the day
of hCG administration, endometrial thickness and the number of
retrieved oocytes. The oocyte unfertilized group exhibited higher
levels of LH on the day of hCG administration when compared with
the pregnant group (Table I).
 | Table I.Clinical and IVF/ICSI patient
characteristics. |
Table I.
Clinical and IVF/ICSI patient
characteristics.
| Characteristic | Pregnant | Non-pregnant | Oocyte
unfertilized |
|---|
| Patients (n) | 64 | 70 | 8 |
| Age (years) | 30.30±4.19 | 31.67±4.42 | 30.62±4.66 |
| HMGB1 protein in FF
(ng/ml) |
7.38±2.02a | 6.14±2.52 | 7.49±1.79 |
| Duration of
infertility (years) | 5.47±3.67 | 4.86±3.13 | 5.28±2.31 |
| BMI
(kg/m2) | 21.69±2.68 | 21.55±2.45 | 20.72±1.62 |
| Basal level |
|
|
|
| E2
(pg/ml) | 46.60±21.89 | 50.70±29.42 | 52.19±22.58 |
| LH
(pmol/l) | 4.41±5.64 | 4.08±2.34 | 3.77±1.49 |
| FSH
(mIU/ml) | 6.05±2.03 | 6.01±2.09 | 5.83±2.78 |
| Antral follicle
count (n) | 12.73±3.66 | 12.09±3.09 | 9.98±2.91 |
| Day of hCG
administration |
|
|
|
| E2
(pg/ml) |
5,169.09±2,264.4 |
4,507.56±2,467.98 |
3,616.55±1,937.16 |
| LH
(pmol/l) |
0.92±1.78a,b | 1.78±2.03 | 1.64±2.29 |
| P
(pmol/l) | 1.50±0.96 | 1.35±0.52 | 1.46±0.48 |
|
Endometrial thickness
(mm) |
10.3±1.3c | 9.7±1.7 | 10.3±0.09 |
| Retrieved oocytes
(n) |
11.00±6.34c | 11.68±6.51 | 11.02±5.09 |
| Fertilization rate
(%) | 71.79±28.00 | 67.14±23.26 | - |
| Grade I/II embryos
(n) | 4.65±4.44 | 3.99±3.45 | - |
| Transferred embryos
(n) | 2.48±1.14 | 2.64±1.12 | - |
FF levels of HMGB1 protein and the
prediction of cycle outcome and oocyte fertilization
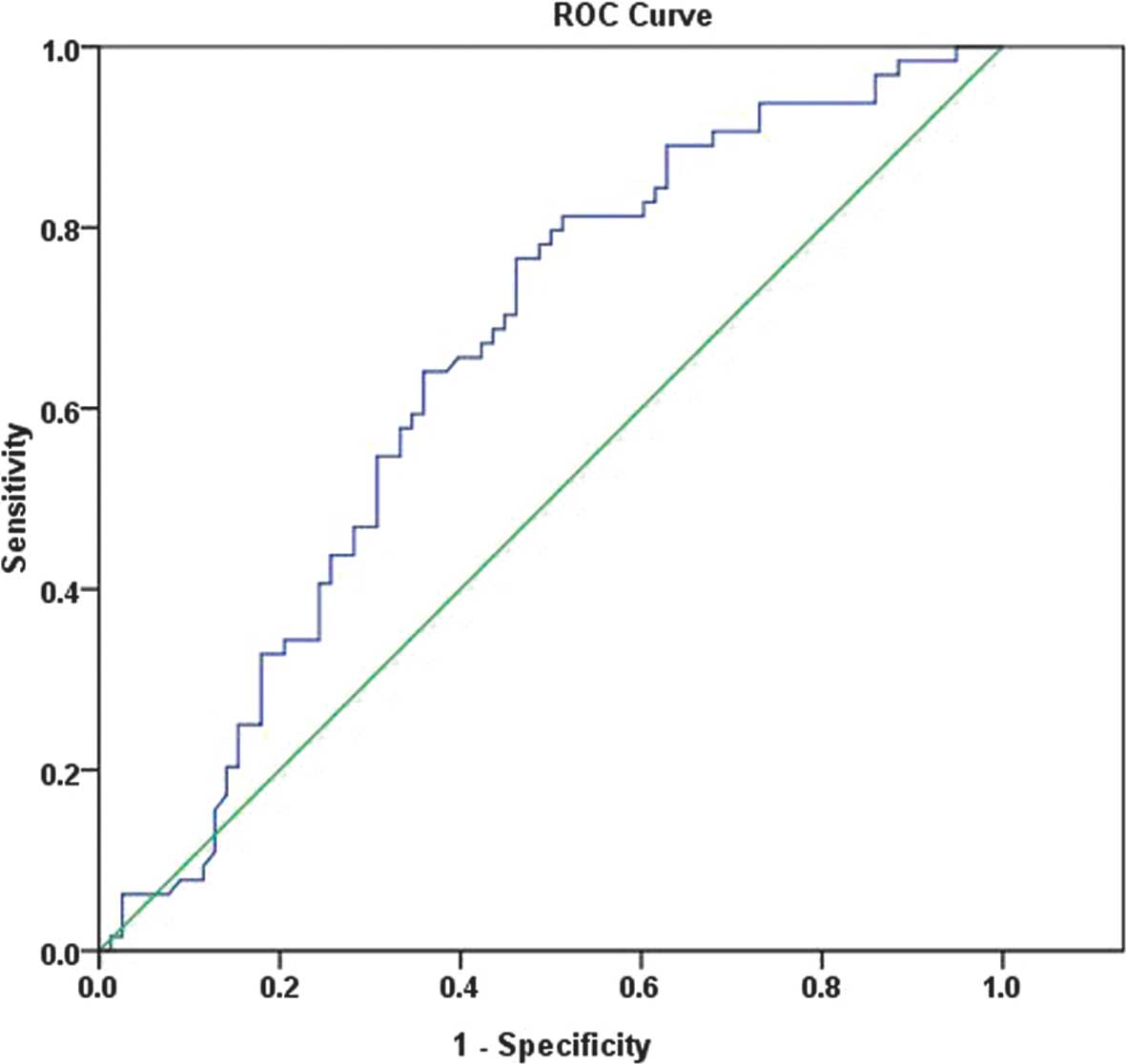
A ROC curve was produced for the prediction of
pregnancy outcomes for patients undergoing IVF/ICSI, based on the
HMGB1 protein level in the FF. The AUCROC was 0.673 (0.581–0.765;
P<0.01), indicating a poor discrimination. The optimal threshold
according to the ROC curve for HMGB1 protein was 5.13 ng/ml, with a
sensitivity of 89.1% and a specificity of 62.9% (Fig. 1). In eight cases, no oocyte was
fertilized. Statistical analyses were conducted to identify any
associations between the measured parameters and the failure of
oocyte fertilization. A ROC curve was produced for predicting the
fertilization outcome of patients undergoing IVF/ICSI, based on the
HMGB1 protein level in the FF. The AUCROC was 0.616 (0.433–0.798;
P=0.273), indicating no statistically significant association.
Correlations between HMGB1 protein
levels and IVF/ICSI data
Spearman's correlation analyses of the HMGB1 protein
level with pregnancy and numerous associated factors are presented
in Table II. Statistically
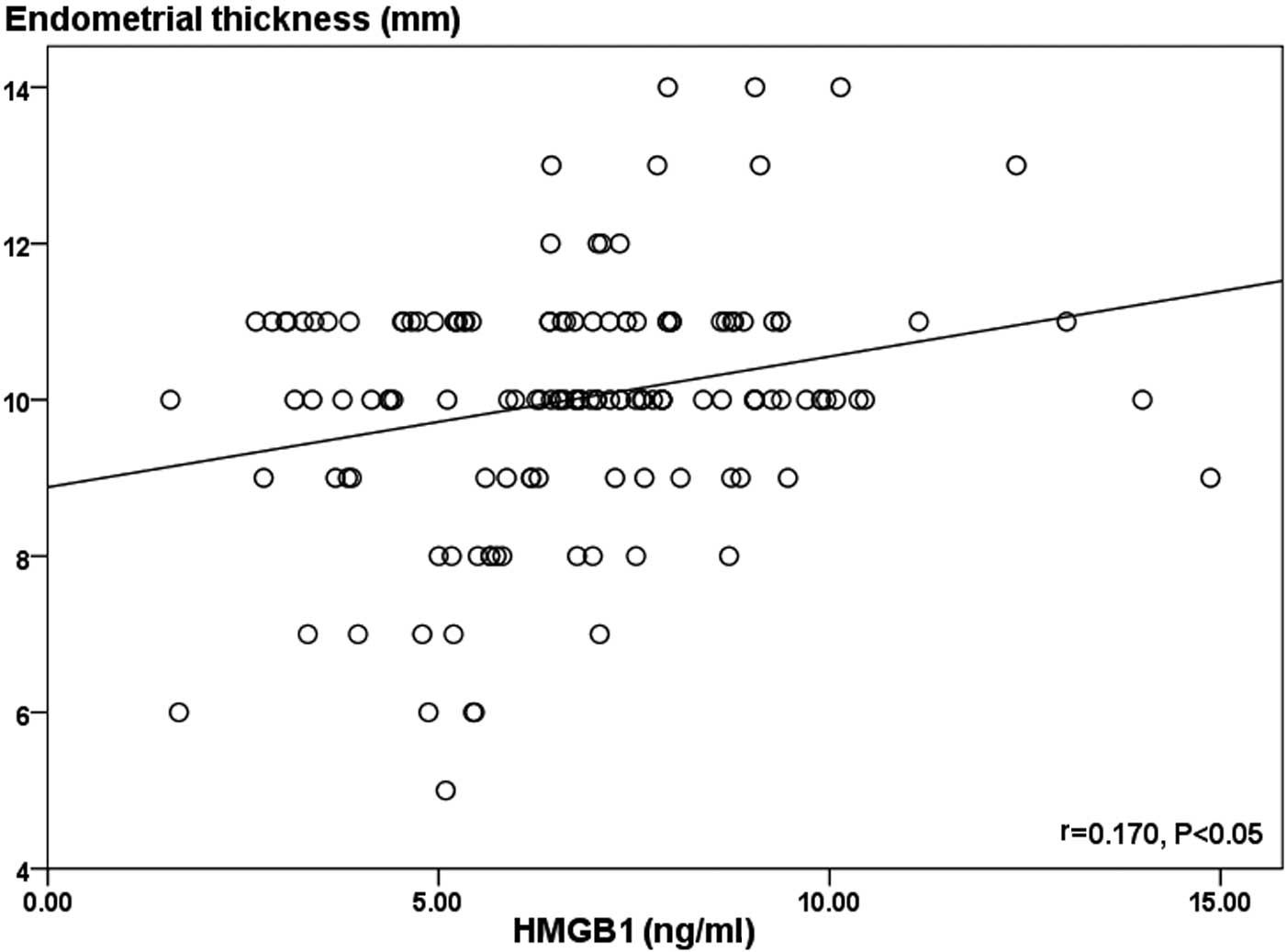
significant correlations were identified when comparing the HMGB1
protein level in the FF with the pregnancy rate and endometrial
thickness on the day of hCG administration (r=0.30; P<0.01 and
r=0.170; P<0.05, respectively; Fig.
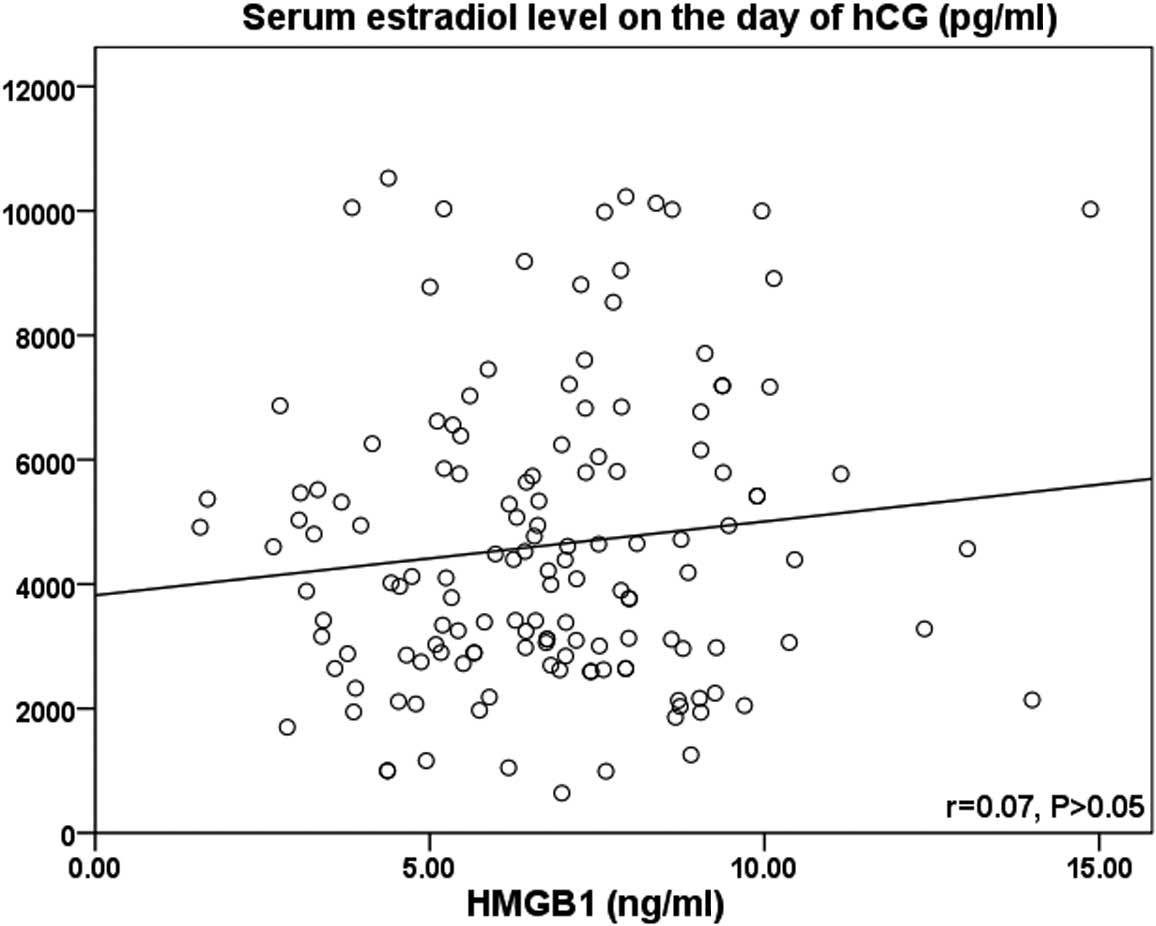
2). However, no significant correlation was observed between
the HMGB1 protein level and the serum E2 level on the day of hCG
administration (r=0.07; P>0.05) (Table II and Fig. 3).
 | Table II.Spearman correlations of the HMGB1
protein level with associated factors. |
Table II.
Spearman correlations of the HMGB1
protein level with associated factors.
| Variable | rs | P-value |
|---|
| Pregnancy | -0.300 | <0.01 |
| BMI | -0.017 | - |
| Day of hCG
administration |
|
|
| E2 | 0.070 | - |
| LH | -0.115 | - |
| P | 0.081 | - |
|
Endometrial thickness | 0.170 | <0.05 |
| Retrieved
oocytes | 0.009 | - |
| Fertilization
rate | -0.067 | - |
| Grade I/II
embryos | -0.031 | - |
Discussion
The present study investigated the association
between HMGB1 protein levels and the treatment outcomes in
infertile patients undergoing induction of superovulation. The
results demonstrated that there were statistically significant
differences in the level of HMGB1 protein in the FF, LH levels on
the day of hCG administration, endometrial thickness and the number
of retrieved oocytes when comparing the pregnant and non-pregnant
groups. To date, there are limited data concerning the correlation
between HMGB1 protein levels and IVF/ICSI outcome.
The present study indicated that LH levels on the
day of hCG administration were higher in the non-pregnant group
compared with the pregnant group (1.78±2.03 vs. 0.92±1.78 pmol/l;
P<0.01). Previous studies have reported elevated LH levels in
patients undergoing IVF/ICSI treatment; however, the observed
effect of these increases on pregnancy rates has differed between
studies. Cantineau and Cohlen (25)
observed an incidence of spontaneously elevated LH levels of 35%
without a significant alteration in pregnancy rates. However,
Huirne et al (26) observed
reduced pregnancy rates in the presence of elevated LH levels at
the time of hCG administration in patients undergoing IVF/ICSI
treatment. Furthermore, no successful pregnancies were observed in
patients with a combined elevation in LH and P levels, which was
consistent with the present study. Elevated LH levels appear to
affect pregnancy rates via mechanisms other than ovulation alone.
LH induces the synthesis of growth factors, cytokines and
chemokines, which exert a variety of effects, including influencing
specific features of the endometrium (27).
HMGB1 protein is a crucial cytokine that mediates
the immune response to infection and inflammation (21). HMGB1 serves a key function in
numerous chronic inflammatory diseases, including rheumatoid
arthritis, systemic lupus erythematosus and inflammatory myositis
(28). To the best of our knowledge,
the present study is the first to report the presence of HMGB1
protein in human FF, demonstrating that FF levels of HMGB1 protein
are significantly increased in pregnant patients. Furthermore, the
present study performed ROC curve analyses to determine if a
threshold concentration of HMGB1 protein in the FF was able to
indicate pregnancy outcomes. The ROC curve indicated that FF levels
of HMGB1 protein were significantly associated with pregnancy
rates, with an AUCROC of 0.673 (0.581–0.765; P<0.01; Fig. 1). In addition, the ROC curve
exhibited discriminating capacity and the optimal threshold,
according to the ROC curve, for HMGB1 protein was 5.13 ng/ml. These
results suggested that FF levels of HMGB1 protein may be a useful
factor to predict the outcome of IVF/ICSI treatment.
A number of studies have evaluated the association
between endometrial thickness and pregnancy rates in patients
undergoing assisted reproductive technology; however, the results
remain controversial (29–38). A number of studies have indicated a
higher pregnancy rate at a particular endometrial thickness
(29–33), while alternative studies indicated no
significant correlation between endometrial thickness and pregnancy
rates in IVF/ICSI patients (34–39). The
present study indicated that clinical pregnancy rates following
embryo transfer are positively correlated with endometrial
thickness, which is consistent with previous studies (39–41).
Growing ovarian follicles produce increasing
quantities of E2, which induces proliferative endometrial
alterations. He et al (42)
reported an estrogen-induced increase in the transcription of HMGB1
in breast cancer cells. This observation suggested that endometrial
HMGB1 expression, and possibly secretion, is steroid-dependent. The
presence of estrogen response elements in the HMGB1 gene supports
this hypothesis (43). This
explanation may account for the current observation that levels of
HMGB1 protein in the FF exhibited a significant positive
correlation with endometrial thickness (r=0.170; P<0.05).
However, no association was observed between the FF levels of HMGB1
protein and serum E2 levels on the day of hCG administration in the
IVF cycle patients (r=0.07; P>0.05). The underlying mechanism
remains unknown and further preliminary and clinical studies are
required.
In the present study, the number of retrieved
oocytes was significantly reduced in the non-pregnant group
compared with the pregnant group on the day of hCG administration
(11.00±6.34 vs. 11.68±6.51; P<0.01). However, the majority of
existing studies reported no association between the number of
oocytes retrieved and the pregnancy rate.
However, there were limitations to the present
study. The study population included a small number of Chinese
patients; thus, future studies with larger cohorts are required.
Furthermore, the precise mechanisms underlying the observations and
their clinical relevance remain unclear and require
elucidation.
In conclusion, the current study reported the
presence of HMGB1 protein in the FF, and indicated elevated levels
of HMGB1 protein in the FF of pregnant patients. In addition, the
level of HMGB1 protein in the FF was shown to positively correlate
with endometrial thickness. Therefore, the FF levels of HMGB1
protein may be a useful factor for predicting the outcome of
IVF/ICSI treatment. Future studies are required to investigate the
precise mechanisms underlying the possible influence of HMGB1
protein on the outcome of IVF/ICSI.
Acknowledgements
The authors thank Su Hong for her assistance during
the sample collection.
References
|
1
|
Driancourt MA and Thuel B: Control of
oocyte growth and maturation by follicular cells and molecules
present in follicular fluid. A review. Reprod Nutr Dev. 38:345–362.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Edwards RG: Follicular fluid. J Reprod
Fertil. 37:189–219. 1974. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hong CY, Chao HT, Lee SL and Wei YH:
Modification of human sperm function by human follicular fluid: A
review. Int J Androl. 16:93–96. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Mahadevan MM and Fleetham J: Relationship
of a human oocyte scoring system to oocyte maturity and fertilizing
capacity. Int J Fertil. 35:240–244. 1990.PubMed/NCBI
|
|
5
|
Wu R, Van der Hoek KH, Ryan NK, Norman RJ
and Robker RL: Macrophage contributions to ovarian function. Hum
Reprod Update. 10:119–133. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Brännström M and Norman RJ: Involvement of
leukocytes and cytokines in the ovulatory process and corpus luteum
function. Hum Reprod. 8:1762–1775. 1993.PubMed/NCBI
|
|
7
|
Smith MP, Flannery GR, Randle BJ, Jenkins
JM and Holmes CH: Leukocyte origin and profile in follicular
aspirates at oocyte retrieval. Hum Reprod. 20:3526–3531. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Moos J, Filova V, Pavelkova J, Moosova M,
Peknicova J and Rezabek K: Follicular fluid and serum levels of
inhibin A and pregnancy-associated plasma protein A in patients
undergoing IVF. Fertil Steril. 91:1739–1744. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Browne RW, Bloom MS, Shelly WB, Ocque AJ,
Huddleston HG and Fujimoto VY: Follicular fluid high density
lipoprotein-associated micronutrient levels are associated with
embryo fragmentation during IVF. J Assist Reprod Genet. 26:557–560.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lédée N, Frydman R, Osipova A, et al:
Levels of follicular G-CSF and interleukin-15 appear as noninvasive
biomarkers of subsequent successful birth in modified natural in
vitro fertilization/intracytoplasmic sperm injection cycles. Fertil
Steril. 95:94–98. 2009. View Article : Google Scholar
|
|
11
|
Hasegawa J, Iwasaki S, Yanaihara A,
Negishi M, Tahara R and Okai T: Correlations between steroids
concentration in follicular fluid, pronuclear morphology and embryo
qualities in vitro fertilization. Reprod Med Biol. 2:171–176. 2003.
View Article : Google Scholar
|
|
12
|
De Mola Loret JR, Flores JP, Baumgardner
GP, Goldfarb JM, Gindlesperger V and Friedlander MA: Elevated IL-6
levels in the ovarian hyperstimulation syndrome: Ovarian
immunohistochemical localization of IL-6 signal. Obstet Gynecol.
87:581–587. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Stewart CL: LIF and the regulation of the
preimplantation development of the mammalian embryo. Mol Reprod
Dev. 39:233–238. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Branisteanu I, Pijnenborg R, Spiessens C,
Van der Auwera I, Keith JC Jr and Van Assche FA: Detection of
immunoreactive interleukin-11 in human follicular fluid:
Correlations with ovarian steroid, insulin-like growth factor I
levels, and follicular maturity. Fertil Steril. 67:1054–1058. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Belayet HM, Kanayama N, Khatun S, Asahina
T, Okada Y, Kitamura K, Kobayashi T and Terao T: Pharmacologic
doses of interleukin 8 suppositories induce follicular maturation
in rabbits. Cytokine. 12:361–367. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Arici A, Oral E, Bukulmez O, Buradagunta
S, Engin O and Olive DL: Interleukin-8 expression and modulation in
human preovulatory follicles and ovarian cells. Endocrinology.
137:3762–3769. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lotze MT and Tracey KJ: High-mobility
group box 1 protein (HMGB1): Nuclear weapon in the immune arsenal.
Nat Rev Immunol. 5:331–342. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang H, Bloom O, Zhang M, et al: HMG-1 as
a late mediator of endotoxin lethality in mice. Science.
285:248–251. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Scaffidi P, Misteli T and Bianchi ME:
Release of chromatin protein HMGB1 by necrotic cells triggers
inflammation. Nature. 418:191–195. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yamada S and Maruyama I: HMGB1, a novel
inflammatory cytokine. Clin Chim Acta. 375:36–42. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Andersson U, Wang H, Palmblad K, et al:
High mobility group 1 protein (HMG-1) stimulates proinflammatory
cytokine synthesis in human monocytes. J Exp Med. 192:565–570.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Erlandsson Harris H and Andersson U:
Mini-review: The nuclear protein HMGB1 as a proinflammatory
mediator. Eur J Immunol. 34:1503–1512. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Andersson U and Harris HE: The role of
HMGB1 in the pathogenesis of rheumatic disease. Biochim Biophys
Acta. 1799:141–148. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Steer CV, Mills CL, Tan SL, Campbell S and
Edwards RG: The cumulative embryo score: A predictive embryo
scoring technique to select the optimal number of embryos to
transfer in vitro fertilization and embryo transfer programme. Hum
Reprod. 7:117–119. 1992.PubMed/NCBI
|
|
25
|
Cantineau AE, Cohlen BJ and Dutch IUI:
Study Group: The prevalence and influence of luteinizing hormone
surges in stimulated cycles combined with intrauterine insemination
during a prospective cohort study. Fertil Steril. 88:107–112. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Huirne JA, van Loenen AC, Schats R,
McDonnell J, Hompes PG, Schoemaker J, Homburg R and Lambalk CB:
Dose-finding study of daily GnRH antagonist for the prevention of
premature LH surges in IVF/ICSI patients: Optimal changes in LH and
progesterone for clinical pregnancy. Hum Reprod. 20:359–367. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Tabibzadeh S: Molecular control of the
implantation window. Hum Reprod Update. 4:465–471. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Abdulahad DA, Westra J, Limburg PC,
Kallenberg CG and Bijl M: HMGB1 in systemic lupus Erythematosus:
Its role in cutaneous lesions development. Autoimmun Rev.
9:661–665. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Check JH, Cohen R, Amui J, Choe JK and
Brasile D: Evidence that the main adverse effect of ganirelix on
pregnancy and implantation rates is on the embryo rather than the
endometrium. Clin Exp Obstet Gynecol. 38:326–327. 2011.PubMed/NCBI
|
|
30
|
Kehila M, Kebaili S, Bougmiza I, Meddeb S,
Boughizane S, Khairi H and Ajina M: Endometrial thickness in in
vitro fertilization. A study of 414 cases. Tunis Med. 88:928–932.
2010.[(In French)]. PubMed/NCBI
|
|
31
|
Chen SL, Wu FR, Luo C, Chen X, Shi XY,
Zheng HY and Ni YP: Combined analysis of endometrial thickness and
pattern in predicting outcome of in vitro fertilization and embryo
transfer: A retrospective cohort study. Reprod Biol Endocrinol.
8:30–36. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Okohue JE, Onuh SO, Ebeigbe P, Shaibu I,
Wada I, Ikimalo JI and Okpere EE: The effect of endometrial
thickness on in vitro fertilization (IVF)-embryo
transfer/intracytoplasmic sperm injection (ICSI) outcome. Afr J
Reprod Health. 13:113–121. 2009.PubMed/NCBI
|
|
33
|
Al-Ghamdi A, Coskun S, Al-Hassan S,
Al-Rejjal R and Awartani K: The correlation between endometrial
thickness and outcome of in vitro fertilization and embryo transfer
(IVF-ET) outcome. Reprod Biol Endocrinol. 6:37–41. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Rashidi BH, Sadeghi M, Jafarabadi M and
Tehrani Nejad ES: Relationships between pregnancy rates following
in vitro fertilization or intracytoplasmic sperm injection and
endometrial thickness and pattern. Eur J Obstet Gynecol Reprod
Biol. 120:179–184. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kovacs P, Matyas S, Boda K and Kaali SG:
The effect of endometrial thickness on IVF/ICSI outcome. Hum
Reprod. 18:2337–2341. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Yuval Y, Lipitz S, Dor J and Achiron R:
The relationships between endometrial thickness, and blood flow and
pregnancy rates in in-vitro fertilization. Hum Reprod.
14:1067–1071. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Csemiczky G, Wramsby H, Johannisson E and
Landgren BM: Endometrial evaluation is not predictive for in vitro
fertilization treatment. J Assist Reprod Genet. 16:113–116. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Oliveira JBA, Baruffi RLR, Mauri AL,
Petersen CG, Borges MC and Franco JG Jr: Endometrial
ultrasonography as a predictor of pregnancy in an in-vitro
fertilization programme after ovarian stimulation and
gonadotrophin-releasing hormone and gonadotrophins. Hum Reprod.
12:2515–2518. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Richter KS, Bugge KR, Bromer JG and Levy
MJ: Relationship between endometrial thickness and embryo
implantation, based on 1,294 cycles of in vitro fertilization with
transfer of two blastocyst-stage embryos. Fertil Steril. 87:53–59.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Kovacs P, Matyas S, Boda K and Kaali SG:
The effect of endometrial thickness on IVF/ICSI outcome. Hum
Reprod. 18:2337–2341. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Traub ML, Van Arsdale A, Pal L, Jindal S
and Santoro N: Endometrial thickness, Caucasian ethnicity, and age
predict clinical pregnancy following fresh blastocyst embryo
transfer: A retrospective cohort. Reprod Biol Endocrinol. 7:332009.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
He Q, Liang CH and Lippard SJ: Steroid
hormones induce HMG1 overexpression and sensitize breast cancer
cells to cisplatin and carboplatin. Proc Natl Acad Sci USA.
97:5768–5772. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Borrmann L, Kim I, Schultheiss D, Rogalla
P and Bullerdiek J: Regulation of the expression of HMG1, a
co-activator of the estrogen receptor. Anticancer Res. 21((1A)):
301–305. 2001.PubMed/NCBI
|