Introduction
Hepatocellular carcinoma (HCC) is the sixth common
cancer and the third most cause of cancer-related mortality in the
world (1,2) with a high incidence of complications.
Between 13 and 23% of HCC patients suffer inferior vena cava (IVC)
obstructions caused by tumor metastasis and/or lymph node invasions
(3), which is frequently associated
with lower extremity edema, ascites, renal dysfunction and even
fatal pulmonary infarction due to cancer thrombus shedding. The
average survival time of HCC patients with IVC obstruction has been
reported to be only 2–3 months without effective treatment
(4). At present, various options are
available for the treatment of HCC with IVC obstruction, including
surgery, transcatheter arterial chemoembolization (TACE), IVC stent
placement, radiotherapy and radioactive iodine-125 seeds. Surgical
resection is often restricted when the tumor is in advanced stage
with poor liver function and tumor invasion of vessels and bile
ducts. The efficacy of TACE for HCC has been widely accepted and
the treatment can significantly relieve obstructions of the IVC,
improve liver function and the quality of life for a short period
when combined with stent implantation; however, the rate of stent
restenosis is high with poor long-term outcomes (5). The main characteristics of iodine-125
are constant low energy X-ray and γ-ray release, which inhibit or
kill tumor cells in a maximum radius of only 17 mm with little
damage to the surrounding tissues. It has also been reported that
iodine-125 is able to inhibit vascular intimal hyperplasia, thus
reducing the incidence of stent restenosis (6,7).
Brachytherapy with iodine-125 has been widely used for the
treatment of prostate cancer and head and neck tumors as well as
liver cancers, mainly by implanting iodine-125 seeds into the tumor
sites (8). The present study is a
retrospective analysis of combined treatment with iodine-125 seed
strands with TACE and stent treatments for IVC obstructions in
patients with HCC in comparison with conventional TACE and stent
treatments.
Patients and methods
Patients
This study has been approved by the hospital ethics
committee (Yancheng Hospital, Dongnan University, Yancheng,
Jiangsu, China) and written informed consent was obtained from all
participants. From March 2009 to June 2013, 52 patients with HCC
and IVC obstruction were treated in the hospital and
retrospectively analyzed. Inclusion criteria were: i) no history of
IVC obstruction treatments, ii) symptoms clearly associated with
IVC obstruction, iii) Child-Pugh score A or B, with an expected
survival time of >3 months. Exclusion criteria were: i)
pulmonary metastasis; ii) tumor thrombus of the right atrium; iii)
right cardiac insufficiency. The symptoms associated with IVC
obstruction were scored with 1 point for mild lower limb edema, 2
points for severe edema, 1 point for abdominal wall superficial
varicose veins and 1 point for scrotal edema. All patients
underwent pre-procedural hepatic and renal function tests, blood
routine and contrast-enhanced abdominal computed tomography (CT)
examinations to assess liver lesions and lymph node metastasis as
well as types (compression, endovascular invasion or tumor
thrombus) of IVC obstructions.
Surgical technique
Main instruments
The stenting instruments were a Z-type metal
self-expandable venous stent (diameter 30 mm and length 100 mm;
Youyan Yijin, Beijing, China) and iodine-125 seeds (type 6711,
length 4.5 mm, radioactivity 0.7 mCi/seed, effective radiation
radius 17 mm; Ningbo Junan Pharmaceutical Technology Company,
Ningbo, China).
Implantation of stent and iodine-125 seed
strands
Following local anesthesia with the patient in a
supine position and electrocardiogram (ECG) monitoring, the right
femoral vein was punctured and a 4-F pigtail catheter was inserted
for IVC venography. This was to obtain diameter and length
measurements for the obstructed segment of the IVC, to determine
the pressure difference between the distal IVC obstructed segment
and the right atrium, and to observe the collateral circulation.
According to pre-stenting CT findings and IVC venography, stents
and iodine-125 seed strands were prepared for transplantation
(stent and seed strands arranged in line were required to cover and
exceed 10 cm on either end of the obstructed segment of the IVC).
For patients with IVC stenosis (tumor invasion or external
pressure) <50%, only a single seed strand was implanted; for
patients with IVC stenosis >50%, two seed strands were used to
ensure treatment efficacy, since the effective radius of iodine-125
is only 17 mm. The required amount of iodine-125 seeds was packaged
in sterile medical plastic 4-F tubes in a line to form iodine-125
seed strands, which were fixed to the stent surface by surgical
suture. For implantation of single seed strands, a 0.035-inch guide
wire was first inserted through the obstructed segment followed by
a 12-F long sheath along the guide wire with the sheath exceeding
the proximal IVC obstructive segment by 10 mm. The stent with
iodine-125 seed strands was then carried into the 12-F long sheath
with an importer under posteroanterior and lateral X-ray
fluoroscopy, pushed to the long sheath head end and rotated along
the sheath so that the iodine-125 seed strand lay close to the
lesion side of the IVC. Finally the long sheath was retreated and
the stent released (Fig. 1). For
implantation of two iodine-125 seed strands, after a 12-F long
sheath with stent and single seed strands was positioned in the
discharge position via a right femoral vein approach, the right
jugular vein was punctured, a 6-F sheath was inserted into the IVC
and the second iodine-125 seed strand was delivered to the targeted
position. The 12-F long sheath was then retreated and released ~2/3
of the length of the stent. Then, the 12-F long sheath was rotated
and the position of the seed strands in the 6-F sheath was adjusted
by the force of the stent in the 12-F sheath under posteroanterior
and lateral X-ray fluoroscopy, to result in two seed strands lying
~1.5 cm apart and close to the obstructed IVC segments. The 12-F
sheath was retreated and the stent completely released. Finally,
the 6-F sheath was retreated and the other seed strands were placed
between the stent and the vessel wall of the IVC. IVC venography
was performed again (Fig. 2) and the
treatment was complete if venography showed that the IVC blood flow
patency was good, the collateral veins disappeared or decreased
significantly, and the diameter of the obstructed segment of the
IVC segment and the pressure difference between the distal
obstructed IVC segment and the right atrium after stent
implantation were acceptable. If not, a balloon of diameter 20 mm
and length 40 mm was inserted to dilate the stent in order to
achieve angiographic images meeting the above criteria.
TACE technique
After right femoral artery puncture, a 4-F glide
catheter was pushed to the coeliac artery for arteriography, and
then into the superior mesenteric artery, left gastric artery and
bilateral phrenic artery for angiography when necessary, in order
to determine the tumor-supplying artery. Next, another catheter was
pushed to the target vessel (a 3-F micro-catheter was used when
selection was difficult). When the catheter was in position, 0.5–1
g 5-fluorouracil and 100–200 mg oxaliplatin plus 10–60 mg
lyophilized pirarubicin added to 5–20 ml Lipiodol was infused for
embolization of the tumor-supplying artery according to the CT
findings prior to the procedure, and the liver function and
angiography results of the patients.
Post-procedural observation and
follow-up
After stenting, all patients received an intravenous
injection of 20 mg furosemide to prevent heart failure and
symptomatic anti-inflammatory and hepatoprotective treatments under
ECG monitoring. At 1 day after stenting, all patients underwent
single-photon emission computed tomography (SPECT)/CT examinations
to examine the stent implantation and position of iodine-125 seed
strands and observe the radiation distribution of implanted
iodine-125. At 3 days after stenting, symptoms associated with IVC
obstruction were re-scored. Patients were followed-up monthly in
the outpatient department, and liver and kidney functions, blood
routine examination as well as upper abdominal CT examinations were
conducted to monitor the IVC patencies and changes of hepatic
lesions after treatment. The tumor mass reduction rate was judged
by response evaluation criteria in solid tumors (RECIST) criteria
(http://www.irrecist.com/recist/): i)
complete response (CR) was complete disappearance of the tumor; ii)
partial response (PR) was shrinkage of the tumor size by ≥30%; iii)
stable disease (SD) was shrinkage of the tumor size by <30% or
an increase of tumor size of <20%; iv) progression of disease
(PD) was an increase of tumor size by ≥20% or the appearance of new
lesions. When existing residual lesions or new lesions were
diagnosed, TACE was performed again 6–8 weeks after the last
treatment.
Statistical analysis
The data are expressed as mean ± standard deviation.
Basic data of the groups, pre- and post-stenting obstruction
indices in the two groups and indices of obstruction between two
groups following the procedure were compared with t-tests. The
tumor response rates between the two groups were compared by
χ2 test. SPSS software, version 13.0 (SPSS, Inc.,
Chicago, IL, USA) was used for data processing, and P<0.05 was
considered to indicate a significant difference.
Results
Basic characteristics of the
patients
Based on the inclusion criteria, 108 patients with
HCC and IVC obstruction were selected and 18 of them accepted TACE
and stents combined with iodine-125 seed strands. From the
remaining 90 patients who accepted TACE and stent treatments but
rejected iodine-125 seeds, 34 subjects with no significant
differences of basic characteristics compared with the above 18
subjects were selected; thus, 52 patients (34 with TACE and stent
as well as 18 with TACE, stent and iodine-125 seed treatments) were
involved in this study (Table I).
The average age was 61±10 years (50–75 years). Liver function
grades in the study population were Child-Pugh A in 43 cases and
Child-Pugh B in 9 cases. Clinical obstruction symptoms were 45
cases of mild edema, 7 cases of severe edema in the lower limbs, 8
cases of abdominal wall superficial varicosity and 2 cases of
scrotal edema, while the average score of obstruction-associated
symptoms prior to treatment was 2.4±0.5 (2,3). Five
patients suffered from IVC obstructions due to external pressure
from retroperitoneal lymph node metastasis; 44 patients suffered
from IVC obstructions due to the invasion of hepatic lesions; and
in 3 patients the IVC obstructions were caused by tumor
thrombus.
 | Table I.Comparison of basic characteristics
between groups with and without iodine-125 seed strand
implantation. |
Table I.
Comparison of basic characteristics
between groups with and without iodine-125 seed strand
implantation.
| Variable | With iodine-125
(n=18) | Without iodine-125
(n=34) |
|---|
| Number of
patients | 18 | 34 |
| Gender, n |
|
|
| Male | 18 | 27 |
|
Female | 0 | 7 |
| Average age,
years | 61±10 | 62±10 |
| Child-Pugh grade |
|
|
| A | 15 | 28 |
| B | 3 | 6 |
| Serum AFP ≥400 ng/ml,
n | 18 | 34 |
| Location of
tumor | Hepatic hilar | Hepatic hilar |
| Score of IVC
obstructive symptom | 2–3 | 2–3 |
| Position of IVC
obstruction | Hepatic segment | Hepatic segment |
| Type of IVC
obstruction, n |
|
|
|
Compression | 1 | 4 |
|
Invasive | 17 | 27 |
| Tumor
thrombus |
| 3 |
| Average diameter of
IVC obstruction, mm | 5±2 | 5±2 |
| Average pressure
difference between the distal site of IVC obstruction and the right
atrium, mmHg | 17.4±2 | 17.3±2 |
Tumor response rates
There was no CR case in either group. In the group
treated with iodine-125 seed strands, there was 1 PR, 16 SD and 1
PD cases; the tumor response rate was 94.4% (17/18). In the group
without iodine-125 treatment, there were 2 PR, 10 SD and 22 PD
cases; the tumor response rate was 35.3% (12/34). The tumor
response rates were significantly different between the two groups
(χ2=16.6, P<0.001).
Comparison of IVC obstructions between
the two groups and within each group
In the iodine-125 seed strand group, all stents and
iodine-125 seed strands were successfully implanted into the
obstructed IVC segments, comprising a total of 18 stents and 20
iodine-125 seed strands (2 iodine-125 seed strands for 2 patients,
and 1 iodine-125 seed strand in the other 16 patients), containing
348 iodine-125 seeds, which was 16.7±6.9 (range, 10–33 seeds) for
each patient in average. In the group without iodine-125 seed
strands, all 34 stents were successfully implanted. The diameter of
the obstructed IVC segments, the pressure difference between the
distal IVC obstructed segment and the right atrium as well as the
IVC obstruction scores did not significantly differ between the two
groups; however, significant differences were found within each
group, between pre-and post-stenting (P<0.05; Table II).
 | Table II.Comparison of IVC obstruction with and
without iodine-125 seed strands. |
Table II.
Comparison of IVC obstruction with and
without iodine-125 seed strands.
|
| With iodine-125 seed
strands | Without iodine-125
seed strands |
|---|
|
|
|
|
|---|
| Variable | Pre-stenting | Post-stenting | Pre-stenting | Post-stenting |
|---|
| Diameter of
obstructed IVC segment, mm | 5±2 | 2±3 | 5±2 | 2±3 |
| Pressure difference
between the distal IVC obstruction and right atrium, mmHg | 17±2 | 6±2 | 17±2 | 6±2 |
| Score of
obstruction | 2–3 | 0–1 | 2–3 | 0–1 |
| P-value for
comparison within each group, between pre- and post-stenting | <0.05 |
| <0.05 |
|
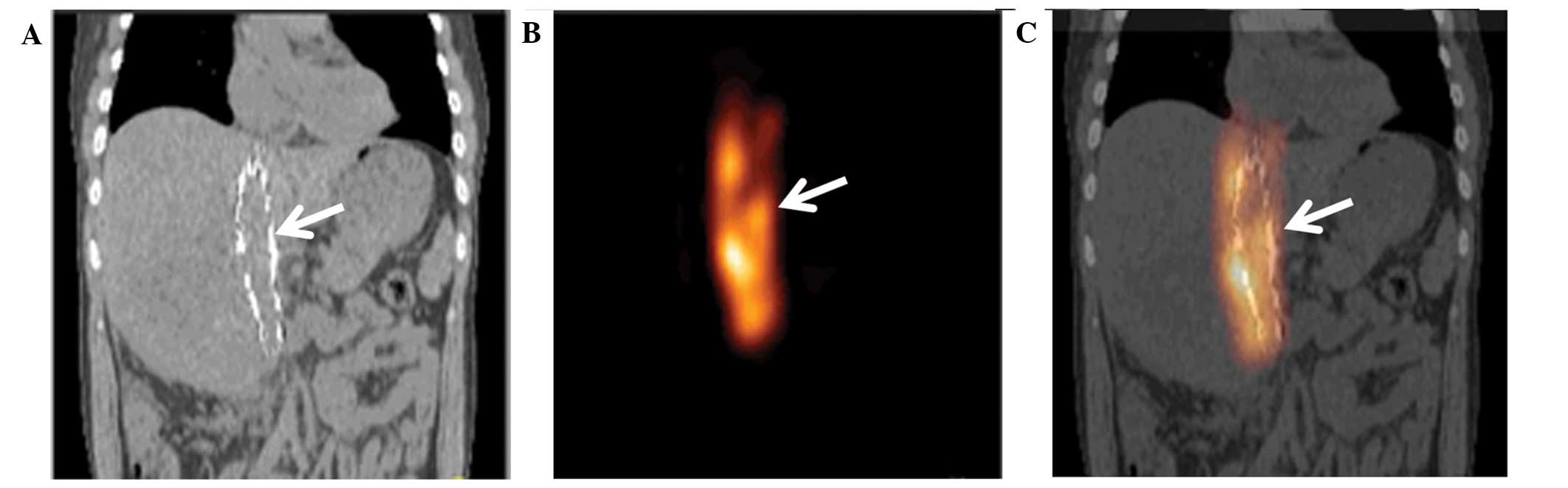
SPECT/CT scanning
At 1 day after the stenting, SPECT/CT images showed
that implanted stents and iodine-125 seed strands were accurately
located in the obstructed IVC segments of all patients without
displacement. The radiation distribution of the iodine-125 seed
strands in the stents was observed to be cylindrical with a radius
of ~1 cm (Fig. 3).
Complications
At 2 days after the procedure, acute renal failure
occurred in 1 patient in the iodine-125 seed strand group with an
increase in the serum creatinine concentration from 54 µmol/l prior
to stenting to 637 µmol/l after stenting; however, the liver
function and blood routine test results remained within the normal
ranges and dialysis alleviated the symptoms. No serious
complications occurred in the other patients.
Discussion
The anti-cancer effect of iodine-125 is thought to
be based on the sustained release of 27.4–31.4 KeV X-rays and 35.5
KeV γ-rays, which kill tumor cells via free oxygen radicals and
ionization (9). The effective
radiation radius of iodine-125 is only 17 mm, which reduces the
damaging effects on normal tissue and adjacent organs (10). Continuous low-dose radioactive
iodine-125 induces the G2/M-phase arrest of tumor cells, which are
then in turn also more sensitive to radiation (11,12). In
addition, implantation of iodine-125 leads to CD3+ and
CD4+ cell activation, thereby producing antitumor immune
responses (13). A randomized
controlled study by Chen et al found that brachytherapy with
iodine-125 following liver resection significantly prolonged the
time to recurrence and overall survival rates (14). Nag et al observed high tumor
complete or partial remission rates for unresectable hepatocellular
carcinoma when using iodine-125 brachytherapy (15) and Chuan-Xing et al reported
that it was effective and safe to perform chemoembolization and
implantation of stents with iodine-125 seeds for patients with HCC
and portal vein thrombus (16). In
the present study, iodine-125 seeds were encapsulated into sterile
plastic 4-F tubes and arranged in a line, thus creating iodine-125
seed strands, which were then placed between the stent and the vena
cava wall. After release, the stent provided sufficient mechanical
expansion force to fix the radioactive seed strands at a
predetermined position. In addition, the continuous adherent effect
of the stent (scaffolding) might reduce the incidence of lung
metastasis and pulmonary embolism caused by tumor thrombus
shedding, which is supported by the fact that no tumor thrombus
shedding was observed in the present study. In this study, the
tumor response rate of patients in the iodine-125 seed strand group
was significantly better than that in the group without iodine-125
(P<0.001). In a previous study (16), it was found that in cases of
hepatocellular carcinoma with portal vein thrombosis, the
intra-stent patency rates were 100, 100 and 84.6% using iodine-125
seeds vs. 100, 93.3 and 53.3% without iodine-125 seeds at 2 months,
6 months and 1 year, respectively, after stent deployment.
Iodine-125 improved stent patencies; however for the difference in
patency improvement to become evident, an extended period is
necessary. In the present study, the average follow-up time of IVC
obstruction was only ~2 months and a prospective study with a
longer follow-up period is required to verify the effect of
iodine-125 on intra-stent restenosis.
Previous studies have reported some complications
associated with iodine-125, such as nausea, vomiting, and decreased
white blood cell and platelet counts; however, the complications
were relatively mild and spontaneous remission was common (17,18). In
the present study, acute renal failure occurred in 1 patient at 1
day after the procedure, but liver function and blood routine test
results were normal. It is hypothesized that the complication was
caused by TACE.
Although the present study has a small sample size
and short follow-up time, it provides a new approach for IVC stent
implantation using iodine-125 seeds. These preliminary experiences
suggest that stents combined with iodine-125 seed strands are
effective and safe for the treatment of HCC with IVC obstruction
after chemoembolization. However, prospective and controlled
studies with larger sample sizes and longer follow-up periods are
required to verify its efficiency.
References
|
1
|
Parkin DM, Bray F, Ferlay J and Pisani P:
Global cancer statistics, 2002. CA Cancer J Clin. 55:74–108. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Tang ZY, Ye SL, Liu YK, et al: A decade's
studies on metastasis of hepatocellular carcinoma. J Cancer Res
Clin Oncol. 130:187–196. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kato Y, Tanaka N, Kobayashi K, Ikeda T,
Hattori N and Nonomura A: Growth of hepatocellular carcinoma into
the right atrium. Report of five cases. Ann Intern Med. 99:472–474.
1983. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zeng ZC, Fan J, Tang ZY, et al: A
comparison of treatment combinations with and without radiotherapy
for hepatocellular carcinoma with portal vein and/or inferior vena
cava tumor thrombus. Int J Radiat Oncol Biol Phys. 61:432–443.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wu LL, Luo JJ, Yan ZP, et al: Comparative
study of portal vein stent and TACE combined therapy with or
without endovascular implantation of iodine-125 seeds strand for
treating patients with hepatocellular carcinoma and main portal
vein tumor thrombus. Zhonghua Gan Zang Bing Za Zhi. 20:915–919.
2012.(In Chinese). PubMed/NCBI
|
|
6
|
Amols HI: Review of endovascular
brachytherapy physics for prevention of restenosis. Cardiovasc
Radiat Med. 1:64–71. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sidawy AN, Weiswasser JM and Waksman R:
Peripheral vascular brachytherapy. J Vasc Surg. 35:1041–1047. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Heysek RV: Modern brachytherapy for
treatment of prostate cancer. Cancer Control. 14:238–243.
2007.PubMed/NCBI
|
|
9
|
Lazarescu GR and Battista JJ: Analysis of
the radiobiology of ytterbium-169 and iodine-125 permanent
brachytherapy implants. Phys Med Biol. 42:1727–1736. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Nath R, Anderson LL, Luxton G, Weaver KA,
Williamson JF and Meigooni AS: Dosimetry of interstitial
brachytherapy sources: Recommendations of the AAPM Radiation
Therapy Committee Task Group No. 43. Med Phys. 22:209–234. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Strasser-Wozak EM, Hartmann BL, Geley S,
et al: Irradiation induces G2/M cell cycle arrest and apoptosis in
p53-deficient lymphoblastic leukemia cells without affecting Bcl-2
and Bax expression. Cell Death Differ. 5:687–693. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhuang HQ, Wang JJ, Liao AY, Wang JD and
Zhao Y: The biological effect of 125I seed continuous
low dose rate irradiation in CL187 cells. J Exp Clin Cancer Res.
28:122009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Xiang GA, Chen KY, Wang HN and Xiao JF:
Immunological influence of iodine-125 implantation in patients with
hepatocellular carcinoma resection. Nan Fang Yi Ke Da Xue Xue Bao.
30:292–294. 2010.(In Chinese). PubMed/NCBI
|
|
14
|
Chen K, Xia Y, Wang H, Xiao F, Xiang G and
Shen F: Adjuvant iodine-125 brachytherapy for hepatocellular
carcinoma after complete hepatectomy: A randomized controlled
trial. PLoS One. 8:e573972013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Nag S, DeHaan M, Scruggs G, Mayr N and
Martin EW: Long-term follow-up of patients of intrahepatic
malignancies treated with iodine-125 brachytherapy. Int J Radiat
Oncol Biol Phys. 64:736–744. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chuan-Xing L, Xu H, Bao-Shan H, et al:
Efficacy of therapy for hepatocellular carcinoma with portal vein
tumor thrombus: Chemoembolization and stent combined with
iodine-125 seed. Cancer Biol Ther. 12:865–871. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Armstrong JG, Anderson LL and Harrison LB:
Treatment of liver metastases from colorectal cancer with
radioactive implants. Cancer. 73:1800–1804. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Martinez-Monge R, Nag S, Nieroda CA and
Martin EW: Iodine-125 brachytherapy in the treatment of colorectal
adenocarcinoma metastatic to the liver. Cancer. 85:1218–1225. 1999.
View Article : Google Scholar : PubMed/NCBI
|