Introduction
Iodine-131 (I-131) therapy and post-therapy I-131
scanning have been accepted as essential components of the overall
management of patients with differentiated thyroid cancer (DTC) for
over six decades (1,2). The finding of any positive lesion on an
I-131 scan is often a decisive indicator for the administration of
subsequent I-131 therapies. Generally, I-131 scanning has a
sensitivity of 60–80% and specificity of >90% (3–6). Despite
this remarkable specificity, false positive uptakes on I-131 scans
do occur, which can be misdiagnosed as residual or metastatic DTC
lesions, and lead to inappropriate I-131 therapies. Since the risk
of secondary malignancies in DTC survivors treated with I-131 is
slightly increased compared with that of DTC survivors not treated
with I-131 (7), and this risk is
dose-related (8), it is important to
identify false positive I-131 scans in order to avoid unnecessary
exposure to radiation.
In the literature, the causes of false positive
results are reported to include contamination, physiological uptake
and unrelated pathological uptake (3,6,9–13).
Regarding saliva, perspiration and urine contamination, with
adequate experience, the clinical practices responsible for this
may be identified. It may be necessary to wash certain body parts
or change contaminated clothes. Physiological uptake in salivary
glands, gastrointestinal and urinary tracts has a characteristic
appearance, and can often be recognized unequivocally. The true
challenge is the diagnosis of pathological false positive I-131
scans. Therefore, the main purpose of this retrospective
investigation was to determine the best imaging modality for
identifying the pathology in patients with pathological false
positive I-131 scans by the investigation of a DTC patient cohort,
and to determine the incidence of pathological false positives.
Materials and methods
Patients
This study was conducted as a collaboration between
the Nuclear Medicine Department and Radiology Department of Tianjin
Medical University General Hospital (Tianjin, China). The data of
patients with DTC archived from January 2008 to January 2010 was
retrieved. The study was approved by the Institutional Review Board
and Ethics Committee of Tianjin Medical University. Written
informed consent was obtained from all patients.
Treatment procedures
The 2006 guidelines (1) and then the 2009 revised guidelines
(2) from the American Thyroid
Association (ATA) were used to treat and monitor these patients.
Briefly, thyroid remnant ablation was performed with I-131
activities of 80–100 mCi. If residual thyroid persisted further
ablations were conducted. Then, empiric I-131 therapy (100–200 mCi)
was administered if cervical, pulmonary or skeletal metastases were
found. I-131 therapy required thyrotropin stimulation for which the
patients were prepared by thyroxine withdrawal for 2–3 weeks along
with a low-iodine diet. Post-therapy I-131 whole body scans were
typically conducted ~1 week after I-131 therapies, using a
dual-detector single-photon emission computed tomography (SPECT)
instrument equipped with high-energy parallel-hole collimators
(Discovery VH; GE Healthcare, Milwaukee, WI, USA). Serum
thyroglobulin (Tg) and thyroglobulin antibody (TgAb) levels were
measured by immunometric assays under thyrotropin-stimulated status
during I-131 therapies (Immulite 2000; Siemens Medical Solutions
Diagnostics, Los Angeles, CA, USA). Simulated levels of Tg <2.0
ng/ml were considered as an indication of DTC lesion-free status,
and Tg ≥2.0 ng/ml as an indication of DTC lesion persistence; this
cutoff value is advocated by ATA guidelines (1,2). A panel
of eight nuclear medicine physicians took direct charge of the
above procedures, and independently interpreted the post-therapy
I-131 scans. Any persistent I-131-avid lesion after complete
thyroid remnant ablation was considered as a positive lesion. The
protocol for any susceptive positive finding was as follows. When
contamination was suspected, another I-131 scan was applied after
washing body parts or changing into new clothes to confirm the
results. Physiological uptake in the salivary glands or in the
gastrointestinal or urinary tract was determined by consensus of
the panel. When pathological positive lesions were identified, they
were marked and later compared with the radiological findings with
more attention.
Imaging procedures
All magnetic resonance imaging (MRI) examinations
were performed with a Signa HDx scanner using a 3-T superconducting
magnet (GE Healthcare). The imaging sequences included T1-,
T2-weighted fast spin echo either with or without fat suppression.
All computed tomography (CT) scans were performed using a 64-slice
spiral CT machine (LightSpeed VCT; GE Healthcare, Waukesha, WI,
USA). Photographic images of scans were captured at windows
appropriate for viewing the mediastinum and the lung.
High-resolution gray-scale ultrasonography (US) was conducted with
real-time equipment (Vivid Five; GE Vingmed Ultrasound, Horten,
Norway), using a 10-MHz linear-array transducer. Positron emission
tomography (PET) was not included as an imaging modality. In
addition to its prohibitive expense, the application of PET is most
suitable for the localization of lesions in patients with DTC who
are Tg-positive and I-131 scan false negative (1,2).
Analysis of false positive scans
The aim of the study was to identify false positive
I-131 scans. A panel of three radiologists took charge of the
radiological procedures, and independently interpreted the images.
Consensus concerning the diagnosis of non-DTC related lesions was
reached by majority. Then radiological images and diagnoses were
supplied to the Nuclear Medicine Panel for further assessments.
Biopsy or needle aspiration results were conducted for patients who
consented to the acquisition of histopathological confirmation.
Finally, the characteristics of the DTC patients
with pathological false positive I-131 scans were summarized and
compared in an attempt to determine the best imaging modality for
diagnosis.
Results
Patient data
From the database, 156 patients with DTC [142
papillary thyroid cancer (PTC) and 14 follicular thyroid cancer
(FTC)] were retrieved. The mean age was 47.23±13.47 years.
Altogether, there were 494 I-131 therapies and the same number of
subsequent post-therapy I-131 scans.
Pathological false positive cases
Among the patients, 6 cases were identified to have
pathological false positive lesions. The incidence of pathological
false positive I-131 scans in the DTC patient cohort was 3.85%
(6/156 cases). All 6 cases had PTC pathology.
Three PTC cases (cases 1–3) had stimulated Tg levels
<2.0 ng/ml, yet exhibited I-131-avid lesions in the anterior
mediastinum on I-131 scans, which were considered as false positive
lesions (Figs. 1–3). Case 1 also demonstrated a false
positive I-131-avid lesion in the right lower abdomen (Fig. 1). Case 4, with a Tg level <2.0
ng/ml, also had a false positive lesion in the right lower abdomen.
Case 5 had a stimulated Tg level of 10.7 ng/ml following complete
thyroid remnant ablation during the second I-131 therapy, when
cervical lesions and a large oropharyngeal lesion were demonstrated
on the I-131 scan. Following three additional I-131 therapies the
cervical lesions almost disappeared yet the oropharyngeal lesion
persisted without change in shape and uptake ability, and was
considered as false positive (Fig.
4). Case 6 had a stimulated Tg level between 3.5 and 4.1 ng/ml,
yet a disproportionately intense I-131-avid cervical lesion was
displayed without any change in two consecutive I-131 therapies
after thyroid remnant ablation (Fig.
5).
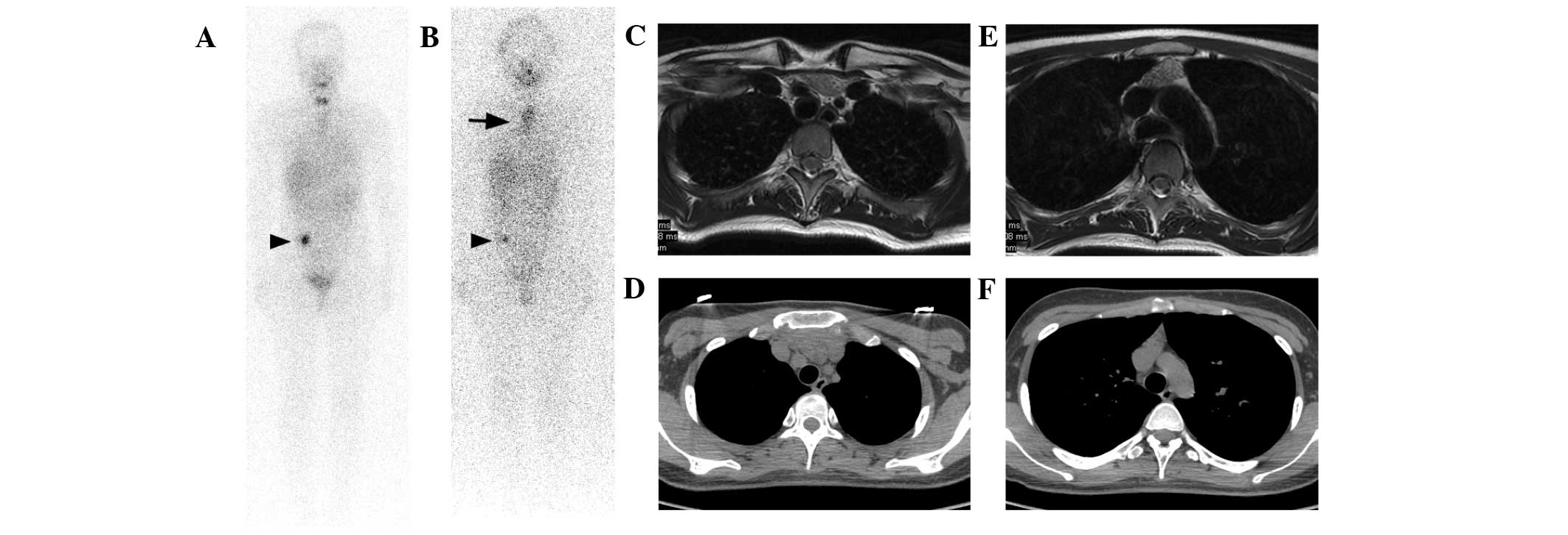 | Figure 1.Case 1. A 22-year-old female PTC
patient underwent three I-131 therapies. A I-131 false positive
lesion was found in the right lower abdomen on the (A) second and
(B) third post-therapy I-131 scans (arrowheads), however no imaging
modality was able to detect and diagnose the lesion. (B) A large
false positive lesion in the mediastinum was identified on the
I-131 scan after the third therapy. The patient had a Tg level of
1.1 ng/ml under TSH stimulation. (C and D) On T2-weighted MRI
images the signal intensity of the lesion was intermediate, which
was lower than that of the adjacent fat tissue. (E and F) On CT
images, the density of the lesion was higher than that of the
adjacent fat tissue. MRI displayed the lesion with better contrast
than that of CT, based on which bilaterally lobed thymic
hyperplasia was diagnosed. Mediastinoscopy with biopsy confirmed
the diagnosis. MRI and CT images were taken during the third I-131
therapy. PTC, papillary thyroid cancer; I-131, iodine-131; Tg,
thyroglobulin; TSH, thyroid-stimulating hormone; MRI, magnetic
resonance imaging; CT, computed tomography. |
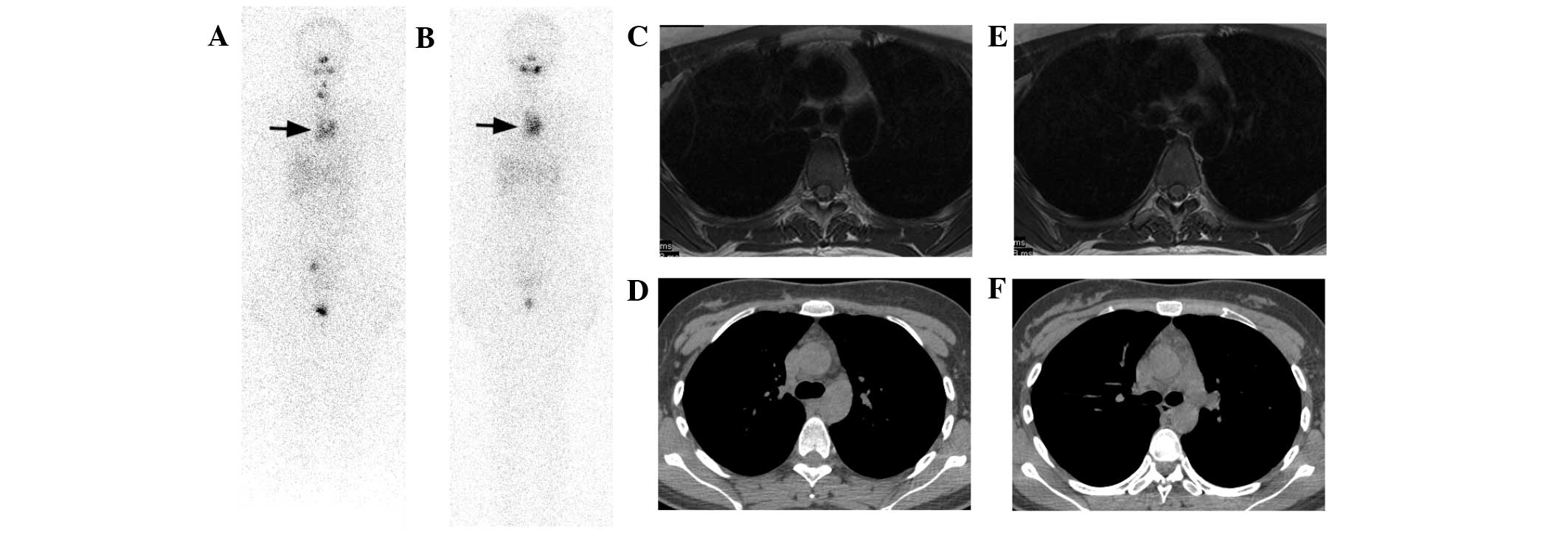 | Figure 3.Case 3. A 23-year-old female PTC
patient received three I-131 therapies. (A) The second post-therapy
I-131 scan demonstrated a small amount of thyroid remnant, yet a
large mediastinum lesion was demonstrated (arrow), which was much
clearer on (B) the third post-therapy I-131 scan when thyroid
remnant was completely ablated (arrow). The TSH-stimulated Tg
levels were 1.2 and 1.0 ng/ml during these I-131 scans. (C and D)
On T2-weighted MRI images, a soft tissue lesion with intermediate
signal intensity tilted to the left side of the anterior superior
mediastinum was shown. (E and F) Although CT images also showed the
lesion, it was obscure compared with MRI and easily overlooked
without careful reading. Thymic hyperplasia was diagnosed primarily
on MRI images. Consent for biopsy was not given. MRI and CT images
were taken during the third I-131 therapy. PTC, papillary thyroid
cancer; I-131, iodine-131; Tg, thyroglobulin; TSH,
thyroid-stimulating hormone; MRI, magnetic resonance imaging; CT,
computed tomography. |
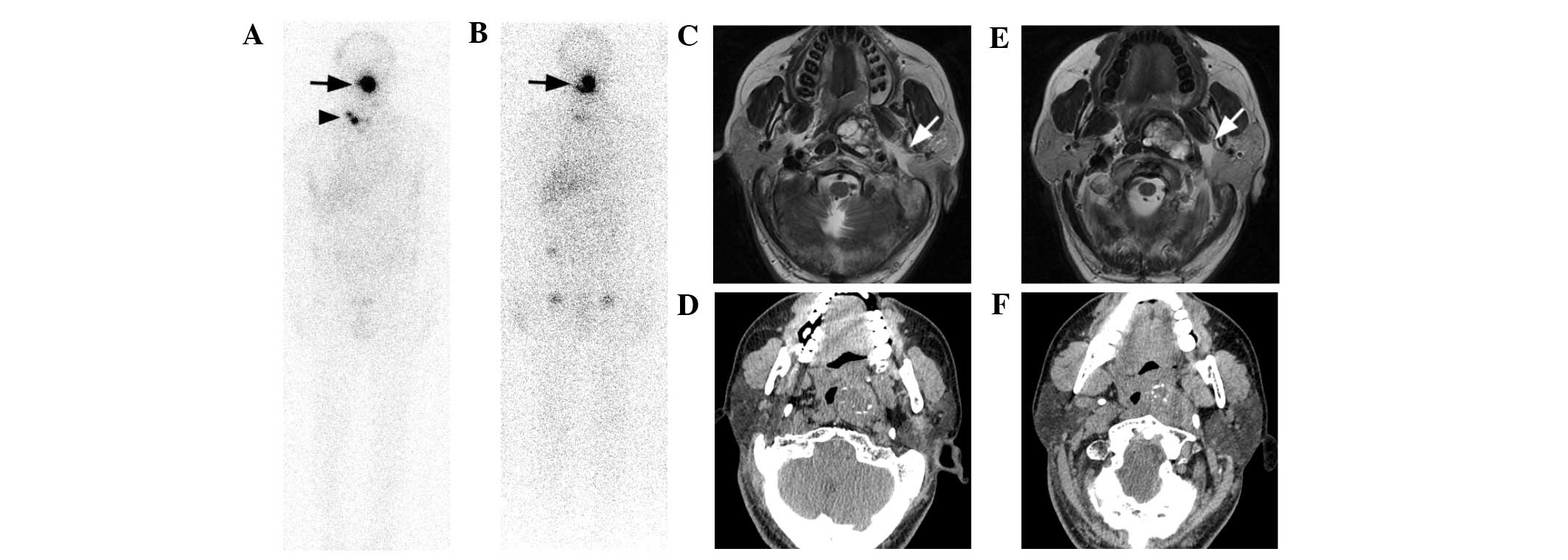 | Figure 4.Case 5. A 43-year-old male PTC patient
was given five I-131 therapies. (A) Cervical lymph node metastases
(arrowhead) and an oropharynx lesion (arrow) were revealed
following complete thyroid remnant ablation on the second
post-therapy I-131 scan while the TSH-stimulated Tg level was 10.7
ng/ml. (B) Another 3 I-131 treatment virtually destroyed all
cervical lymph node metastases reducing the Tg level to 2.8 ng/ml
under stimulation, yet the oropharynx lesion remained the same in
shape and I-131 uptake ability. (C and D) T2-weighted MRI
demonstrated a large oval-shaped lesion with heterogeneous signal
intensity almost obstructing the oropharynx. This lesion was in the
pre-styloid compartment of the parapharyngeal space, and a clear
fatty plane was shown between the lesion and the parotid deep lobe
(white arrows), indicating it was separated from the parotid gland.
(E and F) CT images displayed focal calcifications in the mass and
the position of styloid process, suggesting its location in the
pre-styloid space. Generally, MRI provided more information than CT
about pleomorphic content, location, margins of the lesion and its
relationships with surrounding structures; based on which
pleomorphic adenoma was diagnosed. Biopsy histopathology confirmed
this diagnosis. MRI and CT images were taken during the fifth I-131
therapy. PTC, papillary thyroid cancer; I-131, iodine-131; Tg,
thyroglobulin; TSH, thyroid-stimulating hormone; MRI, magnetic
resonance imaging; CT, computed tomography. |
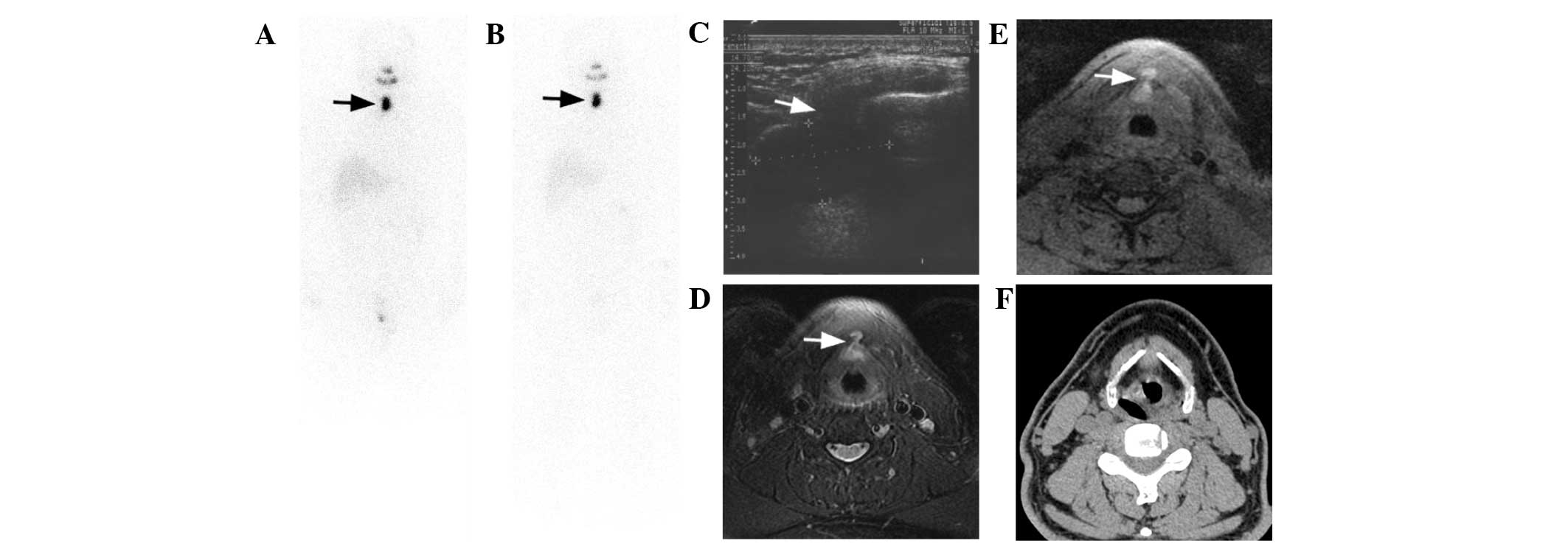 | Figure 5.Case 6. A 44 year-old male PTC patient
was treated with a total of three I-131 therapies. Consequent I-131
scans after the (A) second and (B) third I-131 treatments showed
successful thyroid remnant ablation, yet an oval lesion in the
midline of the neck was exhibited with its location, shape and
intensity unchanged during the follow-up (arrows). TSH-stimulated
Tg levels were 4.1, 3.5 and 3.8 ng/ml for the three therapies. (C)
US demonstrated a dumbbell-shaped cyst lesion with the upper part
posterior to the hyoid bone and the lower part anterior to the
thyroid cartilage and isthmus through thyrohyoid membrane (white
arrow). Due to interference by hyoid bone and thyroid cartilage,
the lesion margins were not so sharp and clear on US images. (D and
E) T1- and T2-weighted MRI images with fat suppression exhibited
the uniquely shaped lesion much more clearly, particularly the
penetration of the lesion isthmus through thyrohyoid membrane
(white arrows). The lesion demonstrated slightly high signal
intensity and high signal intensity on fat-suppressed T1- and
T2-weighted images, respectively, with homogeneous distribution,
reflecting the protein-rich fluid content of the cyst. Thyroglossal
duct cyst was diagnosed by MRI. (F) However, it was difficult to
diagnose by CT alone, because the density of the lesion was almost
identical to that of the adjacent soft tissue structures.
Aspiration pathology confirmed MRI findings concerning the content
of the lesion.MRI, CT and US images were taken during the third
I-131 therapy. PTC, papillary thyroid cancer; I-131, iodine-131;
Tg, thyroglobulin; TSH, thyroid-stimulating hormone; US,
ultrasound; MRI, magnetic resonance imaging; CT, computed
tomography. |
Evaluation of imaging modalities
Characteristics of the false positive I-131 scan
cases are listed in Table I. MRI was
demonstrated as the best imaging modality for diagnosis due to
superior soft tissue resolution. US was shown to be the least
useful, because thymus hyperplasia and parapharyngeal pleomorphic
adenoma were not accessible. No imaging modality was able to locate
false positive sites in the abdomen. MRI, CT or US did not detect
lesions in other DTC patients as non-DTC lesions by the Radiology
Panel, and no other pathological false positive I-131 scans were
identified by the Nuclear Medicine Panel.
 | Table I.Database of DTC patients with
pathological false positive I-131 scans. |
Table I.
Database of DTC patients with
pathological false positive I-131 scans.
| Case no. | Age
(years)/gender | Pathology | Total I-131
therapies | Lesion site | MRI diagnosis | CT diagnosis | US diagnosis | Biopsy or aspiration
pathology |
|---|
| 1 | 22/F | PTC | 3 | Mediastinum | Enlarged thymus | Enlarged thymus | Not accessible | Thymic hyperplasia
(mediastinoscopy biopsy) |
| 1 | 22/F | PTC | 3 | Abdomen | Not detected | Not detected | Not detected | Not consented to |
| 2 | 19/F | PTC | 2 | Mediastinum | Enlarged thymus | Enlarged thymus | Not accessible | Thymic hyperplasia
(mediastinoscopy biopsy) |
| 3 | 23/F | PTC | 3 | Mediastinum | Enlarged thymus | Soft tissue mass,
possibly thymus, yet very obscure | Not accessible | Not consented
to |
| 4 | 59/F | PTC | 3 | Abdomen | Not detected | Not detected | Not detected | Not consented
to |
| 5 | 43/M | PTC | 5 | Oropharynx | Pleomorphic
adenoma | Soft tissue mass
with calcifications, suggestive of pleomorphic adenoma | Not accessible | Pleomorphic adenoma
(biopsy) |
| 6 | 44/M | PTC | 3 | Neck | Thyroglossal duct
cyst | Soft tissue
attenuation lesion, easily overlooked | Thyroglossal duct
cyst | Colloid and mucus
without malignant cells (aspiration) |
Discussion
The trapping of I-131 by DTC lesions allows the
acquisition of scans to identify local and distant metastases. The
challenge to the clinicians is to tailor the treatment to be
vigorous enough to eradicate DTC lesions but not to cause
unnecessary morbidity. The correct interpretation of I-131 scans is
critical in the proper management of patients with DTC, which
requires a thorough knowledge and understanding of all potential
confounding phenomena. False positive findings on I-131 scans can
be classified into contamination, physiological uptake and
pathological uptake (3,4,6,9–13).
Pathological false positive uptake of I-131, ranging from
non-thyroidal neoplasm to inflammation or infection, epitomizes the
diagnostic difficulties that clinicians could confront in this
perspective when managing patients with DTC. Ideally, the
identification of false-positive results should be made through
conservative means whenever possible (3,4,6,9–13).
For patients with DTC, the finding of a mediastinal
mass represents a diagnostic dilemma, and the distinction between
thymic hyperplasia and DTC metastasis must be made correctly.
Notably, Niendorf et al (14)
once reported a high prevalence of 33% for thymic hyperplasia in
patients with DTC on the basis of CT diagnostic criteria, which was
more commonly found in the subgroup of patients with DTC who did
not have metastasis. However, a study by Davidson and Davidson
(15) demonstrated that 6/175 DTC
patients exhibited I-131 uptake by the thymus, and they were found
to be disease-free during the follow-up. These facts indicated that
only some DTC patients with thymic hyperplasia could actually
result in a false-positive conundrum in I-131 imaging. The
mechanism in this respect may be explained by the presence of the
sodium iodine symporter (16);
however, compared with the thyroid gland, the capacity to transport
and concentrate iodine has been found to be diminished in
extrathyroidal tissues, and it appears that the absence of remnant
thyroid or metastasis plus higher I-131 activities could increase
the probability of thymus visualization on post-therapeutic I-131
scans (17,18). In the present study, 3/156 patients
demonstrated a positive uptake of I-131 by the thymus while their
Tg results were negative. All of the patients were young and DTC
lesion-free. Both MRI and CT displayed soft tissue prominences that
affected the bilateral lobes (Fig.
1), were pyramidally shaped (Fig.
2) or deviated to the left of the anterior mediastinum
(Fig. 3), indicating thymic
hyperplasia. However, MRI was superior to CT because of its clearer
soft tissue resolution (e.g., thymic tissue vs. fat tissue) and
distinctive large blood vessel landmarks due to the flow void
phenomenon. However, there is a limitation for MRI, in that the
acquisition time is much longer than that of 64-slice CT, so
misregistration artifacts from respiration might obscure the lesion
on MRI images. Since 64-slice CT data is acquired during one
suspended inspiration, the thoracic cavity is more extended in CT
images than on MRI images. As standard protocols for CT scanning of
the thorax, patients are requested to cross their arms over the
head, yet MRI acquisition requires patients to put their arms
beside their body due to the narrowness of the gantry; this can
often result in alterations in the relative positioning of the
breasts and clavicles on CT images.
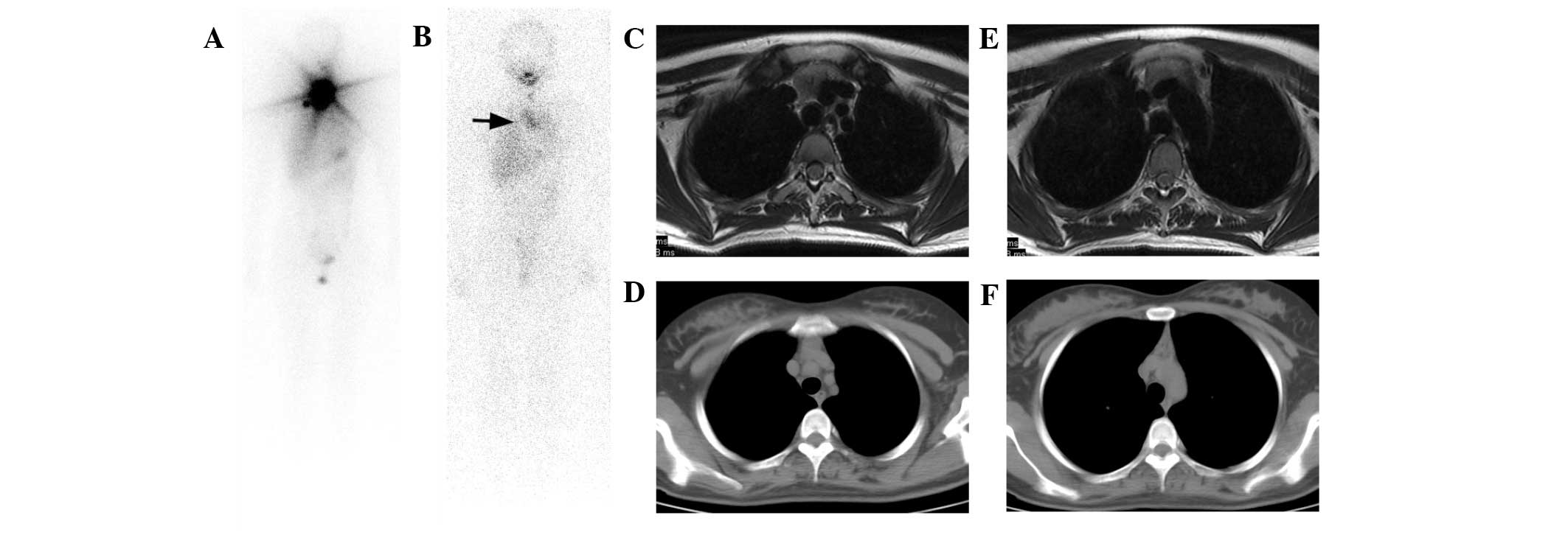 | Figure 2.Case 2. A 19-year-old female PTC
patient received two I-131 therapies. (A) The first post-therapy
I-131 scan demonstrated a large thyroid remnant. (B) The second
post-therapy I-131 scan indicated successful ablation of the
thyroid remnant with a Tg level <0.9 ng/ml under TSH
stimulation, yet a false positive mediastinum lesion was visible
(arrow). (C and D) On T2-weighted MRI images, a pyramidally shaped
lesion with intermediate signal intensity (lower than that of
adjacent fat tissue) was revealed. (E and F) CT images also
exhibited the pyramidally shaped lesion with higher density than
fat tissue. Since CT acquisition was finished during one suspended
inspiration, the thoracic cavity was more extended than that in MRI
images and the pyramidal shape of the lesion seemed more evident.
MRI displayed better resolution of the lesion than CT, and
consequently thymic hyperplasia was diagnosed. Mediastinoscopy
biopsy also confirmed the diagnosis. MRI and CT images were taken
during the second I-131 therapy. PTC, papillary thyroid cancer;
I-131, iodine-131; Tg, thyroglobulin; TSH, thyroid-stimulating
hormone; MRI, magnetic resonance imaging; CT, computed
tomography. |
Tumors in the parapharyngeal space are very rare and
account for only 0.5% of head and neck neoplasms; these usually
present a formidable challenge for preoperative diagnosis as well
as appropriate surgical treatment (19–22).
This space is divided into pre-styloid and post-styloid
compartments by a styloid diaphragm extending from the styloid
process to the tensor-vascular-styloid fascia. In pre-styloid
space, pleomorphic adenoma is the most common benign neoplasm.
Previous studies have demonstrated that MRI is better than CT in
detecting anatomical complexity, tissue diversity, relation to the
internal carotid artery and origin of pleomorphic adenoma, and is
the choice of examination (19–22).
Furthermore, if MRI shows a fatty plane between pleomorphic adenoma
and the parotid deep lobe, as shown in the present case 5 (Fig. 4), it indicates that the tumor is
separated from the parotid deep lobe and originates from a minor
salivary gland. Although CT is slightly inferior to MRI in soft
tissue evaluation, it can better depict the styloid process and
focal calcified degeneration (Fig.
4), which is also often observed in pleomorphic adenoma
(23). When imaging studies
demonstrate a parapharyngeal lesion with no vascular nature, biopsy
or fine needle aspiration can provide very accurate
histopathological diagnosis (19,21–22). In
the present investigation, case 5 was firmly diagnosed as
pleomorphic adenoma by MRI in the pre-styloid space, and CT
provided additional information of focal calcification. Following
complete ablation of the thyroid remnant, cervical lymph node
metastases and a parapharyngeal pleomorphic adenoma were revealed.
When I-131 therapies destroyed almost all cervical metastases, the
pleomorphic adenoma persisted without change in shape and uptake
intensity, causing evident false positive I-131 uptake. To the best
of our knowledge, there are no previous reports of such a
pathological false-positive. Our hypothesis of the underlying
mechanism is, as a benign tumor originating from the salivary
gland, pleomorphic adenoma retains I-131 uptake ability, which is
mediated by the expression of sodium iodine symporter (16).
The concurrence of ectopic thyroid, thyroglossal
duct remnant or thyroglossal duct cyst with DTC has previously been
reported (24–26). Any dormant ectopic thyroid tissue
that is left along the thyroglossal duct during the embryological
development and migration of the thyroid primordium to form the
thyroid gland can be augmented by TSH stimulation under the
hypothyroidism status during I-131 therapy. They can be mistaken
for DTC metastases and the false positive I-131 scans could be
significant diagnostic pitfalls (24–26).
Anatomical imaging modalities are very important for differential
diagnoses, which were compared in case 6 in the present study. Due
to high soft tissue resolution, MRI was able to clearly display the
dumbbell-shaped thyroglossal duct cyst with isthmus penetrating
through the thyrohyoid membrane, particularly in the fat-suppressed
T2-weighted sequence (Fig. 5). Fluid
content rich in protein was diagnosed in the cyst by MRI, which was
confirmed by needle aspiration pathology. US had the great
advantage of detecting cervical lesions, and also revealed the
fluid-content filled cyst in case 6. Although the focal margins of
the lesion were not very sharp owing to interference by hyoid bone
and thyroid cartilage, the diagnosis by US was quite conclusive.
However, it would be relatively difficult to diagnose the
thyroglossal duct cyst by CT alone in case 6, particularly if CT
images were not carefully read or other imaging modality assistance
was not consulted, since the density of the thyroglossal duct cyst
was almost the same as that of the adjacent soft tissue structures
(Fig. 5). However, if the diagnosis
was established, CT would exhibit the advantage of presenting the
complex relationship of the cyst with hyoid bone and thyroid
cartilage.
US is, by far, the most frequently used imaging
procedure in monitoring residual or recurrent thyroid cancer in the
central and lateral neck. However, the diagnostic capabilities of
US in the parapharyngeal, retropharyngeal and mediastinal areas are
limited by interference from bone and air. MRI and CT are of
particular interest since they provide excellent anatomical images
for these areas. Compared with CT, MRI causes negligible exposure
to radiation and provides superior soft tissue resolution, and MRI
avoids the limitation inherent to iodinated contrast agents of CT
in the imaging of patients with DTC who are undertaking I-131
therapies. In fact, MRI has been demonstrated to be a very
efficient modality for the detection of persistent or recurrent DTC
in cervical, mediastinal, parapharyngeal and retropharyngeal spaces
(27–29). In the current investigation, MRI
exhibited superior capability compared with that of CT and US in
the diagnosis of pathological false positive I-131-avid lesions,
namely, thymic hyperplasia in the anterior mediastinum, pleomorphic
adenoma in the parapharyngeal space and thyroglossal duct cyst in
the neck. In addition, all imaging modalities were of limited usage
in the identification of abdominal false positive I-131 lesions in
this cohort (case 1 and 4), probably because the lesions were too
small, although regional inflammation of the right lower abdomen,
such as chronic appendicitis, colitis or oophoritis was
suspected.
A limitation of the present study is the
comparatively small size of the cohort, which intrinsically could
only encompass a restricted number of pathological I-131 false
positive cases. Although the retrospective analysis is very
laborious, a further study over a much longer period is
planned.
In conclusion, pathological false positive I-131
scans occurred with an incidence of 3.85% in the current DTC
patient cohort, which included thymic hyperplasia in the
mediastinum (3 cases), pleomorphic adenoma in the parapharyngeal
space (1 case) and thyroglossal duct cyst in the neck (1 case). MRI
was demonstrated to be the best imaging modality for diagnosing
these pathological false-positives.
Acknowledgements
This investigation was supported by National Key
Clinical Specialty Project (awarded to the Departments of Nuclear
Medicine and Radiology). This study was also supported by Tianjin
Medical University New Century Excellent Talent Program; Young and
Middle-aged Innovative Talent Training Program from Tianjin
Education Committee; and Talent Fostering Program (the 131 Project)
from Tianjin Human Resources and Social Security Bureau (all
awarded to Zhaowei Meng).
References
|
1
|
Cooper DS, Doherty GM, Haugen BR, Kloos
RT, Lee SL, Mandel SJ, Mazzaferri EL, McIver B, Sherman SI and
Tuttle RM: American Thyroid Association Guidelines Taskforce:
Management guidelines for patients with thyroid nodules and
differentiated thyroid cancer. Thyroid. 16:109–142. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
American Thyroid Association (ATA)
Guidelines Taskforce on Thyroid Nodules and Differentiated Thyroid
Cancer. Cooper DS, Doherty GM, Haugen BR, Kloos RT, Lee SL, Mandel
SJ, Mazzaferri EL, McIver B, Pacini F, Schlumberger M, et al:
Revised American Thyroid Association management guidelines for
patients with thyroid nodules and differentiated thyroid cancer.
Thyroid. 19:1167–1214. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Carlisle MR, Lu C and McDougall IR: The
interpretation of 131I scans in the evaluation of
thyroid cancer, with an emphasis on false positive findings. Nucl
Med Commun. 24:715–735. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
van Sorge-van Boxtel RA, van Eck-Smit BL
and Goslings BM: Comparison of serum thyroglobulin, 131I
and 201Tl scintigraphy in the postoperative follow-up of
differentiated thyroid cancer. Nucl Med Commun. 14:365–372. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lubin E, Mechlis-Frish S, Zatz S, Shimoni
A, Segal K, Avraham A, Levy R and Feinmesser R: Serum thyroglobulin
and iodine-131 whole-body scan in the diagnosis and assessment of
treatment for metastatic differentiated thyroid carcinoma. J Nucl
Med. 35:257–262. 1994.PubMed/NCBI
|
|
6
|
McDougall IR: Whole-body scintigraphy with
radioiodine-131. A comprehensive list of false-positives with some
examples. Clin Nucl Med. 20:869–875. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sawka AM, Thabane L, Parlea L,
Ibrahim-Zada I, Tsang RW, Brierley JD, Straus S, Ezzat S and
Goldstein DP: Second primary malignancy risk after radioactive
iodine treatment for thyroid cancer: A systematic review and
meta-analysis. Thyroid. 19:451–457. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Rubino C, de Vathaire F, Dottorini ME,
Hall P, Schvartz C, Couette JE, Dondon MG, Abbas MT, Langlois C and
Schlumberger M: Second primary malignancies in thyroid cancer
patients. Br J Cancer. 89:1638–1644. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Shapiro B, Rufini V, Jarwan A, Geatti O,
Kearfott KJ, Fig LM, Kirkwood ID and Gross MD: Artifacts,
anatomical and physiological variants, and unrelated diseases that
might cause false-positive whole-body 131-I scans in patients with
thyroid cancer. Semin Nucl Med. 30:115–132. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sutter CW, Masilungan BG and Stadalnik RC:
False-positive results of I-131 whole-body scans in patients with
thyroid cancer. Semin Nucl Med. 25:279–282. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Greenler DP and Klein HA: The scope of
false-positive iodine-131 images for thyroid carcinoma. Clin Nucl
Med. 14:111–117. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bakheet SM and Hammami MM: False-positive
radioiodine whole-body scan in thyroid cancer patients due to
unrelated pathology. Clin Nucl Med. 19:325–329. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Mitchell G, Pratt BE, Vini L, McCready VR
and Harmer CL: False positive 131I whole body scans in thyroid
cancer. Br J Radiol. 73:627–635. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Niendorf ER, Parker JA, Yechoor V, Garber
JR and Boiselle PM: Thymic hyperplasia in thyroid cancer patients.
J Thorac Imaging. 20:1–4. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Davidson J and McDougall IR: How
frequently is the thymus seen on whole-body iodine-131 diagnostic
and post-treatment scans? Eur J Nucl Med. 27:425–430. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Spitzweg C, Joba W, Eisenmenger W and
Heufelder AE: Analysis of human sodium iodide symporter gene
expression in extrathyroidal tissues and cloning of its
complementary deoxyribonucleic acids from salivary gland, mammary
gland and gastric mucosa. J Clin Endocrinol Metab. 83:1746–1751.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Meller J and Becker W: The human
sodium-iodine symporter (NIS) as a key for specific thymic
iodine-131 uptake. Eur J Nucl Med. 27:473–474. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Michigishi T, Mizukami Y, Shuke N,
Yokoyama K, Noguchi M, Watanabe Y, Matsui O, Aburano T, Tonami N
and Hisada K: Visualization of the thymus with therapeutic doses of
radioiodine in patients with thyroid cancer. Eur J Nucl Med.
20:75–79. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bozza F, Vigili MG, Ruscito P, Marzetti A
and Marzetti F: Surgical management of parapharyngeal space
tumours: Results of 10-year follow-up. Acta Otorhinolaryngol Ital.
29:10–15. 2009.PubMed/NCBI
|
|
20
|
Dimitrijevic MV, Jesic SD, Mikic AA,
Arsovic NA and Tomanovic NR: Parapharyngeal space tumors: 61 case
reviews. Int J Oral Maxillofac Surg. 39:983–989. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sergi B, Limongelli A, Scarano E, Fetoni
AR and Paludetti G: Giant deep lobe parotid gland pleomorphic
adenoma involving the parapharyngeal space. Report of three cases
and review of the diagnostic and therapeutic approaches. Acta
Otorhinolaryngol Ital. 28:261–265. 2008.PubMed/NCBI
|
|
22
|
Papadogeorgakis N, Petsinis V, Goutzanis
L, Kostakis G and Alexandridis C: Parapharyngeal space tumors:
Surgical approaches in a series of 13 cases. Int J Oral Maxillofac
Surg. 39:243–250. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Shi H, Wang P, Wang S and Yu Q:
Pleomorphic adenoma with extensive ossified and calcified
degeneration: Unusual CT findings in one case. AJNR Am J
Neuroradiol. 29:737–738. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Basaria S, Westra WH and Cooper DS:
Ectopic lingual thyroid masquerading as thyroid cancer metastases.
J Clin Endocrinol Metab. 86:392–395. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zanotti-Fregonara P, Hindié E, Keller I,
Calzada-Nocaudie M and Devaux JY: Scintigraphic visualization of
glossal thyroid tissue during the follow-up of thyroid cancer
patients. Clin Nucl Med. 32:911–914. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Li D, Meng Z, Zhang G, Yu T, Tan J and
Dong F: Visualization of thyroglossal duct cyst in differentiated
thyroid cancer patient. Clin Nucl Med. 35:499–504. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kaplan SL, Mandel SJ, Muller R, Baloch ZW,
Thaler ER and Loevner LA: The role of MR imaging in detecting nodal
disease in thyroidectomy patients with rising thyroglobulin levels.
AJNR Am J Neuroradiol. 30:608–612. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Gross ND, Weissman JL, Talbot JM, Andersen
PE, Wax MK and Cohen JI: MRI detection of cervical metastasis from
differentiated thyroid carcinoma. Laryngoscope. 111:1905–1909.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Toubert ME, Cyna-Gorse F, Zagdanski AM,
Noel-Wekstein S, Cattan P, Billotey C, Sarfati E and Rain JD:
Cervicomediastinal magnetic resonance imaging in persistent or
recurrent papillary thyroid carcinoma: Clinical use and limits.
Thyroid. 9:e-5971999. View Article : Google Scholar
|



















