Introduction
Acute pancreatitis is an inflammatory disease of the
pancreas characterized by acute abdominal pain and increased
concentrations of serum amylase and lipase. In the majority of
cases, it runs a mild course without complications. However, ~25%
patients develop severe acute pancreatitis (SAP), which may result
in systemic inflammatory response syndrome and subsequent multiple
organ dysfunction syndrome, and the mortality rate is up to 40%
(1,2). In recent years, many investigators have
attempted to reveal the pathogenesis of acute pancreatitis;
however, the exact mechanisms are not well understood. Accumulating
evidence indicates that SAP is closely associated with tissue
injury and microcirculation disorders resulting from the production
of proinflammatory cytokines and mediators, including tumor
necrosis factor (TNF)-α, interleukin (IL)-1β, IL-6 and
intercellular adhesion molecule (ICAM)-1 (3,4). In
addition, studies have shown that acute pancreatitis can lead to
activation of the nuclear factor (NF)-κB signaling pathway
(5,6). Therefore, reducing the levels of
proinflammatory cytokines and adhesion molecules to block the
inflammatory cascade may be a valid treatment strategy for acute
pancreatitis.
Thymosin β4 is a highly conserved polypeptide that
was originally isolated from bovine thymus gland extract in 1981
(7). Thymosin β4 contains 43-amino
acid residues with a molecular weight of 4964.5 Da and an
isoelectric point of 4.6 (8). It is
widely distributed in many tissues and cell types and is found in
high concentrations in blood platelets, macrophages and white blood
cells, but not in red blood cells (9). The most prominent physiological role of
thymosin β4 as the major actin-sequestering peptide contributes to
the regulation of actin polymerization in mammalian nucleated cells
(10). There is growing evidence
that thymosin β4 has numerous biological functions in addition to
actin binding and sequestration. It has been shown to downregulate
a number of key inflammatory mediators (e.g., IL-1β and TNF-α) and
decreases the infiltration of inflammatory cells (11–14).
Thymosin β4 has also been found to reduce inflammation by
inhibiting the activation of NF-κB in TNF-α-induced cells (15,16). A
number of studies have suggested that thymosin β4 is capable of
promoting new blood vessel formation via stimulating the
differentiation and directional migration of endothelial cells
(17,18). These activities of thymosin β4 play
an important role in dermal and corneal wound healing and in
recovery from acute myocardial infarction and traumatic brain
injury (11,19–21).
However, whether thymosin β4 is effective in
alleviating inflammatory response and pancreatic tissue injury in
SAP has not yet been elucidated. The present study investigated the
effect of thymosin β4 on pancreatic injury following SAP, to
identify whether it could be a good candidate for attenuating the
severity of SAP.
Materials and methods
Animals
Adult male SPF Sprague-Dawley rats (weight, 200–250
g) were obtained from the Center for Animal Experiments of Wuhan
University (Wuhan, China). All rats were housed in an animal care
facility with a 12 h light/dark cycle and fed with standard
commercial diets and water ad libitum. Experimental
procedures were approved by the ethics committee of Wuhan
University and performed in accordance with the principles of the
1983 Declaration of Helsinki.
Rat model of SAP
Rats were fasted for 12 h prior to the experiment,
but had free access to water. All rats were subjected to the
induction of anesthesia in a closed chamber with 4% isoflurane
(Abbott Laboratories, Shanghai, China) in 2 l/min oxygen, and were
maintained under anesthesia for surgery with 2% isoflurane in 2
l/min oxygen. The SAP rat model was elicited by a standardized
retrograde infusion of 1 ml/kg body weight 5% sodium taurocholate
(STC) solution (Sigma-Aldrich, St. Louis, MO, USA) into the
bile-pancreatic duct. Saline (20 ml/kg) was injected into the back
subcutaneously to compensate for the fluid loss following the
surgery.
Experimental design
The rats were randomly divided into three groups as
follows: i) STC + thymosin β4 group (Tβ4 group, n=24). Thymosin β4
(GL Biochem Ltd., Shanghai, China; 30 mg/kg) was dissolved in
saline at a concentration of 3 mg/ml and was administered
intraperitoneally 30 min prior to STC infusion. ii) STC + saline
(SAP group, n=24). Prior to the induction of SAP, rats in this
group received saline by intraperitoneal injection. iii)
Sham-operated group (SO group, n=24). In this group, rats were
treated with sham surgery (the pancreas and duodenum were flipped a
number of times) instead of STC infusion. Similar doses of thymosin
β4 have been demonstrated to exert neuroprotective and
neurorestorative effects in experimental models of traumatic brain
injury (22).
Rats in the three groups were sacrificed at 3, 6 and
12 h after treatment (n=8 per group at each time-point). Blood
samples were collected by intracardiac puncture at the respective
time-points in the three groups. Samples were centrifuged at 3,000
× g for 15 min, and the serum was stored at −20℃ for subsequent
biochemical and cytokine measurements. Following sacrifice, tissue
from the head of the pancreas was harvested and fixed in 4%
phosphate-buffered formaldehyde for histopathological observation.
The remaining part of the pancreas was snap frozen in liquid
nitrogen and stored at −80℃ for assay.
Histopathological examination
For histological analysis, paraffin-embedded
pancreatic tissues were sliced to a thickness of 4 µm and stained
with hematoxylin and eosin (H&E). The morphology of the
pancreatic tissues was evaluated and documented by two experienced
pathologists who were blinded to the experiment. Pancreatic
histological assessment was scored for the severity of pancreatitis
based on edema, inflammatory cell infiltrate and hemorrhage, and
acinar necrosis according to the scale described by Schmidt et
al (23).
Serum amylase (AMY) and lipase (LIP)
assays
Serum AMY and LIP levels were measured using
standard techniques with a fully automatic chemistry analyzer
(Olympus AU2700 Chemistry-Immuno Analyzer; Olympus Inc., Tokyo,
Japan).
Measurement of inflammatory mediators
and cytokines
The serum concentrations of TNF-α, IL-1β and IL-6
were measured in units of pg/ml by the use of commercially
available enzyme-linked immunosorbent assay (ELISA) kits
(eBioscience Ltd., Vienna, Austria). The absorbance was read by an
automated microplate ELISA reader (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA) and concentrations were calculated by the
standard curve run on each assay plate. All samples were measured
in duplicate.
Myeloperoxidase (MPO)
determination
Neutrophil infiltration in the pancreas was
quantitated by MPO activity. MPO activity was measured
photometrically with 3,3′,5,5′-tetramethylbenzidine as a substrate,
and the reaction was initiated by adding hydrogen peroxide to the
medium. The MPO activity assay was performed with a commercial kit
according to the manufacturer's instructions (Nanjing Jiancheng
Bioengineering Institute, Nanjing, China).
Immunohistochemistry assay
Pancreatic tissue sections were obtained from
paraffin-embedded tissues. Sections of 4 µm thickness were
deparaffinized with xylene, and 0.3% hydrogen peroxide was used to
inactivate endogenous peroxidase activity. Sections were incubated
in 5% normal goat serum diluted in phosphate-buffered saline (PBS).
Endogenous biotin and avidin binding sites were blocked by avidin
and biotin, respectively. The sections were incubated overnight
with rabbit polyclonal anti-rat NF-κB p65 antibody (1:100; C22B4;
Cell Signaling Technology, Inc., Danvers, MA, USA) in a humidity
chamber at 4°C, then counterstained with hematoxylin. Negative
control experiments were performed in which PBS was used instead of
primary antibody.
Western blot analysis
Pancreatic NF-κB p65, I-κBα and ICAM-1 levels at 12
h after STC infusion were determined by Western blot analysis.
Cytoplasmic and nuclear proteins were extracted using the
Nuclear-Cytosol Extraction kit (Applygen Technologies Inc.,
Beijing, China). Concentrations of protein in the samples were
evaluated by the Bradford method with bovine serum albumin as a
standard. Equal amounts of protein samples were electrophoresed
using 8% sodium dodecyl sulfate polyacrylamide gel electrophoresis
(SDS-PAGE) and then transferred to nitrocellulose membranes.
Non-specific binding was blocked with 5% skimmed milk in
Tris-buffered saline and 0.1% Tween 20 (TBST) buffer at room
temperature for 2 h. The cytoplasmic proteins were incubated with
primary antibodies of rabbit polyclonal anti-rat inhibitor of κB
(I-κB)α antibody (1:1,000; sc-371; Santa Cruz Biotechnology, Inc.,
Dallas, TX, USA), actin antibody (1:2,000; sc-130656; Santa Cruz
Biotechnology, Inc.) and ICAM-1 antibody (1:1,000; sc-7891; Santa
Cruz Biotechnology, Inc.). In addition, the nuclear proteins were
incubated with rabbit polyclonal anti-rat NF-κB p65 (1:1,000;
C22B4; Cell Signaling Technology, Inc.) and histone H3 antibody
(1:1,000; sc-10809; Santa Cruz Biotechnology, Inc.) overnight at
4°C. Following extensive rinsing with TBST, the blots were
incubated with horseradish peroxidase-conjugated goat anti-rabbit
secondary antibody (1:5,000; Pierce Biotechnology, Rockford, IL,
USA) for 1 h at room temperature, and developed with the use of an
electrochemiluminescent reagent (Immobilon Western HRP Substrate;
EMD Millipore, Bedford, MA, USA) and images were captured on
light-sensitive imaging film (Kodak, Rochester, NY, USA). The
protein bands were quantified by densitometry using Quantity One
4.5.0 software (Bio-Rad Laboratories, Inc., Richmond, CA, USA).
Statistical analysis
Data was analyzed using SPSS statistical software,
version 16.0 (SPSS Inc., Chicago, IL, USA). All data are expressed
as the mean ± standard deviation. Data were compared between all
groups by one-way analysis of variance (ANOVA). A two-tailed
P-value of less than 0.05 was considered statistically
significant.
Results
Analysis of serum AMY and LIP
levels
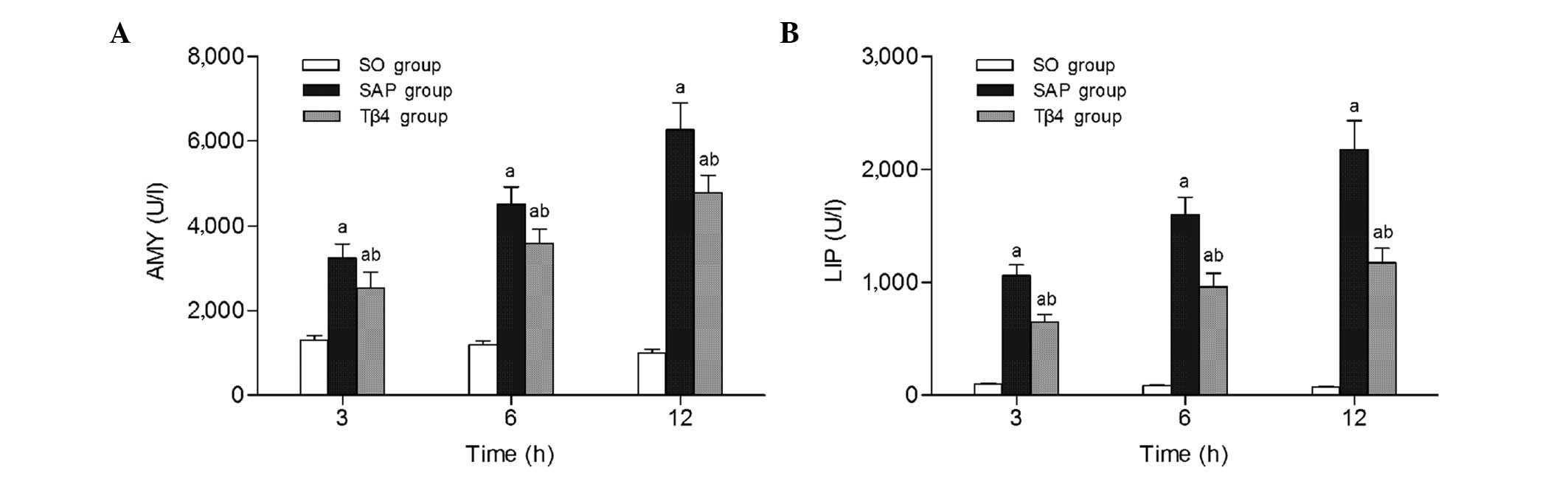
As shown in Fig. 1,
the SAP group had significant increased serum AMY and LIP levels
from 3 h to 12 h compared with those in the SO group (P<0.05).
Compared with the SAP group, treatment with thymosin β4 prior to
STC infusion significantly reduced the serum levels of AMY and LIP
at each time-point (P<0.05).
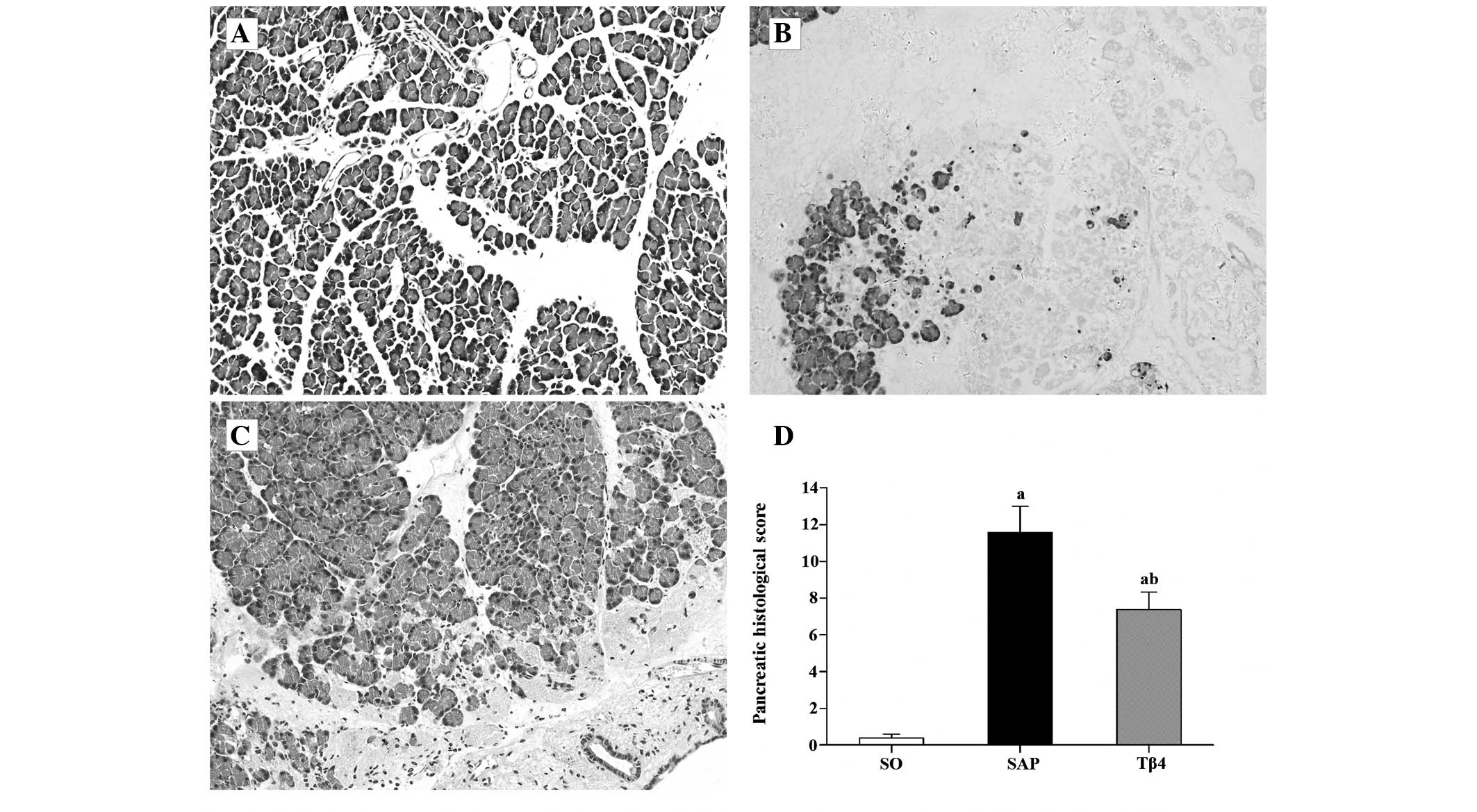
Histopathological assay
Representative pathological sections of the
pancreatic tissue are shown in Fig.
2A–C. In the SAP group, massive areas of acinar necrosis
accompanied by infiltration of inflammatory cells, vacuolization
and hemorrhage were observed. Rats in the SO group showed little
morphological evidence of pancreatic injury, with the exception of
mild interstitial edema. As shown in Fig. 2D, the pancreatic histological score
was significantly decreased in the Tβ4 group compared with the SAP
group.
Analysis of serum proinflammatory
cytokine levels and pancreatic MPO activity
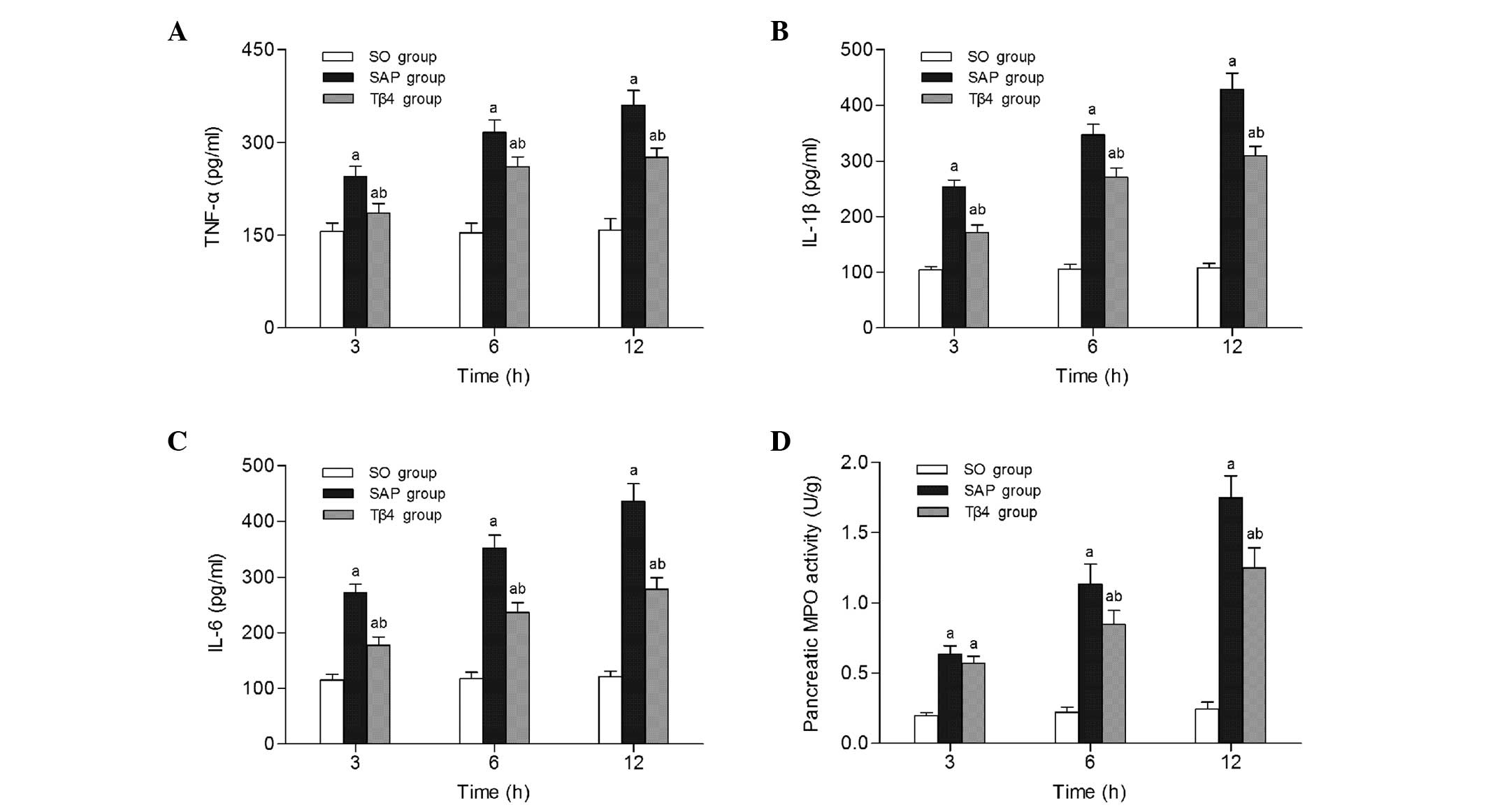
Serum proinflammatory cytokine levels were analyzed
to determine the effect of thymosin β4 on the inflammatory process
of SAP. As illustrated in Fig. 3A–C,
serum concentrations of IL-1β, IL-6 and TNF-α increased
continuously with time. However, the increases in the
concentrations of these cytokines following SAP were markedly
decreased by the administration of thymosin β4 (P<0.05).
Neutrophil infiltration in the pancreas was evaluated by the
measurement of MPO activity after SAP. The MPO activity of the SAP
group increased significantly at each time-point compared with that
in the SO group (P<0.05). When administered prophylactically,
thymosin β4 reduced MPO activity in the pancreatic tissue at 6 to
12 h compared with that in the SAP group (P<0.05; Fig. 3D).
Immunohistochemical analysis
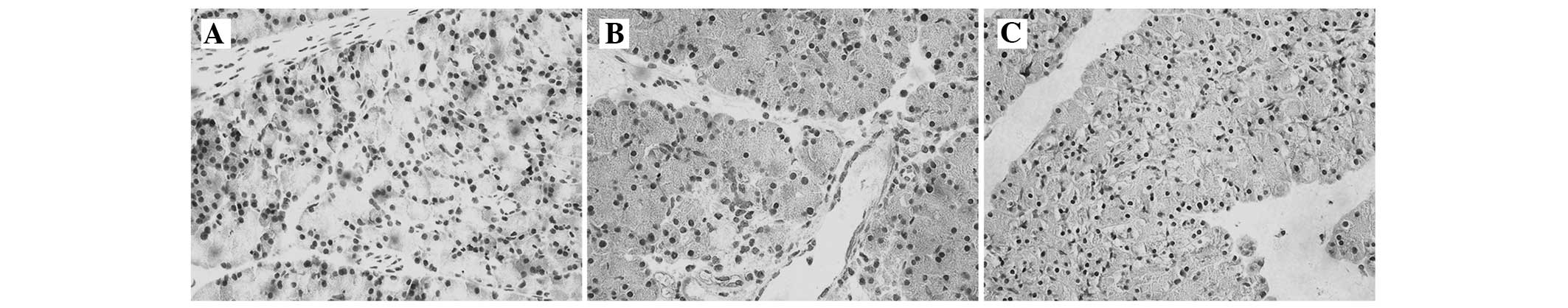
To investigate the localization of NF-κB p65
expression, an immunohistochemical assay was conducted. In the SO
group, NF-κB p65 was expressed mainly in the cytoplasm in
pancreatic tissues (Fig. 4A). NF-κB
p65 immunoreactivity was highly expressed in the nucleus following
the administration of STC (Fig. 4B).
However, a marked reduction in NF-κB p65 staining was observed in
the cell nuclei with thymosin β4 pretreatment, and the staining
increased in the cytoplasm (Fig.
4C).
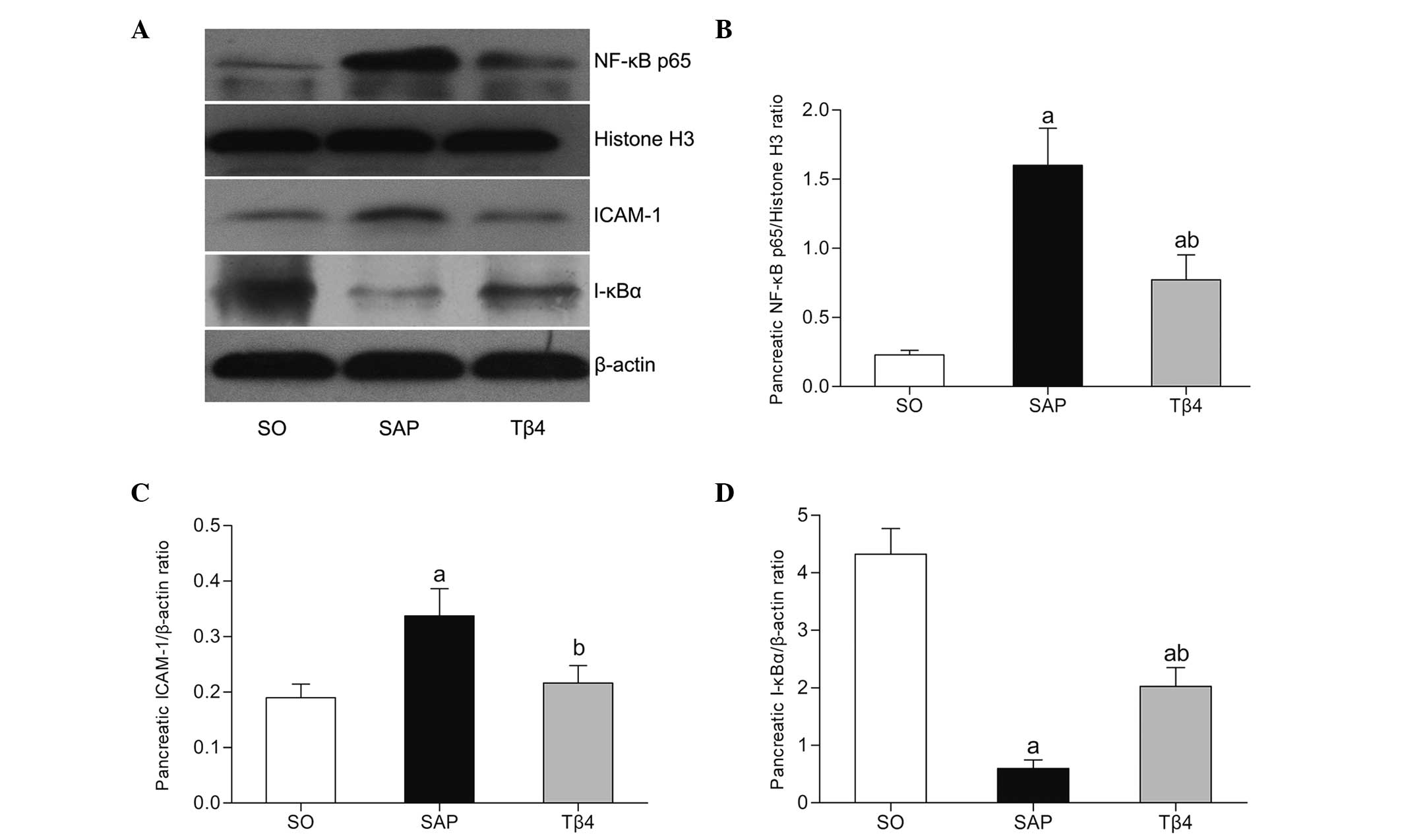
NF-κB p65, I-κBα and ICAM-1 protein
expression
Fig. 5A and B shows
that the expression of NF-κB p65 protein in the pancreas in the SAP
group was higher than that in the SO group at 12 h. In the Tβ4
group, the NF-κB p65 protein was expressed at a much lower level
than in the SAP group (P<0.05). Furthermore, the SAP group
showed a significant reduction of pancreatic I-κBα protein
expression (Fig. 5D). As shown in
Fig. 5C, the expression of
pancreatic ICAM-1 protein in the SAP group was much higher than
that in the SO group. However, ICAM-1 protein expression was much
lower in the Tβ4 group than in the SAP group (P<0.05).
Discussion
Acute pancreatitis causes a release of various
inflammatory mediators, attracts inflammatory cells to the vascular
endothelium and induces the expression of adhesion molecules
(1,24). Studies have shown that NF-κB plays a
critical role in the development of acute pancreatitis (25,26).
Therefore, intervention in NF-κB activation can eliminate the
induced overexpression of proinflammatory cytokines such as TNF-α,
IL-1β and IL-6. As reviewed recently, thymosin β4 has clear
anti-inflammatory and anti-septic shock activities (12,26). In
addition, thymosin β4 can inhibit TNF-α-mediated NF-κB nuclear
translocation and activation (15,16,27).
In the present study, the increased AMY and LIP
levels in the SAP group and the elevated histopathological score of
the pancreas indicate that pancreatic damage increased gradually
following SAP, and that the SAP rat model was successfully induced.
The results also demonstrate that the prophylactic administration
of thymosin β4 alleviated the following: i) serum AMY and LIP
levels; ii) pancreatic neutrophil infiltration; iii) pancreatic
damage; iv) proinflammatory cytokine production; and v) activation
of NF-κB and ICAM-1. These results indicate that pretreatment with
thymosin β4 ameliorates the degree of SAP and exerts potent
anti-inflammatory effects in rats.
Thymosin β4 has shown promising efficacy in animal
models of various diseases, such as myocardial infarction, dermal
wounds, multiple sclerosis, sepsis and endotoxic shock (12,20,28–31).
However, to the best of our knowledge, there have been no reports
concerning thymosin β4 in the treatment of acute pancreatitis until
now. The anti-inflammatory effect of thymosin β4 may be due to the
inhibition of proinflammatory transcription factors such as NF-κB.
Therefore, the present study was conducted to investigate the
alleviating effect of thymosin β4 on pancreatic injury associated
with SAP.
Previous studies have shown that the active RelA/p65
NF-κB subunit plays a critical role in the systemic inflammatory
response in acute pancreatitis (32,33).
NF-κB is normally inactive and resides in the cytoplasm, where it
is sequestered by I-κB (34). Upon
stimulation, I-κB kinase rapidly phosphorylates I-κB proteins,
which leads to their degradation and the release of active NF-κB.
The released NF-κB translocates to the nucleus and activates the
transcription of several important proinflammatory genes: TNF-α,
IL-1β, IL-6 and ICAM-1 (35,36). Inflammatory cytokines such as TNF-α,
IL-1β and IL-6 play an important role in the early stage of acute
pancreatitis and affect the outcome of the disease; in particular,
they are thought to be a trigger of cascading inflammatory response
and multiple organ failure in SAP (37–39). In
the present study, the results also revealed an increase in the
levels of these inflammatory cytokines after acute pancreatitis.
Prophylactic administration of thymosin β4 markedly reduced their
serum levels in rats. This illustrated that thymosin β4 is able to
attenuate the inflammatory response in SAP. The results of
immunohistochemistry detection indicated that NF-κB translocated
from the cytoplasm into the nucleus in the pancreatic tissues upon
activation during pancreatitis. Western blotting results
demonstrated that the I-κBα protein expression levels were reduced
during pancreatitis, and that pretreatment with thymosin β4
inhibited the degradation of I-κBα. These results demonstrate that
thymosin β4 can inhibit NF-κB activation and attenuate pancreatic
injury.
The infiltration of inflammatory cells into the
pancreas is an early and central event in acute pancreatitis that
promotes local damage and systemic complications (40). Studies have demonstrated that the
sequestration of neutrophils plays a key role in the development of
pancreatitis (41). Adhesion
molecules such as ICAM-1 are upregulated in acute pancreatitis, and
cause deleterious effects via neutrophil recruitment to the
pancreas (42). For the optimum
analysis of ICAM-1 activation, measurements were taken at the 12 h
time-point, which coincides with the peak in serum cytokine levels.
The results revealed that the expression of ICAM-1 was
significantly increased in pancreatic tissue following STC
infusion, and pretreatment with thymosin β4 markedly reduced the
expression of ICAM-1 protein in the pancreas.
The increase of ICAM-1 expression in the pancreas
correlated with an increase of leukocyte infiltration measured by
MPO activity. MPO is a peroxidase enzyme produced by azurophilic
granules in neutrophils, which has been used as a biomarker for
neutrophil infiltration in studies of acute pancreatitis (42,43). In
addition, MPO activity can reflect the severity of inflammation
(43). MPO activity noticeably
increased with the induction of pancreatitis, and the increase of
pancreatic MPO activity indicated the progressive aggravation of
pancreatic injury. The thymosin β4 treatment markedly reduced the
increase in pancreatic MPO activity at 6 to 12 h after acute
pancreatitis, which suggests a significant reduction in neutrophil
infiltration into the pancreatic tissues. The finding that thymosin
β4 markedly reduced neutrophil infiltration is consistent with a
previous report demonstrating that treatment with anti-ICAM-1
antibody decreased leukocyte infiltration in pancreas and
attenuated tissue damage (44).
In summary, the present study demonstrates that
thymosin β4 attenuates the severity of pancreatic injury in acute
pancreatitis, through inhibiting NF-κB activation and reducing the
formation of proinflammatory cytokines (TNF-α, IL-1β and IL-6) and
decreasing the expression of the adhesion molecule ICAM-1 and
neutrophil infiltration. However, there are a number of limitations
to this study. For example, the protective effect associated with
thymosin β4 was not investigated in depth, and mechanism by which
thymosin β4 inhibited the NF-κB signal pathway remains unclear. The
present results may serve as a basis for further studies on the
therapeutic potential of thymosin β4 in SAP.
Acknowledgements
The authors thank the Key Laboratory of Hubei
Province for Digestive System Disease, Renmin Hospital of Wuhan
University. This study was supported by the Major Scientific
Research Projects of Health Department of Hubei Province (No.
JX6A07), and the National Natural Science Foundation of China (No.
81300356 and No. 81370562).
References
|
1
|
Bhatia M, Brady M, Shokuhi S, Christmas S,
Neoptolemos JP and Slavin J: Inflammatory mediators in acute
pancreatitis. J Pathol. 190:117–125. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Mofidi R, Duff MD, Wigmore SJ, Madhavan
KK, Garden OJ and Parks RW: Association between early systemic
inflammatory response, severity of multiorgan dysfunction and death
in acute pancreatitis. Br J Surg. 93:738–744. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Granger J and Remick D: Acute
pancreatitis: Models, markers and mediators. Shock. 24:45–51. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Papachristou GI, Clermont G, Sharma A,
Yadav D and Whitcomb DC: Risk and markers of severe acute
pancreatitis. Gastroenterol Clin North Am. 36:277–296. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yu J, Deng W, Wang W, Ding Y, Jin H, Chen
C, Chen X, Xiong X and Xu S: Inhibition of poly (ADP-ribose)
polymerase attenuates acute kidney injury in sodium
taurocholate-induced acute pancreatitis in rats. Pancreas.
41:1299–1305. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Rakonczay Z Jr, Hegyi P, Takács T,
McCarroll J and Saluja AK: The role of NF-kappaB activation in the
pathogenesis of acute pancreatitis. Gut. 57:259–267. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Low TL, Hu SK and Goldstein AL: Complete
amino acid sequence of bovine thymosin beta 4: A thymic hormone
that induces terminal deoxynucleotidyl transferase activity in
thymocyte populations. Proc Natl Acad Sci USA. 78:1162–1166. 1981.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hannappel E: Thymosin beta4 and its
posttranslational modifications. Ann N Y Acad Sci. 1194:27–35.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hannappel E and van Kampen M:
Determination of thymosin beta 4 in human blood cells and serum. J
Chromatogr. 397:279–285. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Goldstein AL, Hannappel E and Kleinman HK:
Thymosin beta4: Actin-sequestering protein moonlights to repair
injured tissues. Trends Mol Med. 11:421–429. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sosne G, Chan CC, Thai K, Kennedy M,
Szliter EA, Hazlett LD and Kleinman HK: Thymosin beta 4 promotes
corneal wound healing and modulates inflammatory mediators in vivo.
Exp Eye Res. 72:605–608. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Badamchian M, Fagarasan MO, Danner RL,
Suffredini AF, Damavandy H and Goldstein AL: Thymosin beta (4)
reduces lethality and down-regulates inflammatory mediators in
endotoxin-induced septic shock. Int Immunopharmacol. 3:1225–1233.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sosne G, Christopherson PL, Barrett RP and
Fridman R: Thymosin-beta4 modulates corneal matrix
metalloproteinase levels and polymorphonuclear cell infiltration
after alkali injury. Invest Ophthalmol Vis Sci. 46:2388–2395. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sosne G, Szliter EA, Barrett R, Kernacki
KA, Kleinman H and Hazlett LD: Thymosin beta 4 promotes corneal
wound healing and decreases inflammation in vivo following alkali
injury. Exp Eye Res. 74:293–299. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sosne G, Qiu P, Christopherson PL and
Wheater MK: Thymosin beta 4 suppression of corneal NFkappaB: A
potential anti-inflammatory pathway. Exp Eye Res. 84:663–669. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Qiu P, Wheater MK, Qiu Y and Sosne G:
Thymosin beta4 inhibits TNF-alpha-induced NF-kappaB activation,
IL-8 expression and the sensitizing effects by its partners PINCH-1
and ILK. FASEB J. 25:1815–1826. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Malinda KM, Goldstein AL and Kleinman HK:
Thymosin beta 4 stimulates directional migration of human umbilical
vein endothelial cells. FASEB J. 11:474–481. 1997.PubMed/NCBI
|
|
18
|
Grant DS, Rose W, Yaen C, Goldstein A,
Martinez J and Kleinman H: Thymosin beta4 enhances endothelial cell
differentiation and angiogenesis. Angiogenesis. 3:125–135. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Treadwell T, Kleinman HK, Crockford D,
Hardy MA, Guarnera GT and Goldstein AL: The regenerative peptide
thymosin β4 accelerates the rate of dermal healing in preclinical
animal models and in patients. Ann N Y Acad Sci. 1270:37–44. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Crockford D: Development of thymosin beta4
for treatment of patients with ischemic heart disease. Ann NY Acad
Sci. 1112:385–395. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Xiong Y, Mahmood A, Meng Y, Zhang Y, Zhang
ZG, Morris DC and Chopp M: Treatment of traumatic brain injury with
thymosin β4 in rats. J Neurosurg. 114:102–115. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Xiong Y, Zhang Y, Mahmood A, Meng Y, Zhang
ZG, Morris DC and Chopp M: Neuroprotective and neurorestorative
effects of thymosin β4 treatment initiated 6 h after traumatic
brain injury in rats. J Neurosurg. 116:1081–1092. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Schmidt J, Rattner DW, Lewandrowski K,
Compton CC, Mandavilli U, Knoefel WT and Warshaw AL: A better model
of acute pancreatitis for evaluating therapy. Ann Surg. 215:44–56.
1992. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Nakae H, Endo S, Sato N, Wakabayashi G,
Inada K and Sato S: Involvement of soluble adhesion molecules in
acute pancreatitis. Eur Surg Res. 33:377–382. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sah RP, Dawra RK and Saluja AK: New
insights into the pathogenesis of pancreatitis. Curr Opin
Gastroenterol. 29:523–530. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Girardi M, Sherling MA, Filler RB, Shires
J, Theodoridis E, Hayday AC and Tigelaar RE: Anti-inflammatory
effects in the skin of thymosin-beta4 splice-variants. Immunology.
109:1–7. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hinkel R, Trenkwalder T and Kupatt C:
Molecular and cellular mechanisms of thymosin β4-mediated
cardioprotection. Ann NY Acad Sci. 1269:102–109. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Bock-Marquette I, Saxena A, White MD,
Dimaio JM and Srivastava D: Thymosin beta4 activates
integrin-linked kinase and promotes cardiac cell migration,
survival and cardiac repair. Nature. 432:466–472. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Shrivastava S, Srivastava D, Olson EN,
DiMaio JM and Bock-Marquette I: Thymosin beta4 and cardiac repair.
Ann NY Acad Sci. 1194:87–96. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Philp D, Badamchian M, Scheremeta B,
Nguyen M, Goldstein AL and Kleinman HK: Thymosin beta 4 and a
synthetic peptide containing its actin-binding domain promote
dermal wound repair in db/db diabetic mice and in aged mice. Wound
Repair Regen. 11:19–24. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhang J, Zhang ZG, Morris D, Li Y, Roberts
C, Elias SB and Chopp M: Neurological functional recovery after
thymosin beta4 treatment in mice with experimental auto
encephalomyelitis. Neuroscience. 164:1887–1893. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Chen X, Ji B, Han B, Ernst SA, Simeone D
and Logsdon CD: NF-kappaB activation in pancreas induces pancreatic
and systemic inflammatory response. Gastroenterology. 122:448–457.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Huang H, Liu Y, Daniluk J, Gaiser S, Chu
J, Wang H, Li ZS, Logsdon CD and Ji B: Activation of nuclear
factor-κB in acinar cells increases the severity of pancreatitis in
mice. Gastroenterology. 144:202–210. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Baldwin AS Jr: The NF-kappaB and I kappaB
proteins: New discoveries and insights. Annu Rev Immunol.
14:649–683. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kim H, Seo JY, Roh KH, Lim JW and Kim KH:
Suppression of NF-kappaB activation and cytokine production by
N-acetylcysteine in pancreatic acinar cells. Free Radic Biol Med.
29:674–683. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Telek G, Ducroc R, Scoazec JY, Pasquier C,
Feldmann G and Rozé C: Differential upregulation of cellular
adhesion molecules at the sites of oxidative stress in experimental
acute pancreatitis. J Surg Res. 96:56–67. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Surbatovic M and Radakovic S: Tumor
necrosis factor-α levels early in severe acute pancreatitis: Is
there predictive value regarding severity and outcome? J Clin
Gastroenterol. 47:637–643. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Malmstrøm ML, Hansen MB, Andersen AM,
Ersbøll AK, Nielsen OH, Jørgensen LN and Novovic S: Cytokines and
organ failure in acute pancreatitis: Inflammatory response in acute
pancreatitis. Pancreas. 41:271–277. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Makhija R and Kingsnorth AN: Cytokine
storm in acute pancreatitis. J Hepatobiliary Pancreat Surg.
9:401–410. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Vonlaufen A, Apte MV, Imhof BA and
Frossard JL: The role of inflammatory and parenchymal cells in
acute pancreatitis. J Pathol. 213:239–248. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Mota R, Sánchez-Bueno F, Berenguer-Pina
JJ, Hernández-Espinosa D, Parrilla P and Yélamos J: Therapeutic
treatment with poly(ADP-ribose) polymerase inhibitors attenuates
the severity of acute pancreatitis and associated liver and lung
injury. Br J Pharmacol. 151:998–1005. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Chen C, Xu S, Wang WX, Ding YM, Yu KH,
Wang B and Chen XY: Rosiglitazone attenuates the severity of sodium
taurocholate-induced acute pancreatitis and pancreatitis-associated
lung injury. Arch Med Res. 40:79–88. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Chooklin S, Pereyaslov A and Bihalskyy I:
Pathogenic role of myeloperoxidase in acute pancreatitis.
Hepatobiliary Pancreat Dis Int. 8:627–631. 2009.PubMed/NCBI
|
|
44
|
Werner J, Z'graggen K, Castillo
Fernández-del C, Lewandrowski KB, Compton CC and Warshaw AL:
Specific therapy for local and systemic complications of acute
pancreatitis with monoclonal antibodies against ICAM-1. Ann Surg.
229:834–842. 1999. View Article : Google Scholar : PubMed/NCBI
|