Introduction
Pterygium is a common ocular surface condition which
may expand on the cornea and thus threaten vision through various
mechanisms, such as visual axis occupation or the induction of
significant astigmatism (1). A
strong association between ophthalmic pterygium and exposure to
solar radiation, particularly to the ultraviolet (UV) light from
the solar spectrum, has been reported (1,2).
Moreover, a number of previous studies have provided evidence of
oncogenic activity in cell populations obtained from pterygium
(1,3–5),
possibly originating from corneo-scleral stem cells (3,4), whereas
the detection of human papilloma virus (HPV) DNA in ophthalmic
pterygia has raised the possibility of viral participation in the
pathogenesis of pterygium (6–9). The
considerable diversity in the mechanisms associated with the
development of pterygium suggests that the same mechanisms may also
be involved in the development of other common ocular surface
conditions. In addition, significant geographical- and
population-related differences in the incidence of pterygium have
been reported, possibly reflecting genetic and environmental
pathogenetic effects (9,10).
Crete is a region with interesting epidemiological
and demographical features associated with pterygium, such as
increased exposure to solar light and a marked diversity in
altitudes of residence (also associated with differences in the
exposure to UV radiation) (5).
Previous studies on pterygia in patients originating from the
Cretan population have detected molecular genetic alterations, such
as the loss of heterozygosity and microsatellite instability, as
well as the involvement of HPV, suggesting a destabilized genetic
background for ocular surface cell populations in affected eyes
(1,4,5). In the
present study, we aimed to determine whether the presence of
ophthalmic pterygium may be associated with an increased likelihood
for the development or presence of other ocular surface lesions in
the same patients from the Cretan population.
Patients and methods
This study analyzed, in a retrospective manner, a
cohort of patients with ophthalmic pterygium treated at the
Department of Ophthalmology of the University Hospital of Heraklion
(Heraklion, Greece) for 8 consecutive years (2006–2014). The
medical history of the patients was examined and clinical data were
recorded, including patient age and gender, the age at which
pterygium was diagnosed for the first time, the surgical history of
pterygium (whether it was primary or recurrent), the location of
pterygium on the ocular surface (nasal, temporal or both) and the
size of pterygium (in mm of advancement on the corneal surface).
Clinical ocular surface images were also examined in order to
assess the degree of pterygium vascularity (on a scale of 1–4). The
concomitant detection of any other ocular surface lesions [which
were present in the eye(s) of the same patient at the time of the
initial clinical examination] was recorded. All chart evaluations
and clinical image assessment were performed by the same
experienced ocular surface surgeon (E.T.D.).
The findings were statistically analyzed using the
statistical package SPSS 8.0 (SPSS, Inc., Chicago, IL, USA). The
incidence of each concomitant condition within the population of
patients with ophthalmic pterygium was recorded. Correlations
between the demographic and clinical characteristics of the
patients in our cohort (such as the age of the patients, age at
which the first occurrence of pterygium was reported, the size and
vascularity of pterygium, as well as a history of recurrence) and
the co-existence of pterygium with other ocular surface lesions
were examined using Pearson's bivariate correlation coefficient.
Statistical significance was set at a value of p<0.05.
Results
Overall, 158 cases of pterygium (96 males, 60.75%)
were included in this study. The age of the patients on their first
examination [mean ± standard deviation (SD), range] was 67.23±12.14
(45–84) years in the male patients in our cohort and 71.38±10.112
(48–86) years in the female patients. The mean age of the patients
when the pterygium first appeared was 37.89±16.24 (25–79) years in
the male patients in our cohort and 42.51±18.08 (28–80) years in
the female patients. Pterygium was located nasally in 140 cases
(88.60%), temporally in 5 cases (3.67%) and on both the nasal and
temporal sides in 13 cases (8.22%) in the affected eyes. On the
first examination at the Department of Ophthalmology of the
University Hospital of Heraklion, pterygium was primary in 114
cases (73.54%). The mean pterygium vascularity was 3.11±2.9
(1–4)
and 2.98±2.56 (1–4) in the male and female patients,
respectively. The mean protrusion of pterygium on the corneal
surface was 3.68±1.99 (1–5) mm and 3.55±2.24 (1–4) mm in
the male and female patients, respectively.
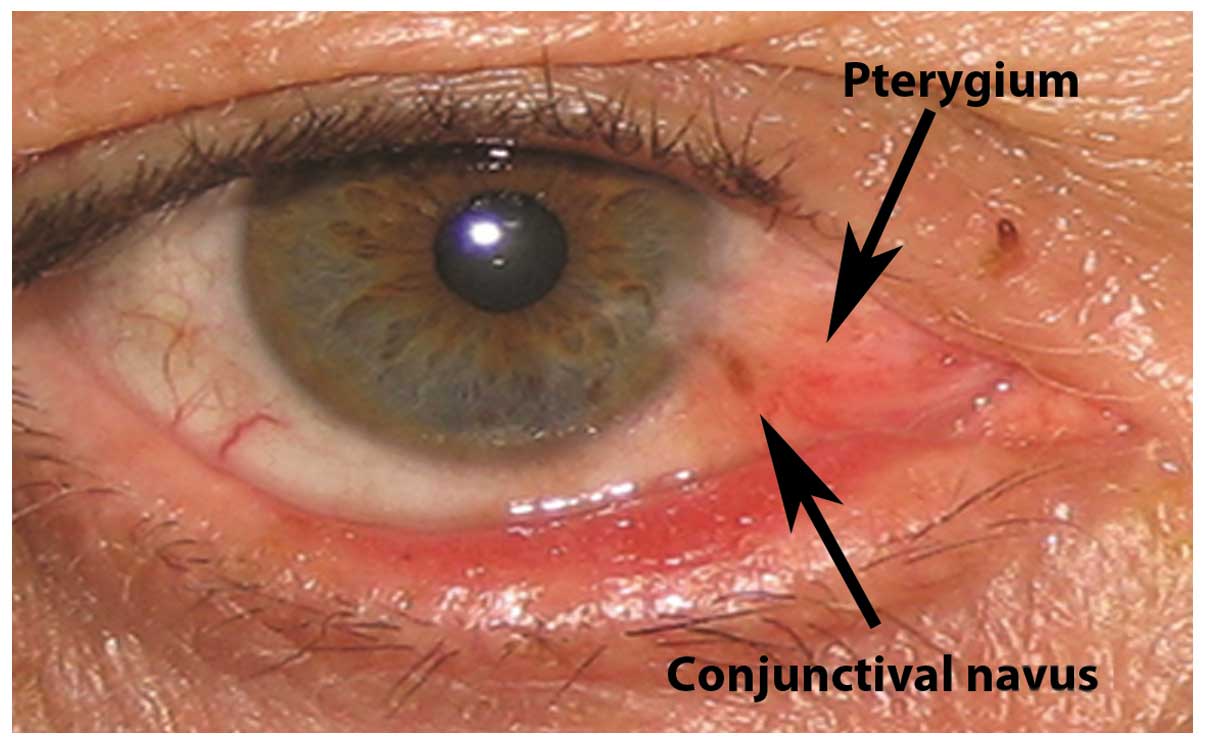
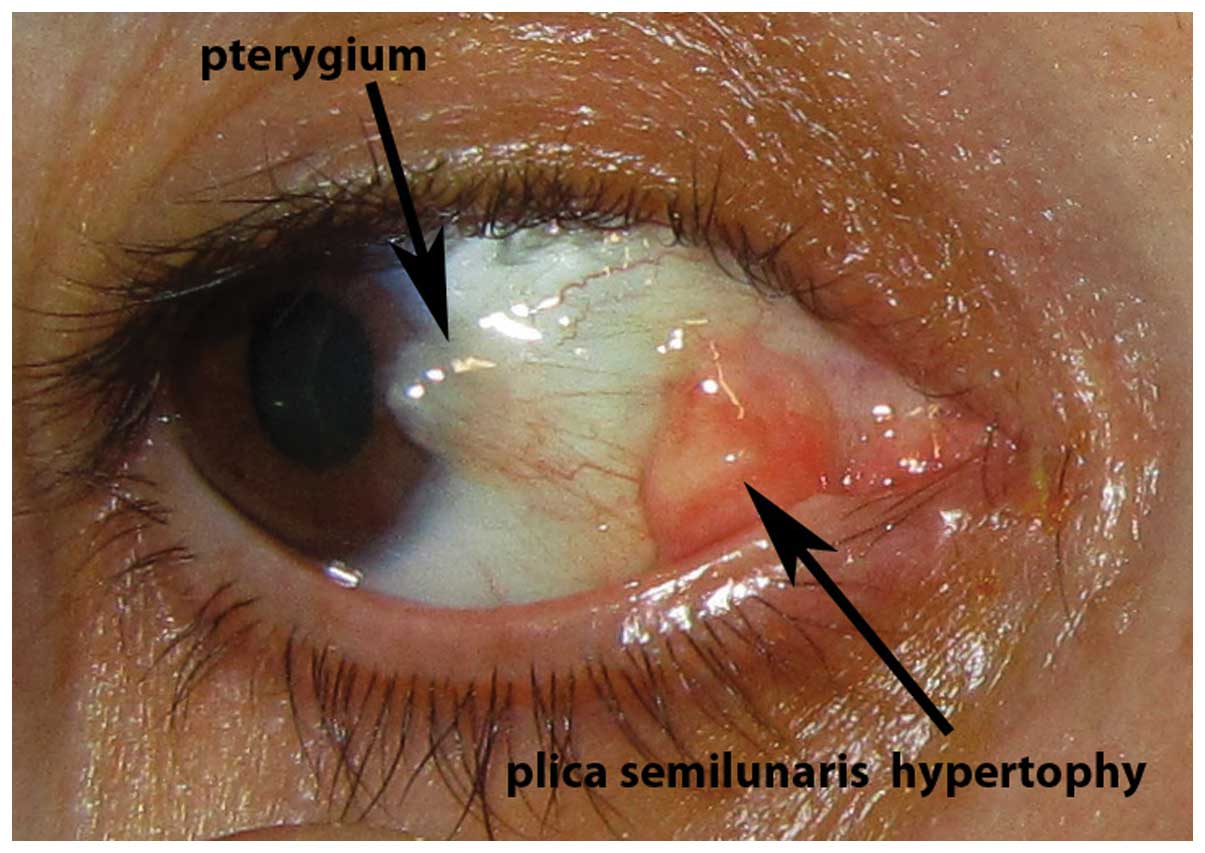
Ocular surface lesions which were recorded to
co-exist with pterygium included conjunctival nevi (5 cases,
3.16%), iris nevi (4 cases, 2.53%), biopsy-proven conjunctival
papillomas (8 cases, 5.06%), biopsy-proven conjunctival
intraepithelial neoplasia (CIN; 4 cases, 2.53%) and hypertophy of
the plica semilunaris (6 cases, 3.79%). Representative cases of
pterygium with concomitant conjunctival nevus, iris nevus,
conjunctival papillomas, biopsy-proven CIN and hypertophy of the
plica semilunaris are presented in Figs.
1–5, respectively. The
correlation between the clinical and demographical parameters
examined (including the patient age, recurrence history, the age at
which pterygium first appeared or pterygium vascularity) and the
presence of concomitant ocular surface lesions in the patients with
pterygium was not statistically significant in all cases. The
values of Pearson's bivariate correlation coefficient for the
correlations between the parameters examined and the presence of
concomitant ocular surface lesions, as well as the respective
p-values are presented in Table I.
In the case of iris nevus, 2 cases were reported in which the nevus
corresponded topographically with pterygium, i.e., the iris nevi
were located on the iris sector corresponding to the corneal
surface occupied by pterygium (Fig.
5). In these cases, iris nevi were reported to be congenital
(in 1 case) or to develop long before to the appearnce of pterygium
(in 1 case).
 | Table I.Pearson's bivariate correlation
coefficient values for the correlations between the clinical and
demographic parameters of the patients with pterygium and
concomitant other ocular surface lesions, as well as respective
p-values. |
Table I.
Pearson's bivariate correlation
coefficient values for the correlations between the clinical and
demographic parameters of the patients with pterygium and
concomitant other ocular surface lesions, as well as respective
p-values.
| Parameter | r-value | p-value |
|---|
| Patient age | −0.09 | 0.78 |
| Male
(67.23±12.14, 45–84 years) |
|
|
| Female
(71.38±10.112, 48–86 years) |
|
|
| Age at which
pterygium first appeared | −0.19 | 0.37 |
| Male
(25–79 years) |
|
|
| Female
(28–80 years) |
|
|
| Pterygium
vascularity | 0.24 | 0.31 |
| Male
(3.11±2.9; 1–4) |
|
|
| Female
(2.98±2.56; 1–4) |
|
|
| Pterygium size | 0.27 | 0.22 |
| Male
(3.68±1.99; 1–5) mm |
|
|
| Female
(3.55±2.24; 1–4) mm |
|
|
| Recurrence
history | 0.25 | 0.29 |
Discussion
In this study, we evaluated the likelihood of the
co-existence between ophthalmic pterygium and other ocular surface
lesions in the Cretan population. The results suggested that
pterygium may co-exist with several other ocular surface conditions
and that pathogenetic links may exist between pterygium and other
concomitant lesions, such as iris nevi.
It has long been known that pterygium may display
unexpected histological characteristics, compatible with neoplastic
lesions (11). Moreover, previous
studies have examined the prevalence of various ocular surface
lesions in patients with pterygium (12,13). CIN
has been reported in 1.89% of patients with pterygium (11), whereas ocular surface melanocytic
lesions have been found in 11.1% of patients with pterygium
(13). The respective prevalences
from the present study are in agreement with these reports. The
potential co-existence of such ocular surface lesions with
pterygium suggests that the clinician managing pterygium cases
should be prepared to detect and adequately address additional
concomitant ocular surface pathologies. These findings also suggest
that pterygium and other concomitant ocular surface conditions may
share common pathogenetic pathways, such as the effects of UV
radiation or the activity of HPV.
The association of pterygium with hypertophy of the
plica semilunaris has previously been mentioned (14) and suggests that inflammatory activity
in the area of the plica semilunaris may play a key role in the
pathogenesis of some pterygia. The association of pterygium with
conjunctival papillomas may reflect the presence of HPV on the
ocular surface, as previously mentioned (1,6,7). In the case of iris nevi, the prevalence
detected in the eyes of patients in our study cohort was similar
with that reported for iris melanocytic lesions in the general
Caucasian population (4–6%) (15),
reflecting the genetic Caucasian profile of the Cretan population.
It should be noted however, that iris nevi were topographically
associated with pterygium in this study in 2 out of 4 cases
recorded, displaying an association between the borders of the iris
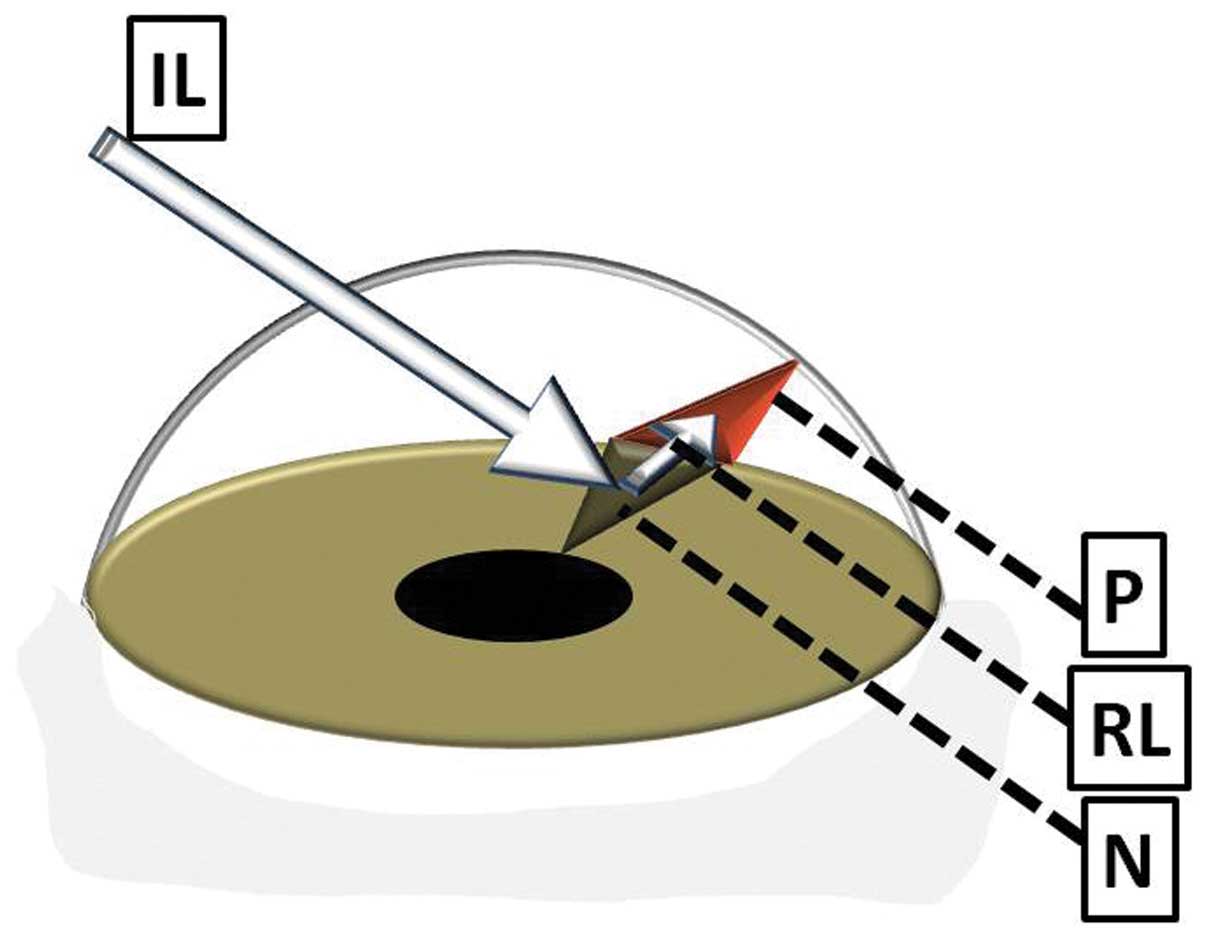
nevus and the borders of the overlying pterygium. One potential
explanation for this finding may be found in the concept of
transcameral light pathways proposed by Coroneo as a pathogenetic
mechanism for the development of pterygium (concept of peripheral
light focusing) (16). In this
proposed model, light scattered in the anterior chamber may deviate
from the transpupillary course and be directed towards the limbus.
The ab interno irradiation of limbal stem cells may cause
genetic destabilization, eventually leading to the development of
pterygium. Accordingly, it can be hypothesized that alterations in
the reflectivity of the anterior iris surface due to the presence
of an iris nevus may enhance the effect of transcameral light
pathways on the iris sector corresponding to the nevus, and may
thus increase the possibility of the development of ipsilateral
pterygium (Fig. 6).
The retrospective design may be considered a
weakness of the present study. Moreover, the overall number of
cases recorded may be considered low and this may possibly explain
the lack of statistically significant correlations between the
recorded clinical and demographic parameters of thee patients in
our cohort and the presence of concomitant pathological conditions.
On the other hand, the fact that all pterygia consecutively managed
at the same reference centre during an 8-year period were included,
whereas the genetic background of the patients studied was
homogeneous (originating from the Cretan population) may enhance
the validity of our results.
In conclusion the findings of the present study
suggest that potential pathogenetic links may exist between
pterygium and other clinically significant ocular surface
pathological conditions. The hypothesis that transcameral light
pathways are associated with iris lesions needs to be further
evaluated in larger multicenter studies, recruiting larger numbers
of cases.
References
|
1
|
Detorakis ET and Spandidos DA:
Pathogenetic mechanisms and treatment options for ophthalmic
pterygium: Trends and perspectives (Review). Int J Mol Med.
23:439–447. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Coroneo MT: Pterygium as an early
indicator of ultraviolet insolation: a hypothesis. Br J Ophthalmol.
77:734–739. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chui J, Coroneo MT, Tat LT, Crouch R,
Wakefield D and Di Girolamo N: Ophthalmic pterygium: a stem cell
disorder with premalignant features. Am J Pathol. 178:817–827.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Detorakis ET, Zaravinos A and Spandidos
DA: Growth factor expression in ophthalmic pterygia and normal
conjunctiva. Int J Mol Med. 25:513–516. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Detorakis ET, Sourvinos G, Tsamparlakis J
and Spandidos DA: Evaluation of loss of heterozygosity and
microsatellite instability in human pterygium: clinical
correlations. Br J Ophthalmol. 82:1324–1328. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Detorakis ET, Drakonaki EE and Spandidos
DA: Molecular genetic alterations and viral presence in ophthalmic
pterygium. Int J Mol Med. 6:35–41. 2000.PubMed/NCBI
|
|
7
|
Detorakis ET, Sourvinos G and Spandidos
DA: Detection of herpes simplex virus and human papilloma virus in
ophthalmic pterygium. Cornea. 20:164–167. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gallagher MJ, Giannoudis A, Herrington CS
and Hiscott P: Human papillomavirus in pterygium. Br J Ophthalmol.
85:782–784. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Piras F, Moore PS, Ugalde J, Perra MT,
Scarpa A and Sirigu P: Detection of human papillomavirus DNA in
pterygia from different geographical regions. Br J Ophthalmol.
87:864–866. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Saw SM and Tan D: Pterygium: prevalence,
demography and risk factors. Ophthalmic Epidemiol. 6:219–228. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Degrassi M, Piantanida A and Nucci P:
Unexpected histological findings in pterygium. Optom Vis Sci.
70:1058–1060. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Artornsombudh P, Sanpavat A,
Tinnungwattana U, Tongkhomsai V, Sansopha L and Tulvatana W:
Prevalence and clinicopathologic findings of conjunctival
epithelial neoplasia in pterygia. Ophthalmology. 120:1337–1340.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Perra MT, Colombari R, Maxia C, Zucca I,
Piras F, Corbu A, Bravo S, Scarpa A and Sirigu P: Finding of
conjunctival melanocytic pigmented lesions within pterygium.
Histopathology. 48:387–393. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Detorakis ET, Halkia A, Tsakalaki V and
Spandidos DA: Association between pterygium and plica semilunaris
morphology. Clin Experiment Ophthalmol. 41:891–892. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Harbour JW, Brantley MA Jr, Hollingsworth
H and Gordon M: Association between posterior uveal melanoma and
iris freckles, iris naevi, and choroidal naevi. Br J Ophthalmol.
88:36–38. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Coroneo M: Ultraviolet radiation and the
anterior eye. Eye Contact Lens. 37:214–224. 2011. View Article : Google Scholar : PubMed/NCBI
|