Introduction
Bladder cancer is among the most common types of
cancer worldwide, with >330,000 new cases reported each year and
>130,000 cases of mortality per year (1). At initial diagnosis, 30% of bladder
cancer cases are diagnosed as muscle-invasive bladder cancer (MIBC)
(2). A proportion of patients with
MIBC require radical cystectomy in order to suppress cancer
invasion and metastasis. However, the quality of life of an MIBC
patient following radical cystectomy remains poor. Therefore,
methods of suppressing MIBC progress to delay the necessity of
surgery are urgently required.
Cancer metastasis comprises a series of complex
processes and one of the initial steps of metastasis is the
detachment and invasive migration of tumor cells from the primary
tumor tissues. The extracellular matrix (ECM) serves a crucial
function in this process (3).
Ras-related protein (Rap) is a type of small GTP-binding protein
that belongs to the Ras protein family. A number of studies have
suggested that Rap signaling is closely associated with cell-ECM
and cell-cell adhesion (4–6). Signal-induced proliferation associated
protein-1 (SIPA-1, previously known as Spa-1) was originally
isolated as a gene product of lymphoid cells activated by mitogenic
stimulation (7). SIPA-1 is a
regulator of Rap1, which is involved in Rap signaling (8). SIPA-1 is able to abrogate Rap1GTP,
which is an active form of Rap1 that maintains cell adhesion, and
then induce the detachment of cells from the matrix (9).
A number of previous studies have indicated that
SIPA-1 is involved in certain processes associated with cancer
activity, including mutation (10,11),
apoptosis (12) and proliferation
(13). Recent studies have indicated
that SIPA-1 is associated with the motility of cancer cells
(14–16). In addition, nuclear SIPA-1 activates
the integrin β1 promoter and promotes the invasion of breast cancer
cells (15). SIPA-1 serves a variety
of functions during disease progression and exerts contrasting
effects on growth and motility of colorectal cancer cells (13). A previous study found that knockdown
of SIPA-1 in colorectal cancer cells resulted in reduced cell
growth in vitro; however, similar knockdown exhibited a
contrasting effect on invasion and migration, which were increased
in SIPA1-knockdown cells compared with the control cells (13). Furthermore, SIPA-1 knockdown
regulated the invasion and metastasis of human prostate cancer
(16). The Ras family includes Ras,
Rap and Ral proteins, of which Ras and Ral have been demonstrated
to have an association with bladder cancer (17,18).
The aim of the present study was to investigate the
capacity of SIPA-1, a regulator of Rap, to regulate bladder cancer
cell invasion and metastasis by modulating cell adhesion.
Materials and methods
Cell culture and reagents
T24 and BIU-87 cells were purchased from American
Type Culture Collection (Manassas, VA, USA). Cells were grown in
RPMI-1640 medium obtained from Life Technologies (Carlsbad, CA,
USA) with 10% fetal bovine serum at 37°C in an atmosphere of 95%
air and 5% CO2. Monoclonal mouse anti-E-cadherin
(#14472) and rabbit anti-zona occludens-1 (ZO-1; #14472) antibodies
were purchased from Cell Signaling Technology, Inc. (Danvers, MA,
USA). Monoclonal mouse anti-SIPA-1 (#166219), anti-Rap1 (#47695)
and rabbit anti-GAPDH (#365062) antibodies were purchased from
Santa Cruz Biotechnology, Inc. (Santa Cruz, CA, USA).
Animals
A total of 48 male, 5-week-old BALB/c nude mice were
purchased from the ABLS-3 laboratory of Wuhan University (Wuhan,
China) and housed under standard conditions. All animal experiments
were conducted in accordance with the UK Animals (Scientific
Procedures) Act 1986 and associated guidelines, and the EEC
Directive of 1986 (86/609/EEC). This study was approved by the
Ethics Committee of Wuhan University School of Medicine.
Gene transfection
BIU-87 cells were transfected with a pcDNA3.1
vector containing the SIPA gene, or a control vector using
opti-MEM (Life Technologies) and Lipofectamine 2000 (Life
Technologies), and were selected using G418 reagent (Life
Technologies) (16). The control
vector consisted of the following sequence: 5′-GAT CCG TAC AGC GGT
CCA ATC ATA GTA GTG CTC CTG GTT GCT ATG ATT GGA CCG CTG TAC TTT TTT
AT-3′. SIPA-1 knockdown in T24 cells was achieved by transfecting
the cells with a pSINsi-hU6 vector containing short hairpin
(sh)RNA targeting SIPA1, or a control vector (16). The sequence of the shRNA for SIPA-1
was as follows: 5′-GAT CCG CTA CTT GCA ACA CCA TTC TTA GTG CTC CTG
GTT GAG AAT GGT GTT GCA AGT AGC TTT TTT AT-3′. Transgene expression
levels were determined using western blot analysis.
Western blot analysis for E-cadherin
and ZO-1
The T24, control-T24, shRNA-SIPA-1-T24 (shT24),
BIU-87, control-BIU-87 and SIPA-1-BIU-87 (S-BIU-87) cells were
pooled and lysed using RIPA buffer. Samples of the lysates were
resolved using SDS-PAGE and subjected to western blot analysis
using standard procedures (19). The
detection of intracellular Rap1GTP was conducted as described in a
previous study (20).
Wound and Transwell assays
A wound assay was performed as previously described
(21). A Transwell migration assay
was conducted with the T24, control-T24 and shT24 cells using a QCM
24-well colorimetric cell migration assay kit (EMD Millipore,
Billerica, MA, USA), following the manufacturer's instructions.
BIU-87, control-BIU-87 and S-BIU-87 cells were also investigated
using the same series of experiments. The invasion assay was
conducted in the same manner as the migration assay, but using a
24-well QCM ECMatrix Cell Invasion assay kit (EMD Millipore), based
on the principle of the Boyden chamber. The results of the invasion
assay were determined by observing six random fields at ×200
magnification, and each experiment was repeated three times.
In vivo metastasis assay
The bladder cancer cells were diluted to
1×107/200 µl. Subsequently, 200-µl cell mixtures were
inoculated into nude mice in the pelvic region. Mice were divided
into six groups, with each receiving a different cell type: Group
I, T24 cells; group II, control-T24 cells; group III, shT24 cells;
group A, BIU-87 cells; group B, control-BIU-87 cells; and group C,
S-BIU-87 cells (n=8 mice per group). The mice were sacrificed at 6
weeks after inoculation via intraperitoneally administered sodium
pentobarbital (60 mg/kg). Resulting tumors were removed for
assay.
Statistical analysis
SPSS software for Windows, version 17.0 (SPSS, Inc.,
Chicago, IL, USA) was used for statistical analysis. Data are
presented as the mean ± standard error of the mean. Statistical
analysis was performed using unpaired t-test and one-way analysis
of variance was used in comparisons of >2 groups. P<0.05 was
considered to indicate a statistically significant difference.
Results
Western blot analysis of E-cadherin
and ZO-1 in T24 and BIU-87 cells
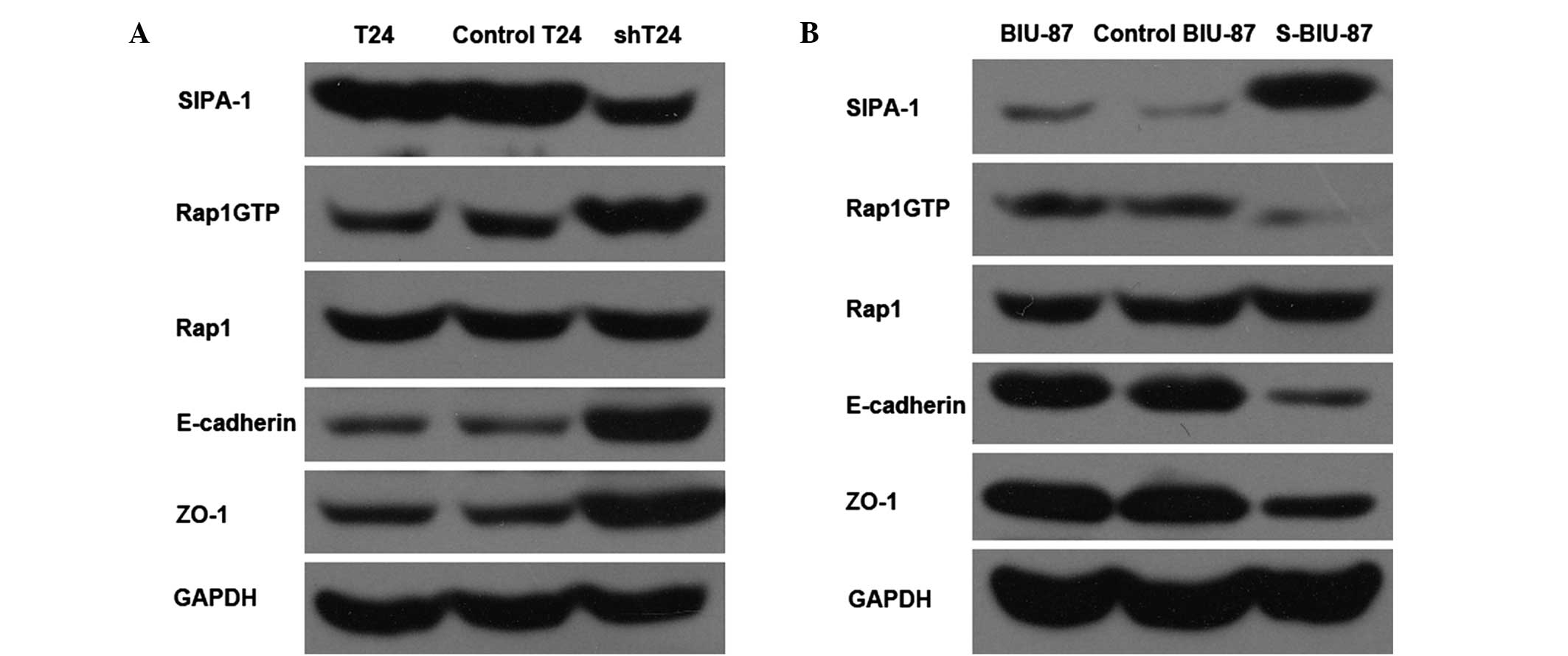
Western blot analysis indicated that the expression
levels of E-cadherin and ZO-1 were increased in the shT24 cells, in
which SIPA-1 expression was suppressed (Fig. 1A). By contrast, the levels of
E-cadherin and ZO-1 were reduced in the S-BIU-87 cells, which were
SIPA-1 enriched (Fig. 1B).
Furthermore, the results indicate that the expression levels of
Rap1GTP were negatively regulated by SIPA-1 (Fig. 1).
Wound and Transwell assays for T24 and
BIU-87 cell migration and invasion
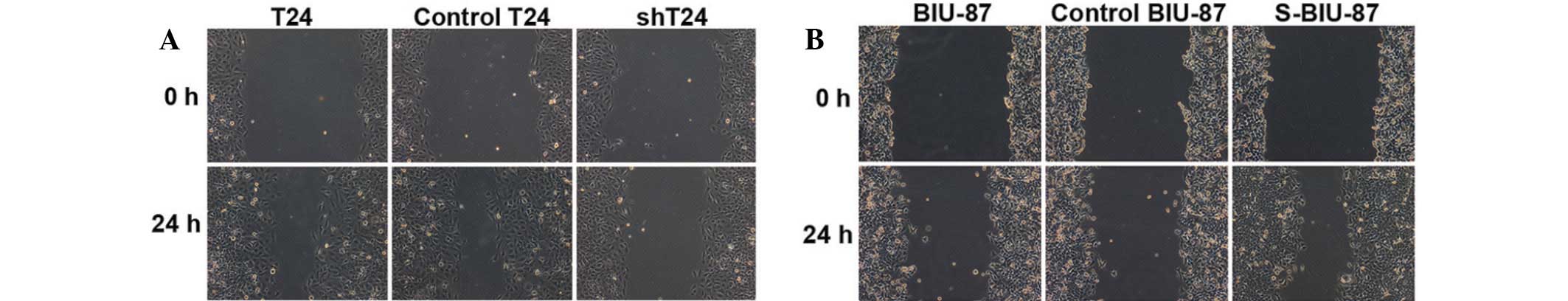
Compared with T24 cells, the shT24 cells with
reduced expression of SIPA-1 showed decreased migration, leaving
the majority of the wound area open (Fig. 2A). Conversely, S-BIU-87 cells with
enhanced levels of SIPA-1 exhibited increased wound healing
(Fig. 2B).
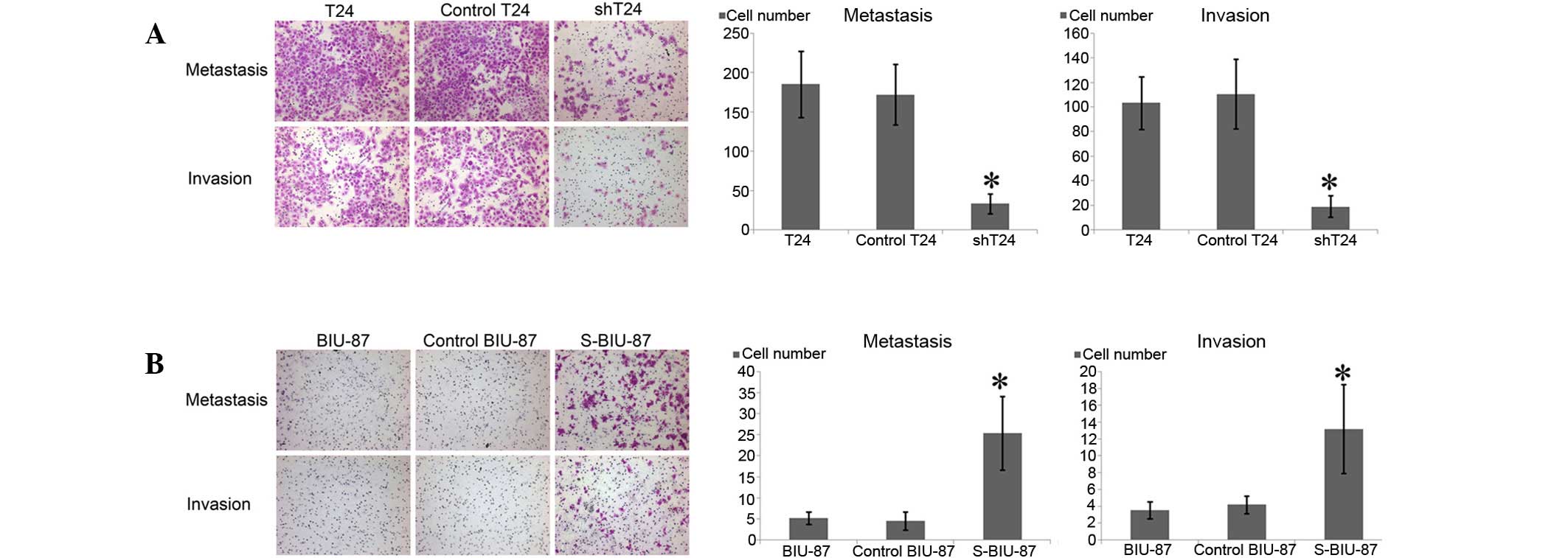
Compared with the T24 and control-T24 cells,
markedly fewer cells crossed the Transwell chamber in the shT24
cell group. In the invasion assay, the shT24 cells that invaded
through the Matrigel membrane were reduced in number (Fig. 3A). By contrast, the S-BIU-87 cells
enriched in SIPA-1 crossed the chamber in greater numbers in the
migration and invasion experiments (Fig.
3B).
Expression of SIPA-1 regulates bladder
cancer cell metastasis in vivo
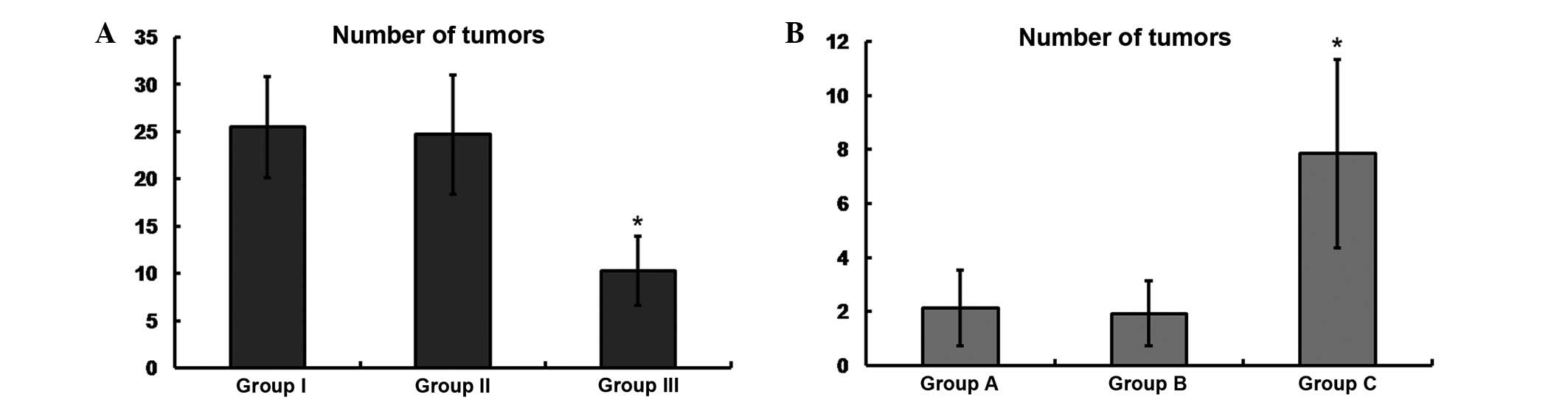
Bladder cancer cells were inoculated into the pelvic
region of nude mice. After 6 weeks, the mice were sacrificed. Mice
of groups I and II exhibited significantly increased numbers of
tumors compared with the SIPA-1-silenced mice of group III
(25.5±5.35 and 24.7±6.32 vs. 10.3±3.66, respectively; Fig. 4A). In the mice of groups A and B, the
numbers of tumors were significantly lower compared with those in
the SIPA-1-overexpressing mice of group C (2.13±1.4 and 1.93±1.21
vs. 7.85±3.5, respectively; Fig.
4B).
Discussion
Metastasis is a type of cell activity that involves
cell adhesion and motility. Therefore, the ability to control
cancer cell adhesion may enable cancer cell metastasis to be
reduced. Rap1 is a member of the Ras family of GTPases, and may
serve a crucial function in the regulation of cell adhesion,
depending on the cellular context (6). In contrast with Ras, which is active on
the cell surface, Rap1 activation is initiated within the cell and
then migrates toward the cell surface (22). Rap1 is rapidly activated in adherent
cells, including cancer cells, as a result of contact with ECM.
Furthermore, basal Rap1 activation appears to be required for the
maintenance of cell adhesion, as the conditional overexpression of
SIPA-1 in adherent cells abrogates basal Rap1GTP and induces the
detachment of cells from the ECM (9). The results of the present study
indicate that Rap1GTP is associated with the expression levels of
SIPA-1, and that metastatic cancer cells have elevated expression
levels of SIPA-1 compared with primary cancer cells (Fig. 1).
The SIPA-1 family consists of numerous
structurally-related but distinct proteins, including SIPA-1,
E6-targeted protein 1 (E6TP1), spine-associated RapGAP (SPAR), and
a number of SPA-1-like proteins (SPA-Ls), each presenting with a
unique cellular distribution in various tissues (6). Previous studies have suggested that
SIPA-1 is involved in certain types of cancer activity, including
mutation (23,24), apoptosis (12,25),
proliferation (4,15), invasion (15,16) and
metastasis (13,26,27). In
the present study, it was observed that SIPA-1 additionally
regulates bladder cancer cell metastasis and invasion (Figs. 2 and 3). Rap1GTP was upregulated, while
E-cadherin and ZO-1 were upregulated as a result of the suppression
of SIPA-1 (Fig. 1). E-cadherin is a
member of the cadherin family, which is related to the
transmembrane glycoproteins responsible for the Ca2+
dependent cell-cell adhesion mechanism that is crucial for the
mutual association of vertebrate cells. A previous study
demonstrated that impairment of the E-cadherin-mediated adhesion
system is characteristic of cells with malignant transformation
(28).
Rap is well known as a critical regulator of
integrin-mediated cell adhesion; however, a previous study showed
that Rap is crucially involved in the regulation of
cadherin-mediated cell-cell adhesion in epithelial cells (29). Furthermore, it has previously been
demonstrated that the important role of Rap in homotypic E-cadherin
interaction is independent of the effects of Rap on integrins
(29). In the present study, T24
cells (with reduced SIPA-1 expression) and BIU-87 cells (with
elevated SIPA-1 expression) were employed to investigate the
association between SIPA-1 and the invasion and metastasis of
bladder cancer cells, in addition to the expression of E-cadherin.
Western blot analysis of the T24 and BIU-87 cells indicated that
E-cadherin was negatively regulated by SIPA-1 (Fig. 1). The molecular mechanisms underlying
the SIPA-1-mediated regulation of E-cadherin remain unclear, and it
has been hypothesized that the protein afadin serves a pivotal
function in the dynamic cyclical activation and inactivation of
Rap1 via the coordinated regulation of SIPA-1 (24,30).
ZO-1 encodes a protein located on the cytoplasmic
membrane surface of intercellular tight junctions. The encoded
protein may be involved in signal transduction at cell-cell
junctions. A previous study reported that the upregulation of ZO-1
correlates with the improved survival of gastrointestinal stromal
tumor cells (31). In the present
experiments, ZO-1 was negatively regulated by SIPA-1, in a similar
manner to E-cadherin (Fig. 1).
Previous studies have suggested that the SIPA-1-induced
downregulation of ZO-1 may involve afadin as a part of its
underlying mechanism (32,33).
The present study is an initial investigation into
the role played by SIPA-1 in bladder cancer, and there were a
number of limitations to this study. For example, the relation
between SIPA-1 and MIBC survival curve will be imortant for
investigat SIPA-1. The present study assessed the effects of SIPA-1
on a limited number of functions in bladder cancer. Future studies
are required to evaluate the impact of SIPA-1 on cell proliferation
and apoptosis, and the mechanistic association between afadin and
SIPA-1 also requires elucidation. In the present in vivo
experiment, metastasis of the cancer cells was reduced following
the suppression SIPA-1 expression (Fig.
4). However, the present animal model may not be the most
appropriate model for indicating clinical outcomes, and cancer
cells inoculated in situ of the bladder may provide a more
accurate model.
In conclusion, SIPA-1 has been demonstrated to
regulate the metastasis and invasion of bladder cancer cells. This
may be achieved by the negative regulation of the expression levels
of E-cadherin and ZO-1. This insight may lead to the identification
of novel therapy targets for the treatment of bladder cancer.
Acknowledgements
This study was supported in part by a grant from the
National Natural Science Foundation of China (no. 81172734).
References
|
1
|
Ploeg M, Aben KK and Kiemeney LA: The
present and future burden of urinary bladder cancer in the world.
World J Urol. 27:289–293. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Vaidya A, Soloway MS, Hawke C, Tiguert R
and Civantos F: De novo muscle invasive bladder cancer: Is
there a change in trend? J Urol. 165:47–50. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Friedl P and Wolf K: Tumour-cell invasion
and migration: Diversity and escape mechanisms. Nat Rev Cancer.
3:362–374. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Minato N: Rap G protein signal in normal
and disordered lymphohematopoiesis. Exp Cell Res. 319:2323–2328.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Minato N and Hattori M: Spa-1 (Sipa1) and
Rap signaling in leukemia and cancer metastasis. Cancer Sci.
100:17–23. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kometani K, Ishida D, Hattori M and Minato
N: Rap1 and SPA-1 in hematologic malignancy. Trends Mol Med.
10:401–408. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hattori M, Tsukamoto N, Nur-e-Kamal MS,
Rubinfeld B, Iwai K, Kubota H, Maruta H and Minato N: Molecular
cloning of a novel mitogen-inducible nuclear protein with a Ran
GTPase-activating domain that affects cell cycle progression. Mol
Cell Biol. 15:552–560. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kurachi H, Wada Y, Tsukamoto N, Maeda M,
Kubota H, Hattori M, Iwai K and Minato N: Human SPA-1 gene product
selectively expressed in lymphoid tissues is a specific
GTPase-activating protein for Rap1 and Rap2. Segregate expression
profiles from a rap1GAP gene product. J Biol Chem. 272:28081–28088.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Tsukamoto N, Hattori M, Yang H, Bos JL and
Minato N: Rap1 GTPase-activating protein SPA-1 negatively regulates
cell adhesion. J Biol Chem. 274:18463–18469. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kim T, Noh M, Lee H, Joo SW, Lee SY and
Lee K: Fluorescence-based detection of point mutation in DNA
sequences by CdS quantum dot aggregation. J Phys Chem B.
113:14487–14490. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yoshida N, Yagasaki H, Takahashi Y, Kudo
K, Manabe A and Kojima S: Mutation analysis of SIPA1 in patients
with juvenile myelomonocytic leukemia. Br J Haematol. 142:850–851.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Katagiri K, Hattori M, Minato N and
Kinashi T: Rap1 functions as a key regulator of T-cell and
antigen-presenting cell interactions and modulates T-cell
responses. Mol Cell Biol. 22:1001–1015. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ji K, Ye L, Toms AM, Hargest R, Martin TA,
Ruge F, Ji J and Jiang WG: Expression of signal-induced
proliferation-associated gene 1 (SIPA1), a RapGTPase-activating
protein, is increased in colorectal cancer and has diverse effects
on functions of colorectal cancer cells. Cancer Genomics
Proteomics. 9:321–327. 2012.PubMed/NCBI
|
|
14
|
Brooks R, Kizer N, Nguyen L, Jaishuen A,
Wanat K, Nugent E, Grigsby P, Allsworth JE and Rader JS:
Polymorphisms in MMP9 and SIPA1 are associated with increased risk
of nodal metastases in early-stage cervical cancer. Gynecologic
Oncol. 116:539–543. 2010. View Article : Google Scholar
|
|
15
|
Zhang Y, Zhang Y, Gong Y, Hu DI, Zhu P,
Wang N, Zhang Q, Wang M, Aldeewan I, Xia H, Qu X, et al: Nuclear
SIPA1 activates integrin beta1 promoter and promotes invasion of
breast cancer cells. Oncogene. 34:1451–1462. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Shimizu Y, Hamazaki Y, Hattori M, Doi K,
Terada N, Kobayashi T, Toda Y, Yamasaki T, Inoue T, Kajita Y, et
al: SPA-1 controls the invasion and metastasis of human prostate
cancer. Cancer Sci. 102:828–836. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Saito R, Shirakawa R, Nishiyama H,
Kobayashi T, Kawato M, Kanno T, Nishizawa K, Matsui Y, Ohbayashi T,
Horiguchi M, et al: Downregulation of Ral GTPase-activating protein
promotes tumor invasion and metastasis of bladder cancer. Oncogene.
32:894–902. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Smith SC, Baras AS, Owens CR, Dancik G and
Theodorescu D: Transcriptional signatures of Ral GTPase are
associated with aggressive clinicopathologic characteristics in
human cancer. Cancer Res. 72:3480–3491. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Roomi MW, Kalinovsky T, Cha J, Roomi NW,
Niedzwiecki A and Rath M: Effects of a nutrient mixture on
immunohistochemical localization of cancer markers in human
cervical cancer HeLa cell tumor xenografts in female nude mice. Exp
Ther Med. 9:294–302. 2015.PubMed/NCBI
|
|
20
|
Reedquist KA and Bos JL: Costimulation
through CD28 suppresses T cell receptor-dependent activation of the
Ras-like small GTPase Rap1 in human T lymphocytes. J Biol Chem.
273:4944–4949. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wang Y, Wang X, Yang Z, Zhu G, Chen D and
Meng Z: Menthol inhibits the proliferation and motility of prostate
cancer DU145 cells. Pathol Oncol Res. 18:903–910. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Mochizuki N, Yamashita S, Kurokawa K, Ohba
Y, Nagai T, Miyawaki A and Matsuda M: Spatio-temporal images of
growth-factor-induced activation of Ras and Rap1. Nature.
411:1065–1068. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
23
|
Tanaka H, Tamura A, Sekai M, Hamazaki Y
and Minato N: Increased c-Myc activity and DNA damage in
hematopoietic progenitors precede myeloproliferative disease in
Spa-1-deficiency. Cancer Sci. 102:784–791. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Miyata M, Rikitake Y, Takahashi M,
Nagamatsu Y, Yamauchi Y, Ogita H, Hirata K and Takai Y: Regulation
by afadin of cyclical activation and inactivation of Rap1, Rac1,
and RhoA small G proteins at leading edges of moving NIH3T3 cells.
J Biol Chem. 284:24595–24609. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Crawford NP, Qian X, Ziogas A, Papageorge
AG, Boersma BJ, Walker RC, Lukes L, Rowe WL, Zhang J, Ambs S, et
al: Rrp1b, a new candidate susceptibility gene for breast cancer
progression and metastasis. PLoS Genet. 3:e2142007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Roberts MR, Hong CC, Edge SB, Yao S,
Bshara W, Higgins MJ, Freudenheim JL and Ambrosone CB: Case-only
analyses of the associations between polymorphisms in the
metastasis-modifying genes BRMS1 and SIPA1 and breast tumor
characteristics, lymph node metastasis, and survival. Breast Cancer
Res Treat. 139:873–885. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Alsarraj J, Faraji F, Geiger TR, Mattaini
KR, Williams M, Wu J, Ha NH, Merlino T, Walker RC, Bosley AD, et
al: BRD4 short isoform interacts with RRP1B, SIPA1 and components
of the LINC complex at the inner face of the nuclear membrane. PLoS
One. 8:e807462013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Shiozaki H, Oka H, Inoue M, Tamura S and
Monden M: E-cadherin mediated adhesion system in cancer cells.
Cancer. 77(Suppl): 1605–1613. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Price LS, Hajdo-Milasinovic A, Zhao J,
Zwartkruis FJ, Collard JG and Bos JL: Rap1 regulates
E-cadherin-mediated cell-cell adhesion. J Biol Chem.
279:35127–35132. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Mandai K, Nakanishi H, Satoh A, Obaishi H,
Wada M, Nishioka H, Itoh M, Mizoguchi A, Aoki T, Fujimoto T, et al:
Afadin: A novel actin filament-binding protein with one PDZ domain
localized at cadherin-based cell-to-cell adherens junction. J Cell
Biol. 139:517–528. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhu H, Lu J, Wang X, Zhang H, Tang X, Zhu
J and Mao Y: Upregulated ZO-1 correlates with favorable survival of
gastrointestinal stromal tumor. Med Oncol. 30:6312013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Birukova AA, Fu P, Wu T, Dubrovskyi O,
Sarich N, Poroyko V and Birukov KG: Afadin controls
p120-catenin-ZO-1 interactions leading to endothelial barrier
enhancement by oxidized phospholipids. J Cell Physiol.
227:1883–1890. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Severson EA, Lee WY, Capaldo CT, Nusrat A
and Parkos CA: Junctional adhesion molecule A interacts with Afadin
and PDZ-GEF2 to activate Rap1A, regulate beta1 integrin levels, and
enhance cell migration. Mol Biol Cell. 20:1916–1925. 2009.
View Article : Google Scholar : PubMed/NCBI
|