Introduction
Lung cancer is associated with the highest mortality
rate (18.2%) of all types of cancer (1), and intervention typically involves
surgical removal of the tumor, with a concomitant lymphadenectomy.
Patients with lung cancer exhibit numerous immune abnormalities,
including cellular immune dysfunction, cytokine alterations,
microcirculatory disturbance and antigen presentation defects
(2,3), which are exacerbated as a result of
surgical trauma and postoperative pain. Furthermore, the immune
dysfunction associated with surgical trauma may predispose patients
to septic complications, multiple organ dysfunction, tumor spread
or metastases, and mortality (4,5);
therefore, it is important to develop strategies that are able to
attenuate perioperative immune dysfunction in patients with lung
cancer.
The activation and differentiation of T lymphocytes
is required for anti-infection and anti-tumor immune responses
(6). In addition, an imbalance in
the relative levels of the various T-helper (Th) cells, including
Th1, Th2 and Th17 cells, and
regulatory T (Treg) cells, has been associated with
immunological disturbances (7). In a
previous study, Th17 cells exhibited potent
pro-inflammatory properties via the secretion of interleukin
(IL)-17, IL-21 and IL-23. Furthermore, immunosuppressive effects of
Treg cells were detected, and were associated with an
immunocompromised state (8). The
identification of Th17 and Treg cells
progressed understanding of the mechanisms underlying immune
dysfunction. An imbalance of Th17/Treg cells
has been widely detected in human cancer, inflammatory and
autoimmune diseases (9); however, to
the best of our knowledge, it has yet to be associated with
thoracotomy in patients with lung cancer.
Transcutaneous acupoint electrical stimulation
(TAES) is a novel analgesic therapy used in the practice of
physiotherapy in order to relieve pain associated with acute and
chronic inflammatory conditions (10). Furthermore, TAES may serve as a
relatively safe and noninvasive alternative to acupuncture, while
providing comparable analgesic effects (11). According to the theory of traditional
Chinese medicine, surgical trauma disrupts the balanced state of
the human body, and disturbs the movement of Qi (vital energy) and
blood (12). It has previously been
suggested that the stimulation of acupoints may restore the balance
of Qi, and facilitate recovery from bodily injury via effects on
the central, autonomic nervous, immune, metabolic and endocrine
systems (13,14). A previous study suggested that
acupuncture may be used alongside existing therapies for the
treatment of cancer and associated symptoms, due to its
‘immune-boosting effects’ (15).
However, to the best of our knowledge, the effects of TAES on the
immune state of patients with lung cancer following thoracotomy,
and the underlying immunomodulatory mechanisms, are yet to be
evaluated.
The present study aimed to evaluate the effects of
TAES on the balance of Th1, Th2,
Th17 and Treg cells, and the expression
levels of associated cytokines and transcription factors, following
thoracotomy of patients with lung cancer.
Materials and methods
Participants and anesthesia
Between May 2012 and May 2013, 311 patients were
diagnosed with lung cancer at the Cancer Hospital of Harbin Medical
University (Harbin, China). A total of 113 patients met the
inclusion criteria of the present study; however, 23 patients
refused participation. Thus, a total of 90 patients (age, 18–65
years), who accepted thoracotomy for the treatment of American
Society of Anesthesiology grade I–III (16), TNM stage I (T1-2N0M0) (17) lung cancer, were evaluated in the
current randomized, controlled trial. Eligible patients had to meet
the following criteria: i) Histologically confirmed lung cancer;
ii) pathologic stage I; iii) no major organ (liver, kidney or
heart) dysfunction; iv) no preoperative anticancer treatment; and
v) no other cancer site besides the lungs. Diagnosis of patients
with lung cancer occurred using histocytological methods, biopsies
were obtained using a bronchofiberscope or thoracoscope. The
exclusion criteria were as follows: i) The detection of a local or
systemic infection; ii) a history of chronic pain or regular opioid
consumption; iii) a body mass index of >40 kg/m2; iv)
evidence of body temperature disturbances; or v) they had
previously experienced acupuncture/TAES therapies. The present
study was approved by the Ethics Committee of Harbin Medical
University, and written informed consent was obtained from all
patients.
Anesthetization and thoracotomy of
patients
Patients were anesthetized using midazolam (0.05
mg/kg; Jiangsu Nhwa Pharmaceutical Co., Ltd., Jiangsu, China),
fentanyl (0.03 mg/kg; Yichang Humanwell Pharmaceutical Co., Ltd.,
Hubei, China) and propofol (2.5–3.0 mg/kg; AstraZeneca,
Macclesfield, UK). Injection of patients with vecuronium bromide
(0.08 mg/kg; Zhejiang Xianju Pharmaceutical Co., Ltd., Zhejiang,
China) facilitated tracheal intubation using a double-lumen
tracheal tube (Portex; Smiths Medical, Dublin, OH, USA). The lungs
were ventilated mechanically (tidal volume, 8 ml/kg; ventilator
frequency, 12–14 bpm; Aestiva 5 7100; 7GE Healthcare Life Support
Solutions, Madison, WI, USA). Anesthesia was maintained using
propofol (4–6 mg/kg/h) and remifentanil (Yichang Humanwell
Pharmaceutical Co., Ltd.). The treatment of patients with
remifentanil was initiated at a rate of 0.1 µg/kg/min, followed by
0.05 µg/kg/min increments or decrements, adjusted according to
hemodynamic variables. If a mean arterial pressure of <60 mmHg
or a heart rate of <45 beats/min were detected for >5 min,
patients were treated with 10 mg ephedrine or 0.5 mg atropine.
Ringer's acetate (Hunan Kelun Pharmaceutical Co., Ltd., Hunan,
China) was administered at a rate of 6–8 ml/kg/h, in order to
maintain basal fluid requirements. All of the patients underwent
thoracotomy (18) with a rib
spreader and wound retractor (tumed-Surgical Instrument &
Hospital Supplies GmbH, Tuttlingen, Germany), and without rib
excision. None of the patients required a perioperative blood
transfusion, and all of the patients were operated on by the same
surgical team, using a consistent operative procedure.
TAES procedure
An anesthesiologist performed TAES at the bilateral
large intestine (LI) 4, pericardium (PC) 6, small intestine (SI) 3
and San Jiao (SJ) 6 acupuncture points. LI 4 is located on the
dorsum surface of the hand, between the first and second metacarpal
bones; PC 6 on the palmar aspect of the forearm, two ‘cuns’ above
the transverse crease of the wrist, between the flexor carpi
radialis and palmaris longus tendons; SI 3 on the ulnar side of the
fifth digit, behind the head of the fifth metacarpal bone, the
starting point of the little finger abductor muscle outer edge; and
SJ 6 on the dorsal aspect of the forearm, two ‘cuns’ above wrist
horizontal stripes between the ulna and radius. One ‘cun’
corresponds to the distance between the interphalangeal creases of
the subject's middle finger. These acupoints were selected on the
basis of the following findings from previous studies: Acupuncture
at LI 4 and PC 6 was able to relieve abdominal pain, whereas it was
associated with enhanced disease resistance and immunomodulation
when performed at SI 3, and a reduction in incision pain associated
with a rib wound when performed at SJ 6 (19–21).
The patients arrived at the laboratory at 9:00 AM on
the assessment day, after which the study design and techniques
were explained. The acupoints of all of the patients were swabbed
with alcohol to reduce skin impedance, and were then covered with
cutaneous self-adhesive electrode pads (size, 16 cm2)
connected to a HANS-200 device (Han's Acupoint Nerve Stimulator;
Neuroscience Research Institute, Peking University, China). The
acupoints were stimulated in the standard dense-and-disperse mode
for 30 min, involving alternate stimulation at 2 Hz and 100 Hz
every 3 sec (frequency, 2/100 Hz). The intensity of stimulation was
set at 4–12 mA to initiate minor muscle contractions. Patients were
informed that they may or may not feel the current.
The subjects were randomized equally into i)
thoracotomy; ii) thoracotomy and sham TAES; and iii) thoracotomy
and TAES groups, using a computer-generated random list with coded
sealed envelopes. Group 3 received TAES at the bilateral LI 4, PC
6, SI 3 and SJ 6 points for 30 min prior to incision, and at 20,
44, 68, 92 and 116 h following surgery. Group 2 received identical
electrical stimulation to group 3; however, TAES was performed at
sham points, which are located 4.0 cm obliquely superior and
lateral to the LI 4, PC 6, SI 3 or SJ 6 acupoints, and are not in
the meridian.
Sample collection and
measurements
Blood samples were collected prior to surgery
(basal), and at 24, 72 and 120 h postoperatively. Peripheral blood
mononuclear cells (PBMCs) were isolated from venous blood using
density gradient centrifugation at 400 × g for 10 min at 4°C and
were analyzed by flow cytometry (FACSCalibur; BD Biosciences, San
Jose, CA, USA). Plasma was stored at −70°C for subsequent
measurement of the expression levels of cytokines and transcription
factors.
Determination of T-cell subsets
To determine the percentages of the various T-cell
subsets, PBMCs were suspended at a density of 2×106
cells/ml in phosphate-buffered saline. Cells were then incubated
with mouse anti-human CD4 (11-0047-42; 1:1,000 dilution), IFN-γ
(12-7319-42; 1:1,000 dilution), IL-4 (12-7049-42; 1:1,000
dilution), IL-17 (12-7178-42; 1:500 dilution), and forkhead box P3
(FoxP3; 12–4777-42; 1:500 dilution) monoclonal antibodies or
isotype-matched immunoglobulin G controls (12-4998-82; 1:1,000
dilution) for 30 min at 37°C. All antibodies were purchased from
eBioscience, Inc. (San Diego, CA, USA). For intracellular IL-17A
staining, the cells were treated with PMA (Sigma-Aldrich, St.
Louis, MO, USA) at 50 ng/ml and ionomycin (Sigma-Aldrich) at 1 µM
in the presence of GolgiStop (BD Pharmingen, San Diego, CA, USA)
for 4 h. For the FoxP3 analysis, the cells were not stimulated. The
stained cells were analyzed using a FACScan Cytometer equipped with
CellQuest 6.0 software (BD FACSAria; BD Biosciences).
ELISA
The protein expression levels of IL-2, IFN-γ, IL-4,
IL-10, IL-17 and transforming growth factor (TGF)-β, were measured
using an ELISA (eBioscience, Inc.). All of the samples were
analyzed in duplicate. The minimum detectable cytokine
concentration was 10 pg/ml for IL-2, 20 pg/ml for IL-4, 2 pg/ml for
IFN-γ, IL-10 and IL-17, and 60 pg/ml for TGF-β.
Reverse transcription-quantitative
polymerase chain reaction
Total RNA was extracted from the PBMCs using the
TRIzol® extraction kit (Invitrogen Life Technologies, Carlsbad, CA,
USA). Total RNA (2 µg) from each sample was reverse transcribed to
cDNA in a 40 µl reaction mixture which contained 8 µl 5X reverse
transcriptase moloney murine leukemia virus (M-MLV) buffer, 1 µl
DTT, 2 µl dNTP mixture, 2 µl random primers, 1 µl reverse
transcriptase M-MLV, 1 µl RNAsafe all Takara Biotechnology Co.,
Ltd., Dalian, China) and 23 µl RNase free ddH2O. TaqMan
primers and probes targeting human T-bet, GATA binding protein 3
(GATA3), RAR-related orphan receptor (ROR)-γt, and FoxP3 were used.
Samples (2 µl cDNA) were analyzed using PrimeScript™ RT-PCR kit
(Takara Biotechnology Co., Ltd.) and the ABI Prism 7900 Sequence
Detection System (Applied Biosystems Life Technologies, Foster
City, CA, USA). The PCR cycling conditions were as follows: 94°C
for 3 min, followed by 94°C for 15 sec, 55°C for 30 sec and 72°C
for 40 sec for 40 cycles, and final extension at 72°C for 10 min.
The primer pairs used were as follows: Forward,
5′-GCTGGAGAAAAGAAGACAAGAAAG-3′ and reverse,
5′-AAGAAAAAACACACACCCACACAC-3′ for T-bet (496 bp); forward,
5′-AGGGAGTGTGTGAACTGTGGG-3′ and reverse,
5′-CTTCGCTTGGGCTTAATGAGG-3′ for GATA3 (253 bp); forward,
5′-GCAATGGAAGTGGTGCTGGTT-3′ and reverse,
5′-AGGATGCTTTGGCGATGAGTC-3′ for RORγt (192 bp); and forward,
5′-CACGCATGTTTGCCTCTTCAGA-3′ and reverse,
5′-GTAGGGTTGGAACACCTGCTGGG-3′ for FoxP3 (235 bp). The mRNA
expression levels of the target genes were normalized against
GAPDH, and relative mRNA expression levels for each cytokine were
calculated. Relative fold changes of gene expression were
calculated using the ∆∆Cq method using ABI Prism® 7900HT software,
and the values were expressed as 2−∆∆Cq (22), relative fold changes of target gene
expression were normalized against GAPDH.
Pain measurement
Postoperative pain at rest and whilst coughing were
assessed using a visual analogue scale (VAS), between 0 (pain free)
and 10 (worst possible pain), prior to surgery (basal), and at 2,
6, 12, 18, 24, 36, 48, 60, 72, 96 and 120 h postoperatively.
Intravenous treatment with 100 mg tramadol (Grünenthal GmbH,
Aachen, Germany) was used for postoperative pain rescue, as
required. Analgesic requirements, adverse events and durations of
hospitalization, were also recorded.
Analysis
Data analysis
Power analysis was based on the results of our
preliminary experiments comparing IL-2 protein expression levels 72
h after surgery among three groups, and yielded a sample size of
n=21 (α=0.05; 1-β=0.9) for each group. Therefore, a sample size of
n=30/group was used in the present study.
Statistical analysis
The normality of quantitative variables was analyzed
by the Kolmogorov-Smirnov test. VAS scales were analyzed by
repeated-measures analysis of variance (ANOVA) for inter-group
comparison. Categorical data were compared using the χ2
test or Fisher's exact test. The remaining data were analyzed using
ANOVA or the Mann-Whitney U test. Statistical analyses were
performed using the SPSS version 13.0 software (SPSS, Inc.,
Chicago, IL, USA). P<0.05 was considered to indicate a
statistically significant difference.
Results
Demographic and surgical
information
Of the 90 patients, nine were excluded: Three due to
serious postoperative complications (two in group 2, and one in
group 3), and six due to incomplete data collection (three in group
1, one in group 2 and two in group 3). Thus, a total of 81 patients
were included in the data analysis. There were no significant
differences in the demographics and surgical information among the
three groups (P>0.05; Table
I).
 | Table I.Demographic and surgical information
(n=27). |
Table I.
Demographic and surgical information
(n=27).
| Characteristic | Group 1 | Group 2 | Group 3 |
|---|
| Age
(year)a |
52.5±24.5 |
57.5±28.3 |
55.5±22.6 |
| Gender
(M/F)b | 14/13 | 11/16 | 12/15 |
| Weight
(kg)a |
64.8±10.9 |
62.8±10.2 | 67.5±8.5 |
| Height
(cm)a | 172.5±6.8 | 176.5±8.2 | 171.5±5.5 |
| TNM stage
(I/IIa/IIb)b | 7/11/9 | 6/13/8 | 6/10/11 |
|
Procedureb |
|
|
|
|
Lobectomy | 16 | 13 | 14 |
|
Pneumonectomy | 6 | 5 | 4 |
|
Bi-lobectomy | 3 | 5 | 6 |
| Wedge
resection | 2 | 4 | 3 |
| Side
(R/L)b | 14/13 | 16/11 | 12/15 |
| Thoracotomy length
(cm)a | 13.2±2.2 | 11.2±1.7 | 12.4±2.2 |
| Blood loss
(ml)a | 157±62 | 145±65 | 167±72 |
| Fluids
(ml)a | 1380±350 | 1220±430 | 1158±270 |
| Lymph nodes
resecteda | 13.5±2.5 | 14.5±3.5 | 16.0±4.5 |
| Duration of surgery
(min)a | 160.5±34.5 | 151.5±27.5 | 145.5±32.5 |
|
Histologyb |
|
|
|
|
Adenocarcinoma (n) | 16 | 14 | 18 |
|
Squamous carcinoma (n) | 9 | 9 | 6 |
| Others
(n) | 2 | 4 | 3 |
Pain intensity
Compared with group 1, VAS scores at rest and during
coughing decreased in group 3 between 6 and 60 h following surgery
(P<0.05. VAS scores between groups 1 and 2 were not
significantly different (P>0.05; Fig.
1). Compared with group 2, VAS scores at rest and during
coughing decreased in group 3 between 6 and 60 h following surgery
(P<0.05). These results indicted that TAES at sham acupoints did
not have any effect on the human body.
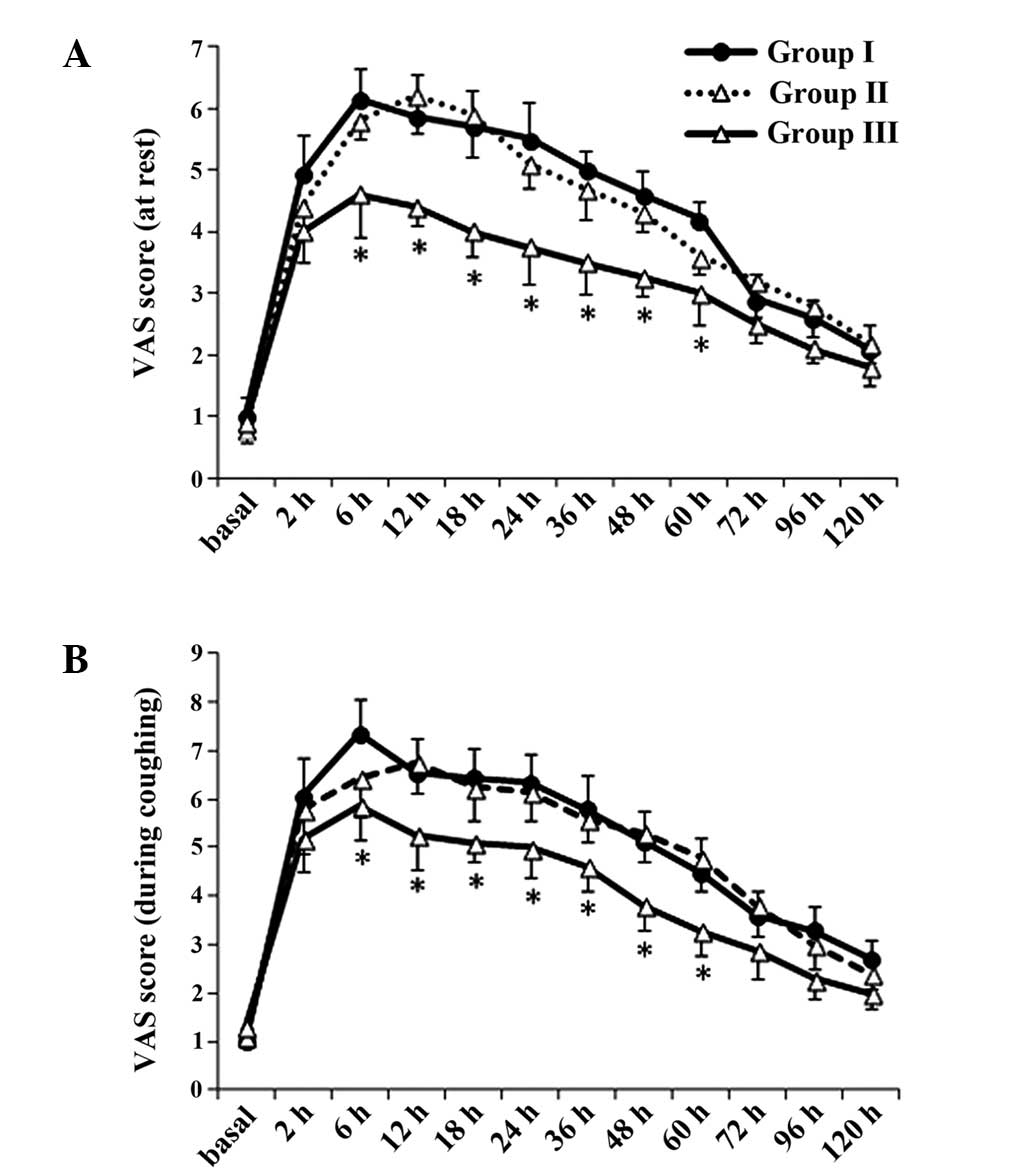 | Figure 1.Postoperative pain (A) at rest and
(B) during coughing assessed using a VAS prior to surgery (basal),
and at 2, 6, 12, 18, 24, 36, 48, 60, 72, 96 and 120 h
postoperatively. Data are presented as the mean ± standard
deviation (n=27/group). *P<0.05 vs. group 1. VAS, visual
analogue scale. |
Percentage of Th cells
Representative binding patterns of the various
CD4+ T cell subsets are presented in Fig. 2. The percentage of Th1
(intracellular antibodies against IFN-γ, 14.4%) and Th17
(intracellular antibodies against IL-17, 2.4%) cells in group 3
were significantly higher 72 h post-operation, as compared with
groups 1 and 2 (9.2%; P<0.05; Fig. 2A
and B). The percentage of Th2 cells (intracellular
antibodies against IL-4, 3.8%) in group 3 were significantly lower,
as compared with in group 1 (8.3%) 72 h post-operation (P<0.05;
Fig. 2A). There were no differences
in the percentage of Treg cells (intracellular
antibodies against FoxP3) among all three groups (P>0.05;
Fig. 2C).
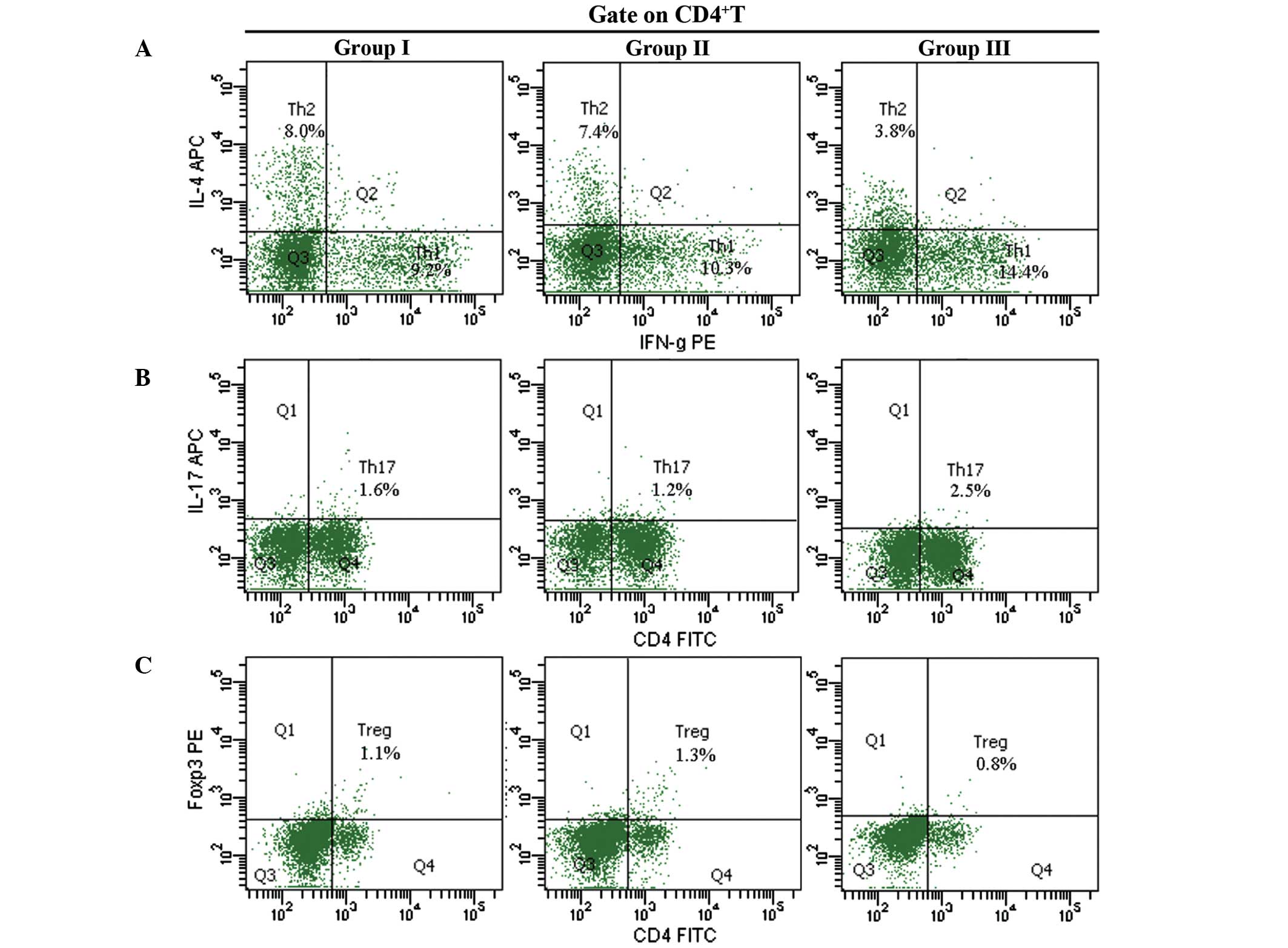 | Figure 2.Percentages of (A) Th1 and
Th2, (B) Th17 and (C) Treg
detected using fluorescence-activated cell sorting. Blood samples
were collected at 72 h postoperatively, and peripheral blood
mononuclear cells were suspended at a density of 2×106
cells/ml. Representative binding patterns of intracellular
monoclonal antibodies against IFN-γ, IL-4, IL-17 and FoxP3 in the
various CD4+ T-cell subsets.*P<0.05 vs. group 1. CD4,
cluster of differentiation 4; IL, interleukin; APC,
allophycocyanin; Th, T-helper cells; IFN, interferon, PE,
phycoerythrin; FoxP3, forkhead box P3; Treg, regulatory
T-cells; FITC, fluorescein isothiocyanate. |
Protein expression levels of Th
cell-associated cytokines
Protein expression levels of IL-2 and IFN-γ were
significantly increased at 72 and 120 h postoperatively in group 3,
compared with group 1 (P<0.05; Fig.
3A and B). By contrast, IL-10 protein expression levels were
significantly decreased at 72 h postoperatively in group 3,
compared with group 1 (P<0.05; Fig.
3C). IL-17 protein expression levels were increased 120 h
postoperatively in group 3, compared with group 1 (P<0.05;
Fig. 3D). There were no significant
differences in the protein expression levels of IL-4 and TGF-β
among the three groups (P>0.05; Fig.
3E and F, respectively).
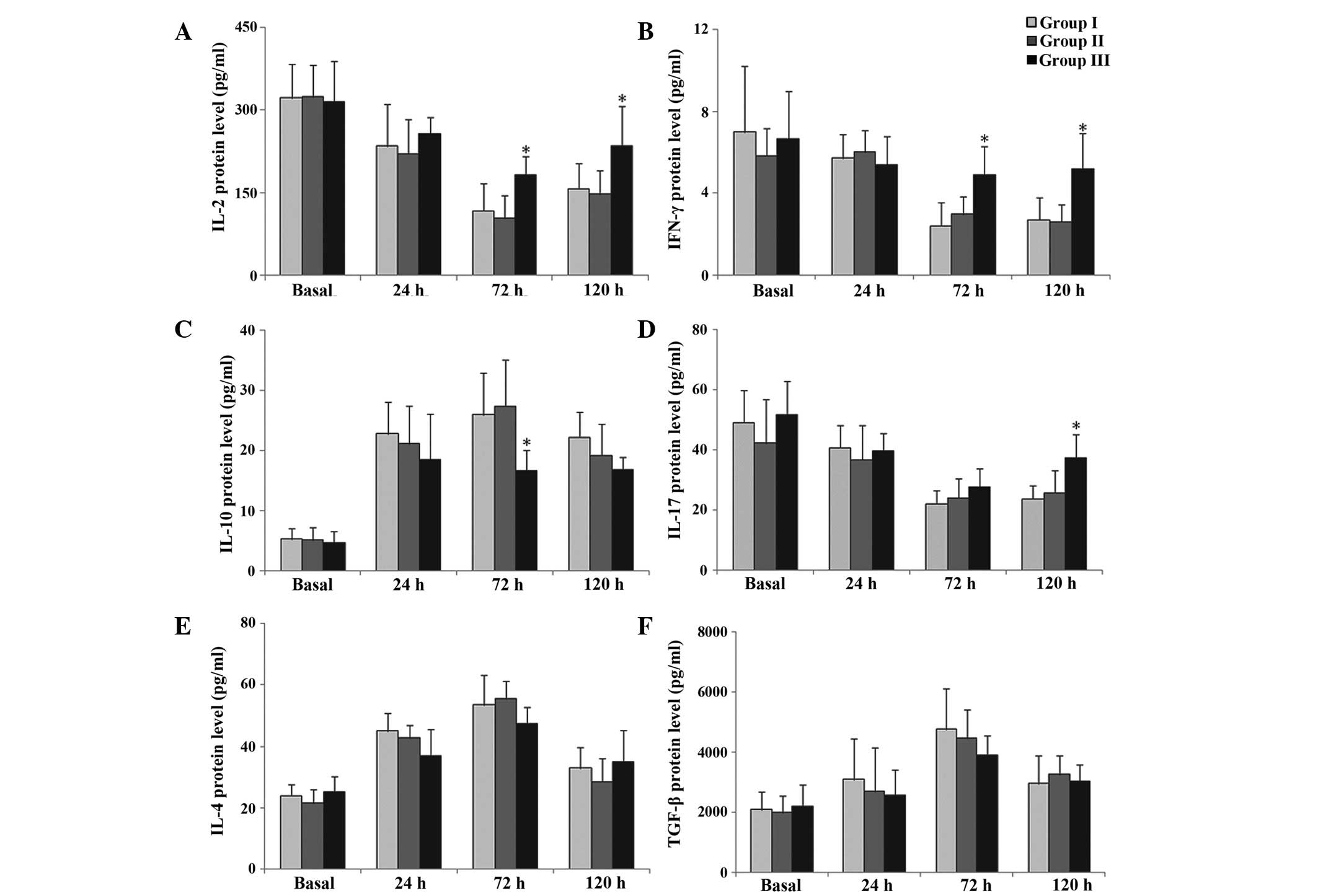 | Figure 3.Protein expression levels of (A)
IL-2, (B) IFN-γ, (C) IL-4, (D) IL-10, (E) IL-17 and (F) TGF-β prior
to surgery (basal), and at 24, 72 and 120 h postoperatively,
measured using ELISA. Data are presented as the mean ± standard
deviation (n=27/group). *P<0.05 vs. group 1. IL, interleukin;
IFN-γ, interferon-γ; TGF-β, transforming growth factor-β. |
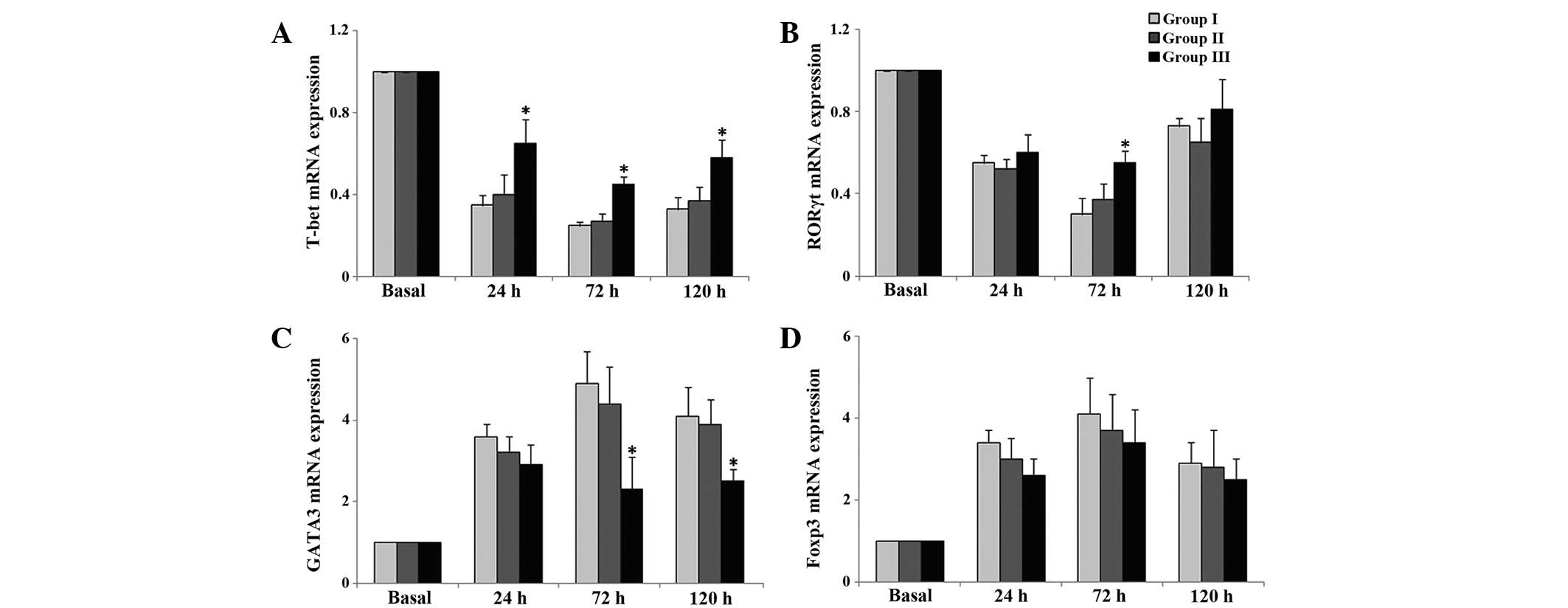
mRNA expression levels of Th
cell-associated transcription factors
T-bet mRNA expression levels were significantly
upregulated at 24, 72 and 120 h postoperatively in group 3,
compared with group 1 (P<0.05; Fig.
4A). RORγt mRNA expression levels were significantly
upregulated at 72 h postoperatively in group 3, compared with group
1 (P<0.05; Fig. 4B). Conversely,
GATA3 mRNA expression levels were downregulated at 72 and 120 h
postoperatively in group 3, compared with group 1 (P<0.05;
Fig. 4C). There were no significant
differences in FoxP3 mRNA expression levels among the three groups
(P>0.05; Fig. 4D).
Rescue analgesic, adverse events and
hospital stays
Rescue analgesic demands were significantly lower in
group 3, compared with group 1 (P<0.05). In addition, the
incidence of postoperative nausea/vomiting and infection was
significantly less in group 3, compared with group I (P<0.05;
Table II). Hospital stays in group
3 (8.7±1.9 days) were marginally shorter, compared with groups 1
and 2 (10.3±1.5 and 11.5±1.6 days, respectively); however, there
were no significant differences among the three groups
(P>0.05).
 | Table II.Postoperative complications. |
Table II.
Postoperative complications.
| Complication | Group 1 | Group 2 | Group 3 |
|---|
|
Nausea/vomiting | 9 (33.3) | 8 (29.6) | 3
(11.1)a |
| Pruritis | 2 (7.4) | 2 (7.4) | 2 (7.4) |
| Hypotension | 5 (18.5) | 4 (14.8) | 4 (14.8) |
| Respiratory
depression | 1 (3.7) | 0 (0.0) | 0 (0.0) |
| Desaturation | 0 (0.0) | 1 (3.7) | 1 (3.7) |
| Pneumonitis | 3 (11.1) | 1 (3.7) | 1 (3.7) |
| Atelectasis | 1 (3.7) | 0 (0.0) | 1 (3.7) |
| Pulmonary
embolism | 0 (0.0) | 1 (3.7) | 0 (0.0) |
| Empyema | 1 (3.7) | 0 (0.0) | 0 (0.0) |
| Infection | 6 (22.2) | 4 (14.8) | 1
(3.7)a |
| Mortality | 0 (0.0) | 0 (0.0) | 0 (0.0) |
Discussion
The present study detected an imbalance in the
percentages of Th1, Th2, Th17 and
Treg cells, which was associated with downregulated
expression levels of Th1/Th17-associated
cytokines and transcription factors (IL-2, IFN-γ, IL-17, T-bet and
RORγt), and upregulated expression levels of
Th2-associated cytokines and transcription factors
(IL-10, GATA3), following thoracotomy of patients with lung cancer.
TAES treatment was able to partially restore the imbalance in the
various CD4+ T-cell subsets, which may have contributed
to attenuation of the postoperative immunosuppression in patients
with lung cancer.
Postoperative pain is the most important
consideration in the care of thoracic surgical patients. In
previous studies, increased expression levels of endogenous
catecholamines, as a result of surgical trauma, stress responses
and pain, were associated with suppression of cellular immune
responses and an increased probability of metastasis (23,24). It
has previously been suggested that TAES is able to inhibit pain
signals via the descending pathway and the dorsal horn cell and the
spinothalamic tract, and by blocking the release of
neurotransmitters, including β-endorphins, enkephalins and
dynorphin (25). The results of the
present study indicated that TAES was able to alleviate the
postoperative pain of thoracic surgical patients with lung cancer.
Numerous studies have suggested that the use of an effective
analgesia may attenuate the occurrence of postoperative
immunosuppression, which may explain why TAES was able to exert
immunomodulatory effects.
Th17 and Treg cells are the
most recently discovered CD4+ T-cell subsets. The
percentage of Th17 cells and expression levels of RORγt
were previously demonstrated to be decreased in the peripheral
blood of patients with lung cancer (26); however, little is known about the
postoperative balance of Th17 and Treg cells
in patients with lung cancer. In the present study, the balance of
Th1, Th2, Th17 and Treg
cells was disrupted following thoracotomy of patients with lung
cancer; thus suggesting that this imbalance may contribute to the
postoperative immunosuppression commonly observed in these
patients. Treatment with TAES increased Th1 and
Th17 cells, and decreased Th2 cells, and this
may have partially restored their balance. The results of the
present study suggested that an imbalance in the numbers of the
various CD4+ T-cell subtypes in patients with lung
cancer following surgery, may lead to postoperative immune
depression, and that restoration of this imbalance may be the
underlying mechanism of the TAES immunomodulatory effects.
Cytokines are important Th cell-polarization
factors; therefore, the profiles of specific cytokines may be
informative in the role of T cell dynamics in immune dysfunction.
In our previous study, surgical trauma was associated with
decreased expression levels of IL-2 and IFN-γ, increased expression
levels of IL-4 and IL-10, and immunosuppression in a surgical
trauma rat model (27). In the
present study, the expression levels of Th17-associated
cytokines in patients with lung cancer were decreased following
thoracotomy, which may have promoted an imbalance in numbers of
Th1, Th2, Th17 and Treg
cells, and contributed to postoperative immunosuppression.
Treatment with TAES increased the expression levels of IL-2, IFN-γ
and IL-17, and decreased IL-10 secretion; thus suggesting that TAES
was able to attenuate the postoperative immune impairment of
patients with lung cancer via altering the expression of Th
cell-associated cytokines. The results of the present study are in
line with a previous study, in which electroacupuncture was able to
improve surgery-suppressed immune function (28). Furthermore, this study reported that
acupuncture and TAES were able to affect the immune system of
patients undergoing major abdominal surgery (28).
Numerous studies have demonstrated that
cytokine-mediated signals are predominantly transduced via specific
transcription factors; for example, T-bet regulates the
transcriptional initiation of Th1 cytokines; GATA3
controls that of Th2 cytokines; RORγt is an important
transcription factor for the differentiation of Th17
cells; and Foxp3 is the master transcription factor in
Treg cells. In the present study, T-bet and RORγt mRNA
expression levels were decreased and the mRNA expression levels of
GATA3 were increased, following thoracotomy; thus suggesting that
an imbalance in the expression of Th cell-associated transcription
factors may have a role in the pathogenesis of postoperative immune
suppression. Treatment with TAES increased T-bet and RORγt mRNA
expression levels and decreased the mRNA expression levels of GATA3
in patients with lung cancer; thus suggesting that TAES is able to
regulate the balance of Th cell-associated transcription
factors.
In the present study, Treg cells, and
their associated cytokines and transcription factors, were not
significantly altered following thoracotomy of patients with lung
cancer. This may be due to the predominant use of lung cancer
patients with early stage cancer: In previous studies, the
percentages of Treg cells in patients with early stage
cancer increased marginally or did not increase at all
postoperatively [preoperative vs. postoperative: Stage I (2.34 vs.
1.77%), stage II (2.72 vs. 1.94%)], whereas the postoperative
Treg percentage in patients with advanced stage (III+IV)
cancer remained high (preoperative vs. postoperative: 1.61 vs.
3.52%) (29).
In the present study, acupoint stimulation occurred
for 30 min, at a frequency setting of 2/100 Hz, and this model was
selected for numerous reasons. First, a long duration of acupoint
stimulation has been associated with enhanced patient discomfort,
whereas a stimulation time that was too short had unclear
therapeutic effects (30). In
addition, low frequency-TAES triggered µ-and δ-opioid receptors,
and β-endorphin production, whereas high frequency (100 Hz)
stimulation was demonstrated to stimulate the κ-opioid receptor and
resulted in the release of dynorphin (31,32).
Future studies should endeavor to optimize the TAES model in order
to maximize its effects.
In the present study, patients treated with TAES
required less analgesic treatment, which was associated with fewer
side effects, including nausea/vomiting and infection. The reduced
incidence of postoperative complications may have facilitated early
recovery following thoracotomy of patients with lung cancer.
In conclusion, TAES was able to partially restore
the postoperative immunosuppression of patients with lung cancer by
altering the balance of Th1, Th2,
Th17 and Treg cells, and their associated
cytokines and transcription factors. Therefore, TAES may provide a
novel therapeutic intervention strategy for clinical immune
dysfunction. Future studies should expand the application of
acupuncture in clinical practice, in order to determine its
immunomodulatory effects.
Acknowledgements
The present study was funded by grants from The
Science and Technology Innovation Talents of Harbin City (grant no.
2013RFXYJ031), the research project of The Department of Science
and Technology, Heilongjiang (grant no. GB05C402-14), Harbin City
Technology Bureau Outstanding Subject Leaders (grant no.
2012RFXXS041), and The National Nature Science Fund of China (grant
no. 81401584).
Glossary
Abbreviations
Abbreviations:
|
TAES
|
transcutaneous acupoint electrical
stimulation
|
|
Th cells
|
T-helper cells
|
|
Treg cells
|
regulatory T-cells
|
|
VAS
|
visual analogue scale
|
References
|
1
|
Ferlay J, Shin HR, Bray F, Forman D,
Mathers C and Parkin DM: Estimates of worldwide burden of cancer in
2008: GLOBOCAN 2008. Int J Cancer. 127:2893–2917. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Dasanu CA, Sethi N and Ahmed N: Immune
alterations and emerging immunotherapeutic approaches in lung
cancer. Expert Opin Biol Ther. 12:923–937. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Micheli DC, Fernandes PC Jr, Cruvinel JC,
Nomelini ID, Murta EF and Tavares-Murta BM: Circulating cytokines
and nitric oxide are involved in the Inhibition of Neutrophil
Migration in patients with Uterine Cervical Neoplasia. Clin Med
Insights Oncol. 6:233–242. 2012.PubMed/NCBI
|
|
4
|
Bobocea AC, Trandafir B, Bolca C and
Cordoş I: Minimally invasive surgery in cancer. Histopathology.
Chirurgia (Bucur). 107:154–157. 2012.PubMed/NCBI
|
|
5
|
Leaver HA, Craig SR, Yap PL and Walker WS:
Lymphocyte responses following open and minimally invasive thoracic
surgery. Eur J Clin Invest. 30:230–238. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ren XF, Li WZ, Meng FY and Lin CF:
Differential effects of propofol and isoflurane on the activation
of T-helper cells in lung cancer patients. Anaesthesia. 65:478–482.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Dai H, Sun T, Liu Z, Zhang J and Zhou M:
The imbalance between regulatory and IL-17-secreting
CD4+T cells in multiple-trauma rat. Injury.
44:1521–1527. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kong QF, Sun B, Bai SS, Zhai DX, Wang GY,
Liu YM, Zhang SJ, Li R, Zhao W, Sun YY, et al: Administration of
bone marrow stromal cells ameliorates experimental autoimmune
myasthenia gravis by altering the balance of Th1/Th2/Th17/Treg cell
subsets through the secretion of TGF-beta. J Neuroimmunol.
207:83–91. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ye J, Liu H, Zhang G, Li P, Wang Z, Huang
S, Yang Q and Li Y: The treg/th17 imbalance in patients with
obstructive sleep apnoea syndrome. Mediators Inflamm.
2012:8153082012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yang L, Yang L and Gao X: Transcutaneous
electrical nerve stimulation on Yongquan acupoint reduces
CFA-induced thermal hyperalgesia of rats via down-regulation of
ERK2 phosphorylation and c-Fos expression. Anat Rec (Hoboken).
293:1207–1213. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zheng CH, Zhang J, Wu J and Zhang MM: The
effect of transcutaneous electrical acupoint stimulation on
pregnancy rates in women undergoing in vitro fertilization:
A study protocol for a randomized controlled trial. Trials.
15:1622014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chapman CR, Schimek F, Gehrig JD, Gerlach
R and Colpitts YH: Effects of nitrous oxide, transcutaneous
electrical stimulation, and their combination on brain potentials
elicited by painful stimulation. Anesthesiology. 58:250–256. 1983.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hopwood V: Acupuncture in Physiotherapy:
Key Concepts and Evidence Based-Practice. Allen H and Edwards R:
(1st). Oxford UK: Butterworth-Heinemann. 2004. View Article : Google Scholar
|
|
14
|
Kondo T and Kawamoto M: Acupuncture and
moxibustion for stress-related disorders. Biopsychosoc Med.
8:72014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
McQuade JL, Meng Z, Chen Z, Wei Q, Zhang
Y, Bei W, Palmer JL and Cohen L: Utilization of and attitudes
towards Traditional Chinese Medicine therapies in a Chinese cancer
hospital, A survey of patients and physicians. Evid Based
Complement Alternat Med. 2012:5045072012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Little JP: Consitency of ASA grading.
Anaesthesia. 50:658–659. 1995.PubMed/NCBI
|
|
17
|
Goldstraw P, Crowley J, Chansky K, Giroux
DJ, Groome PA, Rami-Porta R, Postmus PE, Rusch V and Sobin L:
International Association for the Study of Lung Cancer
International Staging Committee; Participating Institutions: The
IASLC Lung Cancer Staging Project. Proposals for the revision of
the TNM stage groupings in the forthcoming (seventh) edition of the
TNM Classification of malignant tumours. J Thorac Oncol. 2:706–714.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Alfara Fibla JJ: GómezS ebastián G, Farina
Ríos C, Carvajal Carrasco A, Estrada Saló G and León González C:
Lobectomy versus limited resection to treat non-small cell lung
cancer in stage I: A study of 78 cases. Arch Bronconeumol.
39:217–220. 2003.PubMed/NCBI
|
|
19
|
Chen LL, Hsu SF, Wang MH, Chen CL, Lin YD
and Lai JS: Use of acupressure to improve gastrointestinal motility
in women after trans-abdominal hysterectomy. Am J Chin Med.
31:781–790. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Xu HY, Yang F, Zhu J, He ZP and Yan C:
Effect of electroacupuncture at Hegu (LI 4) and Sanyinjiao (SP 6)
on short-term adverse effects of drug-induced abortion. Zhongguo
Zhen Jiu. 27:103–105. 2007.(In Chinese). PubMed/NCBI
|
|
21
|
Fu GQ, Zhou J, Tong QY, et al: Observation
on the anti-stress effect of acupuncture-assisted anesthesia for
pulmonary lobectomy patients. Zhen Ci Yan Jiu. 36:361–365.
2011.PubMed/NCBI
|
|
22
|
Pfaffl MW: A new mathematical model for
relative quantification in real-time RT-PCR. Nucleic Acids Res.
29:e452001. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hunter JD: Effects of anaesthesia on the
human immune system. Hosp Med. 60:658–663. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Engers R, Mueller M, Walter A, Collard JG,
Willers R and Gabbert HE: Prognostic relevance of Tiam1 protein
expression in prostate carcinomas. Br J Cancer. 95:1081–1086. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chernyak GV and Sessler DI: Perioperative
acupuncture and related techniques. Anesthesiology. 102:1031–1049.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhao L, Yang J, Wang HP and Liu RY:
Imbalance in the Th17/Treg and cytokine environment in peripheral
blood of patients with adenocarcinoma and squamous cell carcinoma.
Med Oncol. 30:4612013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wang K, Wu H, Wang G, Li M, Zhang Z and Gu
G: The effects of electroacupuncture on TH1/TH2 cytokine mRNA
expression and mitogen-activated protein kinase signaling pathways
in the splenic T cells of traumatized rats. Anesth Analg.
109:1666–1673. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Li G, Li S, Wang B and An L: The effect of
electroacupuncture on postoperative immunoinflammatory response in
patients undergoing supratentorial craniotomy. Exp Ther Med.
6:699–702. 2013.PubMed/NCBI
|
|
29
|
Chen C, Chen D, Zhang Y, Chen Z, Zhu W,
Zhang B, Wang Z and Le H: Changes of
CD4+CD25+FOXP3+ and
CD8+CD28− regulatory T cells in non-small
cell lung cancer patients undergoing surgery. Int Immunopharmacol.
18:255–261. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lv JQ, Feng RZ and Li N: P6 acupoint
stimulation for prevention of postoperative nausea and vomiting in
patients undergoing craniotomy, Study protocol for a randomized
controlled trial. Trials. 14:1532013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hughes GS Jr, Lichstein PR, Whitlock D and
Harker C: Response of plasma beta-endorphins to transcutaneous
electrical nerve stimulation in healthy subjects. Phys Ther.
64:1062–1066. 1984.PubMed/NCBI
|
|
32
|
Ngai SP, Jones AY, Hui-Chan CW, Ko FW and
Hui DS: Effect of 4 weeks of Acu-TENS on functional capacity and
beta-endorphin level in subjects with chronic obstructive pulmonary
disease, A randomized controlled trial. Respir Physiol Neurobiol.
173:29–36. 2010. View Article : Google Scholar : PubMed/NCBI
|