Introduction
Pulmonary hypertension (PH) is a type of lung
disease that is severely detrimental to human health, and is often
characterized by a progressive increase in pulmonary arterial
pressure, eventually resulting in right ventricular overload and
finally, heart failure (1). Despite
current treatments, including the use of targeted medicine such as
sildenafil, assisting in relief of the clinical symptoms of PH, the
long-term prognosis remains poor (2). For example, the 5-year survival rate of
cystic fibrosis patients with PH is 40.8% (3); however, even following lung
transplantation, the median survival rate is only ~7 years
(4). Therefore, there is an urgent
requirement for the development of more effective therapeutic
agents for the treatment of PH.
Numerous studies have served to elucidate the
mechanism of PH, highlighting the importance of inflammation and
the calcineurin (CaN)/nuclear factor of activated T cells (NFAT)
signaling pathway (5–13). It has been determined in experimental
animal models and in human patients that inflammatory cells are
present in the region of remodeled pulmonary arteries (5–8).
Furthermore, it has been explicated that the perivascular
accumulation of inflammatory cells is essential to pulmonary
vascular remodeling (9). Therefore,
elevated production of inflammatory cytokines has the potential to
be utilized as a predictive marker of survival in patients with PH
(9). It remains uncertain as to
whether inflammation initiates pulmonary vascular remodeling, or
whether it is a bystander effect. However, it is clear that
inflammation and associated inflammatory cytokines are involved in
the progression of PH. Of the various inflammatory cytokines, the
role of tumor necrosis factor-α (TNF-α) in the overproliferation of
pulmonary artery smooth muscle cells (PASMCs) and in vascular
remodeling, appears to be significant. TNF-α has the ability to
cause calcium influx and promote the proliferation of SMCs
(10); the activation of the
CaN/NFAT signaling pathway is a crucial step within this process
(11–13). Therefore, we hypothesize that
inflammation-associated overproliferation of PASMCs is a
therapeutic target in the treatment of PH.
Mesenchymal stem cells (MSCs), as primitive cells
capable of multilineage differentiation and self-renewal, have been
observed to effectively inhibit the production of inflammatory
cytokines (14), and improve
hypoxia-induced PH in experimental animal models (15). However, there are a number ethical
factors that may serve to impede the progress of MSCs into the
clinic. Our previous study indicated that the immunosuppressive
effect of MSCs are strongly associated with their ability to
express immuno-regulatory cytokines and secrete prostaglandin E2
(PGE2) (14). It was also revealed
that conditioned media from MSCs could produce cytoprotective
effects (15). However, the
mechanism by which MSC-conditioned media (CM) suppresses the
inflammation-associated overproliferation of PASMCs remains
unknown.
In the present study, a rat model of PH and a
co-culture system comprised of PASMCs and activated T cells were
used to assess the ability of MSC-CM to suppress the
overproliferation of PASMCs. Associated factors, including the
expression levels of TNF-α in the rat model and co-culture system,
and the expression levels and activation of CaN/NFAT in PASMCs,
were also evaluated, in order to better understand the mechanism by
which MSC-CM leads to the overproliferation of PASMCs in PH.
Materials and methods
Preparation of MSC-CM
All studies were approved by the Institutional
Review Board of Guiyang Medical College (Guiyang, China) and
written informed consent was obtained from all donors. In June
2013, 3 umbilical cords were obtained following normal deliveries
from healthy, post-partum women (age, 26–30 years; gestational age,
37–42 weeks) at the Tianjin Central Hospital of Gynecology and
Obstetrics (Nankai, China). From these, human umbilical
cord-derived MSCs were isolated and cultured in Dulbecco's modified
Eagle's medium (DMEM)/F-12 medium (Gibco; Thermo Fisher Scientific,
Inc., Waltham, MA, USA) supplemented with 10% fetal bovine serum
(FBS; GE Healthcare Life Sciences, Logan, UT, USA) and Gibco
penicillin-streptomycin (100 U/ml; Thermo Fisher Scientific, Inc.),
as described in a previous protocol (16). MSCs were identified by analyzing the
expression of cell-surface markers or internal cell markers was
assessed using a BD FACSCalibur flow cytometer (BD Biosciences,
Franklin Lakes, NJ, USA), including cluster of differentiation
(CD)11b, CD29, CD34, CD44, CD45, CD54, CD73, CD80, CD86, CD90,
CD105, human leukocyte antigen (HLA)-DR, HLA-ABC, Nestin and SRY
box-2 (Sox-2). The osteogenic and adipogenic differentiation in
vitro were also assessed (16).
Briefly, MSCs were seeded in 24-well plates at a density of
104 cells/ml (1 ml/well); after 24 h of culture, the
medium was replaced with osteogenic or adipogenic induction medium.
For osteogenic induction, this medium consisted of DMEM/F-12 medium
supplemented with 10% FBS, 100 nmol/l dexamethasone, 10 mmol/l
β-glycerophosphate and 0.2 mmol/l L-ascorbic acid-2-phosphate (all
Sigma-Aldrich, St. Louis, MO, USA). For adipogenic induction, the
medium consisted of DMEM/F-12 medium supplemented with 10% FBS, 5
g/ml insulin, 1 mmol/l dexamethasone, 60 mmol/l indomethacin, and
0.5 mmol/l isobutylmethylxanthine (all Sigma-Aldrich). After 2
weeks of inducted culture, osteogenic and adipogenic
differentiation were identified using Alizarin Red S and Oil Red O
stain (Sigma-Aldrich), respectively. MSCs passaged 8–10 times were
washed thoroughly with phosphate-buffered saline (PBS; BD
Biosciences) and incubated in new medium for 24 h. The MSC-CM was
collected by centrifugation at 4°C, at 2,000 × g for 10 min, then
stored at −80°C. For administration to rats, MSC-CM prepared
according to the aforementioned protocol was replaced with
serum-free TheraPEAK MSCGM-CD medium (Lonza Group Ltd., Basel,
Switzerland) at passage 3.
Experimental animals
All animal studies were approved by the
Institutional Animal Care and Use Committee of Guiyang Medical
College. Female Sprague-Dawley (SD) rats (age, 8–10 weeks; n=18)
with body weights of ~200 g were purchased from and housed in
specific pathogen-free units of the Laboratory Animals Center at
Tianjin Blood Diseases Hospital (Tianjin, China). The rats were
maintained at ~25°C, a relative humidity of 70% and with a 12-h
light/dark cycle.
The rats were randomly divided into three equal
groups (n=6 per group), as follows: A PH model group, a MSC-CM
administration group and a control group. The PH model was induced
by a single subcutaneous injection with monocrotaline (MCT; 60
mg/kg; Sigma-Aldrich), in accordance with a previous study
(17). On days 5–9 after injection
with MCT, 500 µl serum-free MSCGM-CD was subcutaneously injected
into the MSC-CM group. The control group was injected with 500 µl
PBS alone. Rats were anesthetized by intraperitoneal injection of
pentobarbital (50 mg/kg; Sigma-Aldrich) 21 days after
administration, and right ventricular systolic pressure (RVSP) and
mean aortic pressure (MAoP) were determined, according the protocol
detailed in a previous study (18).
Subsequent to the aforementioned procedures, rats
were sacrificed by decapitation, lung tissues were removed and
fixed in 10% paraformaldehyde at room temperature for 24 h. Serial
sections (5 µm) were stained with hematoxylin and eosin (Yuanmu
Biotechnology Co., Ltd., Shanghai, China), and the medial wall
thickness (WT) of pulmonary arterioles was observed under an
Olympus BX53 microscope (Olympus Corporation, Tokyo, Japan) and
expressed as: WT (%) = [(medial thickness × 2) / external diameter]
× 100 (19).
Immunohistochemical staining for TNF-α
in lung tissue
Serial sections (5 µm) were fixed on gelatin-coated
slides. Following deparaffinization with two changes of xylene,
rehydration with graded ethanol and sequential incubation for 5 min
at room temperature with 0.3% Triton X-100 (Sigma-Aldrich) and 3%
hydrogen peroxide (Santa Cruz Biotechnology, Inc., Dallas, TX,
USA), the sections were incubated with goat polyclonal primary
antibody against TNF-α (1:400 dilution; cat. no. sc-1350; Santa
Cruz Biotechnology, Inc.) for 12 h at 4°C. Following three washes
with PBS, the sections were incubated for 30 min at room
temperature with biotinylated rabbit anti-goat monoclonal antibody
(1:100 dilution; cat. no. BA-1006; Wuhan Boster Biological
Technology, Ltd., Wuhan, China), and the immunoreactivity detected
with a 3-amino-9-ethylcarbazole peroxidase substrate kit (Wuhan
Boster Biological Technology, Ltd.). The sections were
counterstained with hematoxylin, and observed under the Olympus
BX53 microscope. Mean optical density (OD) was subsequently
calculated using Image-Pro Plus Software 6.0 (Media Cybernetics,
Rockville, MD, USA).
Isolation of PASMCs and T cells
A total of 4 SD rats with body weights of ~100 g
were sacrificed by decapitation prior to harvesting of pulmonary
arteries for PASMC culture using the tissue explants method
(20). DMEM/F-12 media supplemented
with 10% FBS and 100 U/ml penicillin-streptomycin was used. PASMCs
were identified by immunofluorescent staining for α-smooth muscle
actin as follows: PASMCs were sequentially fixed with 4%
paraformaldehyde for 15 min, incubated with 0.3% Triton X-100 and
1% bovine serum albumin for 20 min at room temperature and
incubated with rabbit α-smooth muscle actin polyclonal antibody
(α-SMA; 1:100 dilution; cat. no. 14395-1-AP; ProteinTech Group,
Inc., Chicago, IL, USA) overnight at 4°C. Subsequent to washing
with PBS, cells were incubated with fluorescein
isothiocyanate-conjugated goat anti-rabbit immunoglobulin G (1:100
dilution; cat. no. SA00003-2; ProteinTech Group, Inc.) for 2 h at
room temperature in the dark. Finally, cells were incubated with
DAPI (1 µg/ml) for 5 sec. To extract T cells, the spleens were
harvested and T cells were isolated contingent on their
non-adherence to nylon wool (21)
and stored at −80°C prior to use.
Co-culture of PASMCs and T cells
The cells were divided into three groups, as
follows: Group A (PASMCs alone), Group B (PASMCs + T cells) and
Group C (PASMCs + T cells + MSC-CM). All cells were maintained at
37°C in a humidified environment with 5% CO2. PASMCs at
passage 5 were seeded into 24-well plates at a density of
104 cells/ml (1 ml/well). In Group A, the media was
renewed after 24 h of culture. In Group B, 0.9 ml of medium was
removed, and 0.9 ml T cell suspension at a density of
105 cells/ml was added and stimulated by concanavalin A
(ConA; 10 µg/ml; Sigma-Aldrich). For Group C, the total volume of
supernatant was removed and ConA-stimulated T cells were added at
the same density as those in Group B. Furthermore, an additional
0.1 ml MSC-CM was added.
TNF-α protein expression levels in
co-culture systems
Following 3 days of co-culture, the cell-free
supernatant was collected from each cell group by centrifugation
(4°C, 2,000 × g, 10 min), and expression levels of TNF-α were
measured using a Rat TNF-α Mini ABTS ELISA Development kit
(PeproTech, Inc., Rocky Hill, NJ, USA), according to the
manufacturer's protocol.
Proliferation of PASMCs
Following collection of the supernatant from all
three groups, T cells were removed by washing three times with PBS.
Next, PASMCs were incubated for 4 h in 300 µl new medium containing
15 µl 3-(4,
5-diethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-etrazolium,
inner salt (MTS; Promega Corporation, Madison, WI, USA) at 37°C/5%
CO2. The OD of the supernatant was read at 490 nm using
a Model 680 microplate reader (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA).
mRNA expression of CaN and NFATc2
After 3 days of co-culture, total RNA of PASMCs was
extracted from each of the three groups using an E.Z.N.A. Total RNA
I kit (Omega Biotek, Inc., Norcross, GA, USA), and reverse
transcribed to cDNA using a Moloney Murine Leukemia Virus Reverse
Transcriptase kit (Invitrogen; Thermo Fisher Scientific, Inc.).
Following this, the cDNA was amplified and the expression of CaN
and NFATc2 were analyzed by quantitative PCR (qPCR) using an
Applied Biosystems 7300 Real-Time PCR System with Invitrogen
Platinum SYBR Green qPCR SuperMix-UDG w/ROX (Thermo Fisher
Scientific, Inc.). The PCR cycling conditions were as follows: 95°C
for 2 min, 95°C for 15 sec and 60°C for 30 sec; this was repeated
for 40 cycles, as per our previous study (22). Relative expression levels were
quantified by the 2−ΔΔCq normalization method (23). The primers used are listed in
Table I.
 | Table I.Rat gene primers for RT-qPCR. |
Table I.
Rat gene primers for RT-qPCR.
| Primer | Sequence |
|---|
| GAPDH F |
5′-CCATTCTTCCACCTTTGATGCT-3′ |
| GAPDH R |
5′-TGTTGCTGTAGCCATATTCATTGT-3′ |
| CaN F |
5′-CAGAGGGTGCTTCGATTCTC-3′ |
| CaN R |
5′-CCCCTAAGAAGAGGTAGCGA-3′ |
| NFATc2 F |
5′-CAGCAGATTTGGGAGATGGAAG-3′ |
| NFATc2 R |
5′-GACTGGGTGGTAAGTAAAGTGC-3′ |
CaN activity
PASMCs from each of the three groups were lysed in 1
ml lysate buffer composed of 50 mmol/l Tris, 0.5 mmol/l DTT, 50
µg/ml PMSF, 50 µg/ml soybean trypsin inhibitor, 5 µg/ml leupeptin
and 5 µg/ml aprotinin (Beijing Dingguo Changsheng Biotechnology
Co., Ltd., Beijing, China). Following three freeze-thaw cycles, the
supernatant was collected by centrifugation (4°C, 2,000 × g, 10
min) for measurement of CaN phosphatase activity using a
Calcineurin assay kit (Nanjing Jiancheng Bioengineering Institute,
Nanjing, China).
NFATc2 activation
After 3 days of co-culture, PASMCs from each of the
three groups were sequentially fixed with 4% paraformaldehyde (15
min), incubated with 0.3% Triton X-100 and 1% bovine serum albumin
(20 min), and then incubated with mouse anti-rat NFATc2 monoclonal
antibody (1:100 dilution; cat. no. NB300-504; Novus Biologicals,
LLC, Littleton, CO, USA) overnight at 4°C. Subsequent to washing
with PBS, cells were incubated with fluorescein
isothiocyanate-conjugated goat anti-mouse immunoglobulin G (1:100
dilution; cat. no. SA00003-1; ProteinTech Group, Inc.) for 2 h at
room temperature in the dark. Finally, cells were incubated with
DAPI (1 µg/ml) for 5 sec and assessed with a TCS SP5 confocal laser
scanning microscope (Leica Microsystems GmbH, Wetzlar,
Germany).
Statistical analysis
Data are presented as mean ± standard deviation and
SPSS software (version 17.0; SPSS, Inc., Chicago, IL, USA) was used
for statistical analysis. Differences were compared using an
independent-samples t-test or one-way analysis of variance.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Identification of MSCs and PASMCs
Human MSCs grown in the serum-free medium presented
with a typical fibroblastic shape (Fig.
1A), whilst those cultured in the specific conditioned medium
displayed osteogenic (Fig. 1B) and
adipogenic (Fig. 1C)
differentiation. The MSCs were positive for HLA-ABC (99.73%),
Nestin (68.39%), Sox-2 (78.46%), CD29 (100%), CD44 (100%), CD54
(86.98%), CD73 (99.79%), CD90 (100%) and CD105 (97.33%), and
negative for CD34, CD45, CD80, CD86, HLA-DR and CD11b (Fig. 1D).
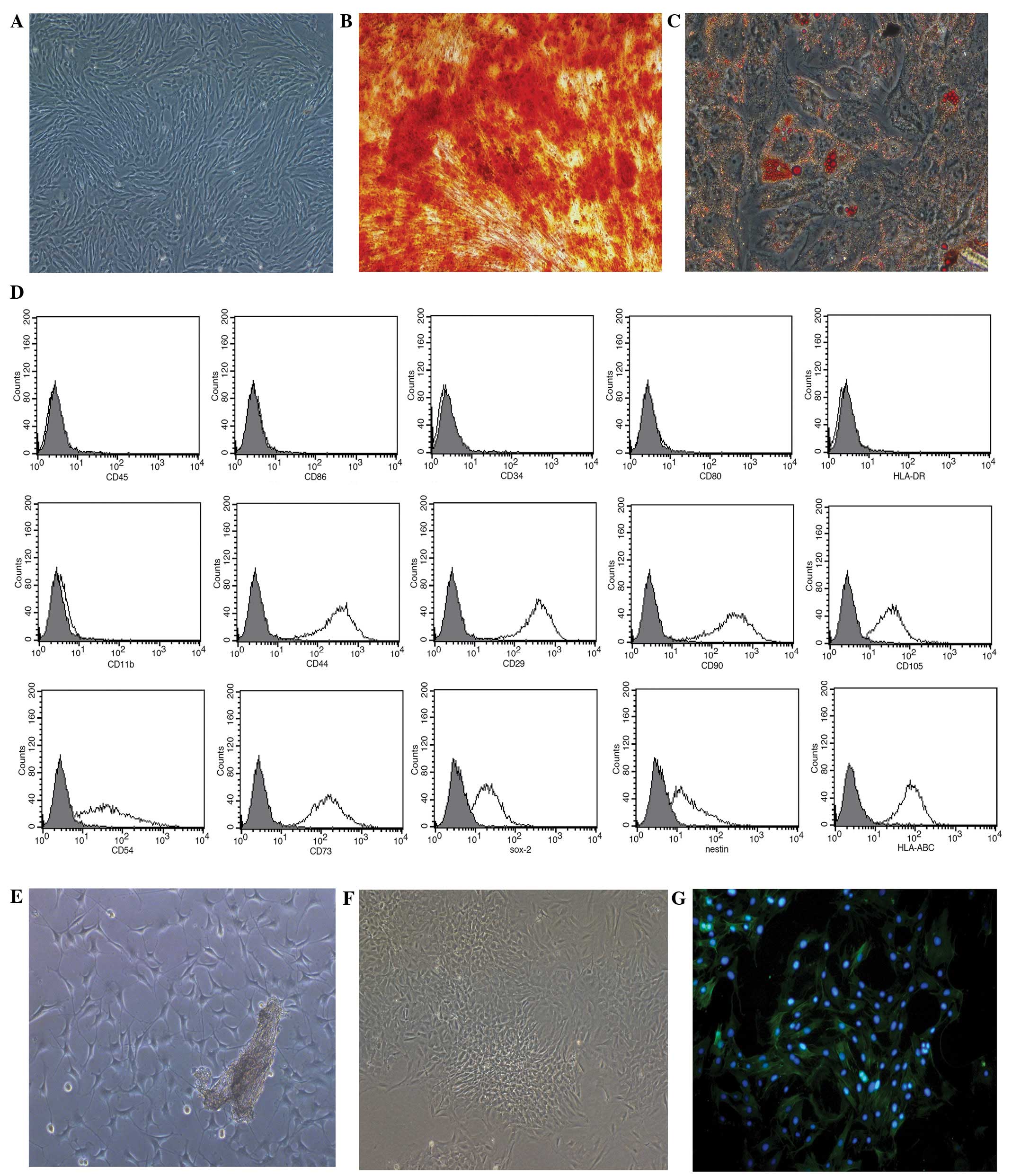 | Figure 1.Growth of mesenchymal stem cells
(MSCs) and pulmonary artery smooth muscle cells (PASMCs) in
vitro. (A) MSCs culured in serum-free medium at passage 3
(magnification, ×100). (B) Osteogenic and (C) adipogenic
differentiation of MSCs cultured in MSC-conditioned media
identified using Alizarin red S or Oil Red O stain, respectively
(magnification, ×200). (D) Phenotypic analysis of MSCs, assessed
using flow cytometry. (E) PASMC tissue explant cultured for 5 days
(magnification, ×100). (F) PASMCs at passage 3 (magnification,
×100). (G) PASMC immunofluorescent staining for α-smooth muscle
actin (magnification, ×200). CD, cluster of differentiation; Sox-2,
SRY box-2; HLA, human leukocyte antigen. |
After 5 days of culture using the tissue explants
method, PASMCs harvested from rats were observed surrounding the
tissue explants (Fig. 1E). Following
a further 3 passages of culture, the cells displayed the typical
‘hill and valley’ growth pattern (Fig.
1F), and were positive for α-SMA, according to
immunofluorescent staining (Fig.
1G).
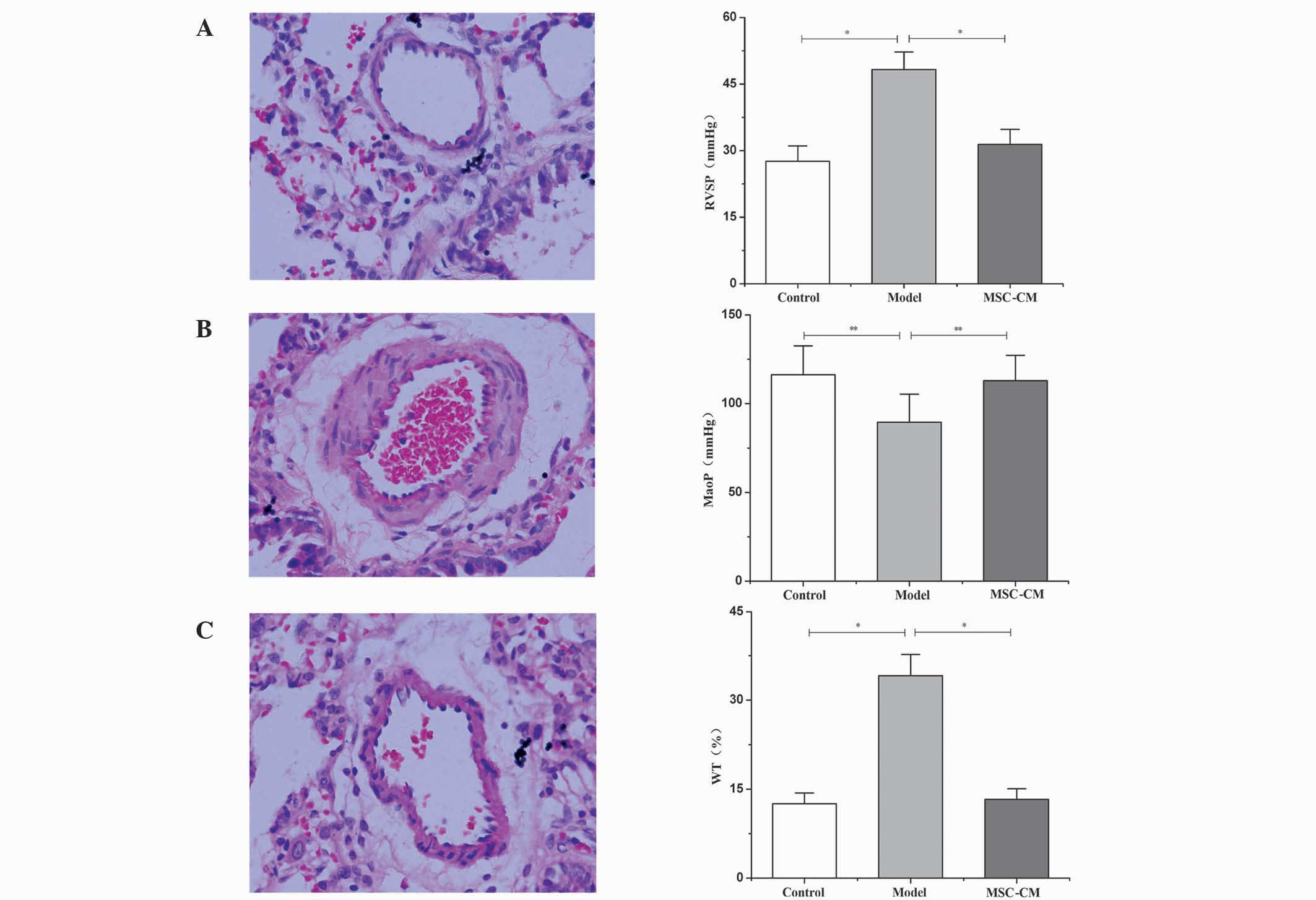
MSC-CM improves PH
The examination of hemodynamics 21 days after
injection of MCT indicated a significant increase in RVSP compared
with the control group (48.26±3.93 vs. 27.58±3.47 mmHg; P<0.01;
Fig. 2A), and a significant decrease
in MAoP compared with the control group (89.54±15.81 vs.
116.37±16.17 mmHg; P<0.05; Fig.
2B). In accordance with this finding, histological examination
also indicated that, 21 days after injection with MCT, medial
hypertrophy of pulmonary muscular arterioles was evident in the
model group (Fig. 2B). Additionally,
WT levels were significantly increased in the model group compared
with the control (34.12±3.59 vs. 12.53±1.82%; P<0.01; Fig. 2C). These changes indicated the
development of PH in the experimental rat.
Administration of MSC-CM leads to the
improvement of abnormal hemodynamics
RVSP decreased to 31.42±3.38 mmHg (Fig. 2A) and MAoP increased to 112.97±14.26
mmHg (Fig. 2B) following MSC-CM
treatment; these values were significantly different compared with
the model group (P<0.05). In addition, the medial hypertrophy of
pulmonary muscular arterioles was improved (Fig. 2C), and the WT was significantly
decreased (13.28±1.78%) compared with that in the model group
(34.12±3.59%; P<0.01; Fig.
2C).
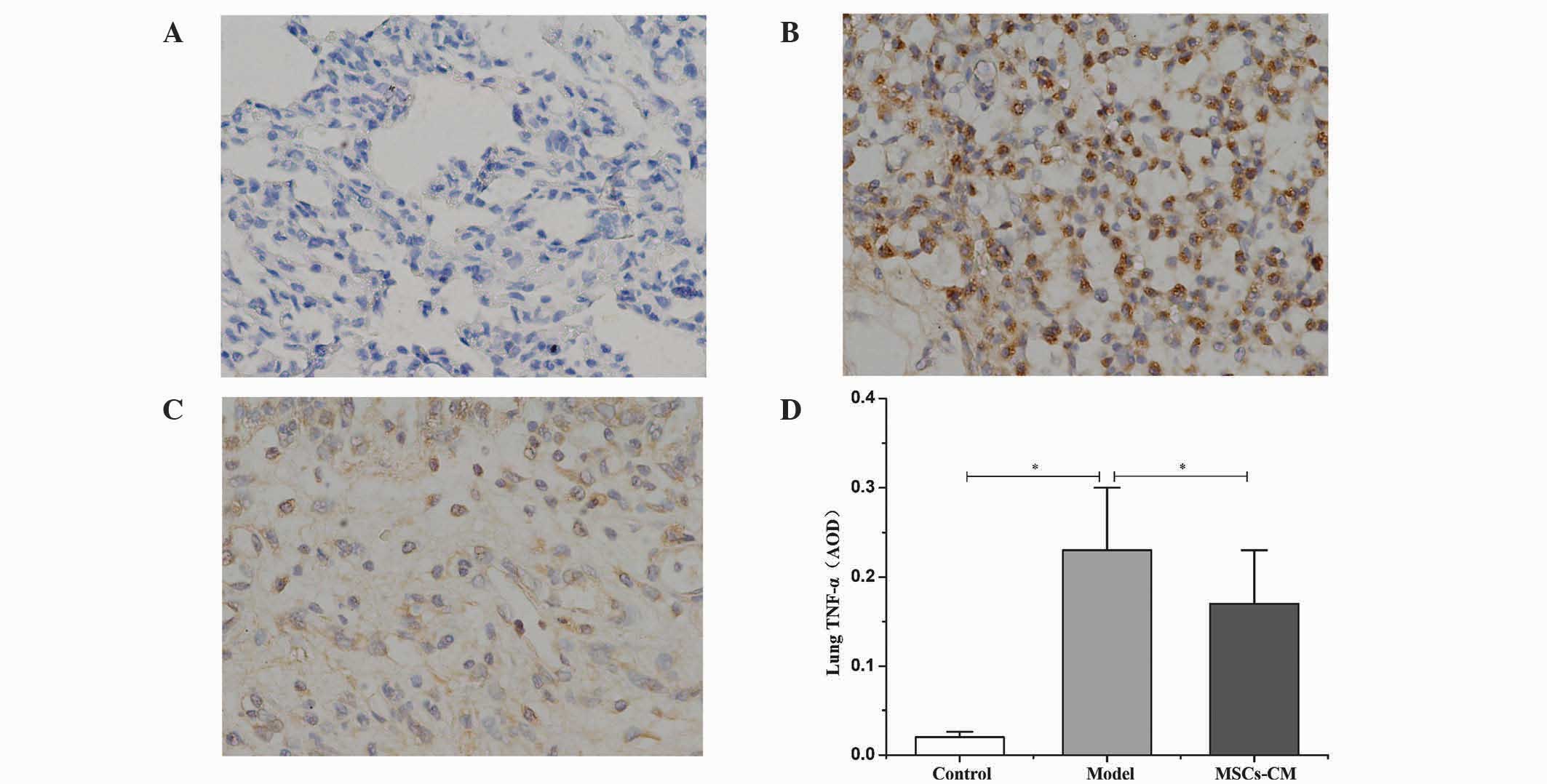
MSC-CM decreases the protein
expression levels of TNF-α in lung tissue
Compared with the control group (Fig. 3A), immunohistochemical staining
revealed that an abundance of cells were positive for TNF-α within
the lung tissue 21 days after injection of MCT in the model group
(Fig. 3B). Following the
administration of MSC-CM, the number of cells positive for TNF-α
decreased (Fig. 3C), and the average
OD significantly decreased from 0.23±0.07 in the model group to
0.17±0.06 in the MSC-CM treatment group (P<0.01; Fig. 3D).
MSC-CM suppresses the production of
TNF-α and proliferation of PASMCs in co-culture systems
After 3 days of culture, levels of TNF-α in the
supernatant of the co-culture system were analyzed by ELISA. The
results revealed that high levels of TNF-α (762.51±42.35 pg/ml)
were produced by T cells under stimulation with ConA (Fig. 1A). However, in the presence of
MSC-CM, the production of TNF-α was significantly inhibited
(218.25±19.33 pg/ml; P<0.01; Fig.
4A). Additionally, the proliferation of PASMCs, as assessed by
an MTS assay, revealed that the proliferation of PASMCs
significantly increased when co-cultured with ConA-stimulated T
cells, compared with PASMCs cultured alone (0.94±0.05 vs.
0.73±0.06; P<0.01; Fig. 4B).
Furthermore, the ability of T cells in promoting the proliferation
of PASMCs was significantly inhibited by treatment with MSC-CM
(0.78±0.07; P<0.01; Fig. 4B).
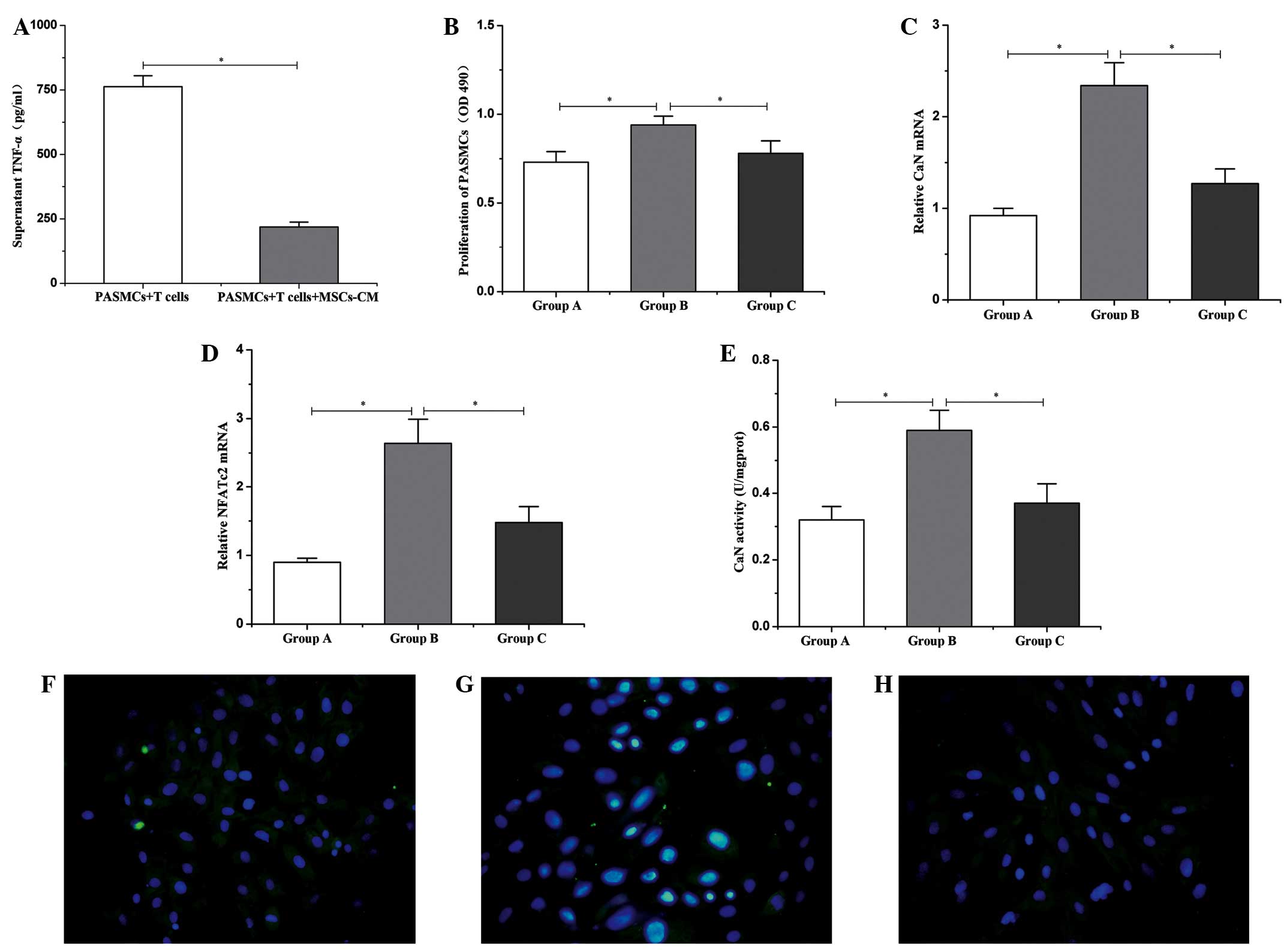 | Figure 4.Changes in TNF-α, CaN and NFATc2 in
the co-culture system. (A) Protein expression levels of TNF-α in
the supernatant. (B) Proliferation of PASMCs. mRNA expression of
(C) CaN and (D) NFATc2 in PASMCs. (E) CaN activity in PASMCs. Group
A, PASMCs alone; Group B, PASMCs + T cells; Group C, PASMCs +T
cells + MSC-CM. Data are presented as mean ± standard deviation
(n=6). *P<0.01. Activation of NFATc2 in PASMCs in the co-culture
system for (F) group A; (G) group B, and (H) group C (fluorescein
isothiocyanate/DAPI staining; magnification, ×400). TNF-α, tumor
necrosis factor-α; PASMCs, pulmonary artery smooth muscle cells;
MSC-CM, mesenchymal stem cell-conditioned media; OD, optical
density; CaN, calcineurin; NFATc2, nuclear factor of activated T
cells c2. |
MSC-CM downregulates the expression of
CaN and NFATc2
CaN and NFATc2 are critical regulatory factors of
SMC proliferation, thus, the expression levels of both were
assessed by reverse transcription-quantitative polymerase chain
reaction (11). The results
indicated that, after 3 days of co-culture with ConA-stimulated T
cells, the expression levels of CaN and NFATc2 in PASMCs were
significantly upregulated compared with the expression in PASMCs
alone. Furthermore, MSC-CM was able to significantly reduce the
expression levels of CaN and NFATc2 (P<0.01) that were increased
by the promoting effects of the activated T cells (Fig. 4C and D).
MSC-CM suppresses CaN activity and
NFATc2 activation in PASMCs
In the co-culture system, it was observed that
intracellular CaN activity was significantly upregulated in PASMCs
cultured for 3 days with ConA-stimulated T cells, compared with
PASMCs cultured alone (0.59±0.06 vs. 0.32±0.04 U/mgprot; P<0.01;
Fig. 4E). Furthermore, the majority
of NFATc2 was translocated to the nucleus, indicating the
activation of NFATc2 (Fig. 4G).
Following the addition of MSC-CM, the intracellular CaN activity of
PASMCs was significantly decreased (0.37±0.06 U/mgprot; P<0.01;
Fig. 4E). When PASMCs were cultured
independently, the expression of NFATc2 was predominantly localized
to the cytoplasm (Fig. 4F). Finally,
addition of MSC-CM also led to suppression of NFATc2 activation
(Fig. 4H).
Discussion
The present study demonstrated that the
administration of MSC-CM decreases protein expression levels of
TNF-α in the lungs of rat models with MCT-induced PH. In addition,
improvements in hemodynamic and histological abnormalities were
also observed. A co-culture system comprised of activated T cells
and PASMCs was established to further reveal the therapeutic
mechanism of MSC-CM in PH. The in vitro co-culture system
revealed that the high levels of TNF-α produced as a result of
activated T cells leads to the upregulation of CaN and NFATc2
expression. Additional effects observed included an increase in
intracellular CaN activity and NFATc2 activation in PASMCs, thus
promoting the proliferation of PASMCs. MSC-CM was able to
effectively suppress the production of high levels of TNF-α by T
cells, which led to the downregulation of expression levels of CaN
and NFATc2 in PASMCs. This had the effect of decreasing CaN
activity and preventing NFATc2 activation, which consequently
resulted in suppression of the proliferation of PASMCs.
Persistent contraction and hypertrophy of pulmonary
arterioles are considered to be defining characteristics of PH
(24). Inflammation and the
overproliferation of PASMCs are also hypothesized to have an
involvement in the pathological process, thus rendering them a
therapeutic target for the treatment of PH (12). MSCs, as a subset of stromal stem
cells, have attracted much attention as potential therapeutic
vehicles for the treatment of PH and other diseases of the lung,
due to their potent immunomodulatory, anti-inflammatory and
anti-fibrotic properties (25–27).
Previously, it has been demonstrated that MSCs have the ability to
suppress activated CD4+ T cells, thereby preventing the
production of inflammatory cytokines when co-cultured (14). Furthermore, the immunosuppressive
activity of MSCs is highly associated with the secretion of PGE2
and immunoregulatory cytokines (14). Due to the extensive level of
inflammation involved in the pathological mechanism of PH, the
immunosuppressive effects of MSCs may be beneficial to the
treatment of PH. The exact mechanism detailing the involvement of
MSCs in PH, however, remains unclear. A former hypothesis
suggesting that MSCs had the ability to differentiate into various
lung tissue types following transplantation has been somewhat
discredited due to the existence of the MSC niche, which may serve
to hinder the homing and differentiation of MSCs (28). Furthermore, due to the ability of
MSC-CM to attenuate hypoxia-induced pulmonary injury (15), the paracrine mechanism is generally
considered to have greater plausibility in explaining the
protective effects of MSCs. With the additional benefit of no
ethical restrictions against its use, MSC-CM, which possesses
numerous therapeutic effects, may be a promising vehicle in the
clinic. In the present study, a rat model of MCT-induced PH was
used to assess the immunoregulatory capabilities and therapeutic
effects of MSC-CM. The results demonstrated that expression levels
of TNF-α within the lung tissue were decreased by the
immunosuppressive activity of MSC-CM. As a result, hemodynamic
abnormalities, including increased RVSP and decreased MAoP, were
improved and approaching normal levels. Further examination of the
hemodynamics also indicated that the medial hypertrophy and
remodeling of pulmonary arterioles were prevented. These results
may indicate the mechanisms by which the immunoregulatory
properties of MSC-CM are responsible for potential therapeutic
benefits in the treatment of PH.
The NFAT family of proteins contains a series of
pivotal regulators of immune response that are widely expressed in
a host of tissues and organs (29),
including smooth muscle cells (30).
Typically, NFAT proteins are predominately distributed in the
cytoplasm, where they are inactive. Upon dephosphorylation by the
Ca2+-responsive phosphatase CaN, NFAT proteins are
activated and translocated to the nucleus in order to exert their
regulatory actions (29). It has
previously been identified that the activation of CaN and NFAT are
involved in the pathogenesis of PH (9).
NFAT activation has been observed in PASCs obtained
from patients with PH (12) and in
experimental animal models, such as MCT-induced (12) and hypoxia-induced (13) PH models. Furthermore, inhibition of
CaN or NFAT may result in a decrease in the proliferation of
PASMCs, and an increase the levels of apoptosis (12), thereby inhibiting hypoxia-induced PH
and right ventricular hypertrophy (31). In order to further elucidate the
exact therapeutic mechanism of MSC-CM in PH, the present study used
an in vitro co-culture system containing T cells and PASMCs.
The supernatant protein expression levels of TNF-α, mRNA expression
levels of CaN and NFAT, as well as CaN activity and NFATc2
activation in PASMCs were assessed to better understand the
mechanisms pertaining to the regulatory effects of MSC-CM on the
inflammation-associated overproliferation of PASMCs. The results
indicated that, under the stimulation of ConA, T cells produced
high levels of TNF-α, resulting in the upregulation of CaN and NFAT
mRNA expression levels, increased CaN activity, and promoted
activation of NFATc2, thus accelerating the proliferation of
PASMCs. As a result of the immunosuppressive effects of MSC-CM,
TNF-α production levels significantly decreased. This
immunosuppressive effect was also associated with downregulated
expression levels of CaN and NFAT in PASMCs; in agreement, CaN
activity and NFATc2 activation levels were observed to have
decreased. Consequentially, the overproliferation of PASMCs was
effectively suppressed by MSC-CM.
In conclusion, the present study provides new
evidence highlighting the ability of conditioned media from MSCs to
suppress the overproliferation of PASMCs, and thus have possible
therapeutic benefits in the treatment of PH. The predominant reason
for this effect was a result of the suppression of TNF-α, and the
subsequent inhibitory action on the expression levels of CaN and
NFAT, CaN activity, and the activation of NFATc2 in PASMCs.
However, the long-term impacts and potential side-effects of this
prospective treatment require further investigation.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (grant nos. 30560159, 30960412
and 31360285).
References
|
1
|
Bazan IS and Fares WH: Pulmonary
hypertension: Diagnostic and therapeutic challenges. Ther Clin Risk
Manag. 11:1221–1233. 2015.PubMed/NCBI
|
|
2
|
Tonelli AR: Pulmonary hypertension
survival effects and treatment options in cystic fibrosis. Curr
Opin Pulm Med. 19:652–661. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Tonelli AR, Fernandez-Bussy S, Lodhi S,
Akindipe OA, Carrie RD, Hamilton K, Mubarak K and Baz MA:
Prevalence of pulmonary hypertension in end-stage cystic fibrosis
and correlation with survival. J Heart Lung Transplant. 29:865–872.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hayes D Jr, Higgins RS, Kirkby S, McCoy
KS, Wehr AM, Lehman AM and Whitson BA: Impact of pulmonary
hypertension on survival in patients with cystic fibrosis
undergoing lung transplantation, An analysis of the UNOS registry.
J Cyst Fibros. 13:416–423. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Pinto RF: HiguchiM de L and Aiello VD:
Decreased numbers of T-lymphocytes and predominance of recently
recruited macrophages in the walls of peripheral pulmonary arteries
from 26 patients with pulmonary hypertension secondary to
congenital cardiac shunts. Cardiovasc Pathol. 13:268–275. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Perros F, Dorfmüller P, Montani D, Hammad
H, Waelput W, Girerd B, Raymond N, Mercier O, Mussot S,
Cohen-Kaminsky S, et al: Pulmonary lymphoid neogenesis in
idiopathic pulmonary arterial hypertension. Am J Respir Crit Care
Med. 185:311–321. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Heath D and Edwards JE: The pathology of
hypertensive pulmonary vascular disease; a description of six
grades of structural changes in the pulmonary arteries with special
reference to congenital cardiac septal defects. Circulation.
18:533–547. 1958. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Soon E, Holmes AM, Treacy CM, Doughty NJ,
Southgate L, Machado RD, Trembath RC, Jennings S, Barker L, Nicklin
P, et al: Elevated levels of inflammatory cytokines predict
survival in idiopathic and familial pulmonary arterial
hypertension. Circulation. 122:920–927. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Frid MG, Brunetti JA, Burke DL, Carpenter
TC, Davie NJ, Reeves JT and Roedersheimer MT: vanR ooijen N and
Stenmark KR: Hypoxia-induced pulmonary vascular remodeling requires
recruitment of circulating mesenchymal precursors of a
monocyte/macrophage lineage. Am J Pathol. 168:659–669. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Rowlands DJ, Islam MN, Das SR, Huertas A,
Quadri SK, Horiuchi K, Inamdar N, Emin MT, Lindert J, Ten VS, et
al: Activation of TNFR1 ectodomain shedding by mitochondrial
Ca2+ determines the severity of inflammation in mouse
lung microvessels. J Clin Invest. 121:1986–1999. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Said SI, Hamidi SA and Gonzalez Bosc L:
Asthma and pulmonary arterial, hypertension. Do they share a key
mechanism of pathogenesis? Eur Respir J. 35:730–734. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bonnet S, Rochefort G, Sutendra G, Archer
SL, Haromy A, Webster L, Hashimoto K, Bonnet SN and Michelakis ED:
The nuclear factor of activated T cells in pulmonary arterial
hypertension can be therapeutically targeted. Proc Natl Acad Sci
USA. 104:11418–11423. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
de Frutos S, Spangler R, Alò D and Bosc
LV: NFATc3 mediates chronic hypoxia-induced pulmonary arterial
remodeling with alpha-actin up-regulation. J Biol Chem.
282:15081–15089. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yang ZX, Han ZB, Ji YR, Wang YW, Liang L,
Chi Y, Yang SG, Li LN, Luo WF, Li JP, et al: CD106 identifies a
subpopulation of mesenchymal stem cells with unique
immunomodulatory properties. PLoS One. 8:e593542013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Aslam M, Baveja R, Liang OD,
Fernandez-Gonzalez A, Lee C, Mitsialis SA and Kourembanas S: Bone
marrow stromal cells attenuate lung injury in a murine model of
neonatal chronic lung disease. Am J Respir Crit Care Med.
180:1122–1130. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lu LL, Liu YJ, Yang SG, Zhao QJ, Wang X,
Gong W, Han ZB, Xu ZS, Lu YX, Liu D, et al: Isolation and
characterization of human umbilical cord mesenchymal stem cells
with hematopoiesis-supportive function and other potentials.
Haematologica. 91:1017–1026. 2006.PubMed/NCBI
|
|
17
|
Matsuda Y, Hoshikawa Y, Ameshima S, Suzuki
S, Okada Y, Tabata T, Sugawara T, Matsumura Y and Kondo T: Effects
of peroxisome proliferator-activated receptor gamma ligands on
monocrotaline-induced pulmonary hypertension in rats. Nihon Kokyuki
Gakkai Zasshi. 43:283–288. 2005.(In Japanese). PubMed/NCBI
|
|
18
|
Liu JF, DU ZD, Chen Z, Han ZC and He ZX:
Granulocyte colony-stimulating factor attenuates
monocrotaline-induced pulmonary hypertension by upregulating
endothelial progenitor cells via the nitric oxide system. Exp Ther
Med. 6:1402–1408. 2013.PubMed/NCBI
|
|
19
|
Maruyama H, Watanabe S, Kimura T, Liang J,
Nagasawa T, Onodera M, Aonuma K and Yamaguchi I: Granulocyte
colony-stimulating factor prevents progression of
monocrotaline-induced pulmonary arterial hypertension in rats. Circ
J. 71:138–143. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Woodrum DT, Ford JW, Cho BS, Hannawa KK,
Stanley JC, Henke PK and Upchurch GR Jr: Differential effect of
17-beta-estradiol on smooth muscle cell and aortic explant MMP2. J
Surg Res. 155:48–53. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ahmad S, Choudhry MA, Shankar R and Sayeed
MM: Transforming growth factor-beta negatively modulates T-cell
responses in sepsis. FEBS Lett. 402:213–218. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Liu JF, Han ZB, Han ZC and He ZX:
Mesenchymal stem cells suppress CaN/NFAT expression in the
pulmonary arteries of rats with pulmonary hypertension. Exp Ther
Med. 10:1657–1664. 2015.PubMed/NCBI
|
|
23
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tuder RM, Marecki JC, Richter A,
Fijalkowska I and Flores S: Pathology of pulmonary hypertension.
Clin Chest Med. 28:23–42. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Weiss DJ, Bertoncello I, Borok Z, Kim C,
Panoskaltsis-Mortari A, Reynolds S, Rojas M, Stripp B, Warburton D
and Prockop DJ: Stem cells and cell therapies in lung biology and
lung diseases. Proc Am Thorac Soc. 8:223–72. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ortiz LA, Gambelli F, McBride C, Gaupp D,
Baddoo M, Kaminski N and Phinney DG: Mesenchymal stem cell
engraftment in lung is enhanced in response to bleomycin exposure
and ameliorates its fibrotic effects. Proc Natl Acad Sci USA.
100:8407–8411. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hansmann G, Fernandez-Gonzalez A, Aslam M,
Vitali SH, Martin T, Mitsialis SA and Kourembanas S: Mesenchymal
stem cell-mediated reversal of bronchopulmonary dysplasia and
associated pulmonary hypertension. Pulm Circ. 2:170–81. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Jun D, Garat C, West J, Thorn N, Chow K,
Cleaver T, Sullivan T, Torchia EC, Childs C, Shade T, et al: The
pathology of bleomycin-induced fibrosis is associated with loss of
resident lung mesenchymal stem cells that regulate effector T cell
proliferation. Stem Cells. 29:725–735. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Pan MG, Xiong Y and Chen F: NFAT gene
family in inflammation and cancer. Curr Mol Med. 13:543–554. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kudryavtseva O, Aalkjaer C and Matchkov
VV: Vascular smooth muscle cell phenotype is defined by
Ca2+-dependent transcription factors. FEBS J.
280:5488–5499. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Koulmann N, Novel-Chaté V, Peinnequin A,
Chapot R, Serrurier B, Simler N, Richard H, Ventura-Clapier R and
Bigard X: Cyclosporin A inhibits hypoxia-induced pulmonary
hypertension and right ventricle hypertrophy. Am J Respir Crit Care
Med. 174:699–705. 2006. View Article : Google Scholar : PubMed/NCBI
|