Introduction
Chronic kidney disease (CKD) is a major challenge
for global public health care, due to its irreversible progression,
low awareness and high cost (1). Due
to the progressive aging of the general population, and the
emerging epidemic of obesity and diabetes, CKD is now one of the
three leading causes of mortality worldwide (1,2). Studies
regarding the pathogenesis of CKD have predominantly focused on
glomerular sclerosis and interstitial fibrosis, with disturbance of
extracellular matrix (ECM) homeostasis (i.e. synthesis and
degradation) a common factor (3).
Mesangial lesions, caused by mesangial cell (MC) proliferation and
accumulation of ECM, are universal morphological manifestations of
renal diseases that involve the mesangium. There are numerous
growth factors, cytokines and signaling pathways that are involved
in the process of mesangial fibrosis, among which transforming
growth factor-β1 (TGF-β1) is the most versatile, with functions in
cell growth, apoptosis, proliferation, and ECM production (4). TGF-β1 is able to affect MC
proliferation, and stimulate the synthesis and inhibit the
degradation of ECM in an autocrine or paracrine manner, leading to
relentless progression of glomerular sclerosis (5).
The Wnt signaling pathway is a key regulator of
embryonic development, tissue homeostasis, cell apoptosis,
proliferation and differentiation (6–8). There
are 19 known Wnts, which mediate at least three divergent signaling
pathways: The β-catenin dependent pathway (canonical Wnt pathway),
the Ca2+-pathway, and the planar cell polarity-pathway;
the latter two pathways are also known as non-canonical Wnt
pathways. In the canonical Wnt pathway, Wnt proteins bind to a
receptor complex formed by Frizzled and low-density lipoprotein
receptor-related protein 5 or 6, which leads to disassembly of the
β-catenin destruction complex, which is composed of axin,
adenomatous polyposis coli protein, and glycogen synthase kinase-3β
(8). Disassembly of the β-catenin
destruction complex hinders the normal phosphorylation and
ubiquitination process of cytoplasmic β-catenin, allowing it to
accumulate in the cytosol and translocate into the nucleus, where
it stimulates the transcription of its target genes, including
Twist, Snail, fibronectin, and matrix metalloproteinase-7 (9). The Wnt pathway has previously been
demonstrated to be involved in renal tubular atrophy and
interstitial fibrosis of an obstructed renal model (10,11),
podocyte apoptosis and dysfunction, albuminuria and slit diaphragm
abnormalities (12–14), and high glucose-induced MC apoptosis
(15,16). Interactions between Wnt/β-catenin and
TGF-β signaling pathways have been shown to be important in the
pathogenesis of fibrosis (17,18). In
addition, manipulation of the Wnt pathway may rescue TGF-β1
mediated fibrosis.
Yi Qi Qing Re Gao (YQ) is a traditional Chinese
medicine mixture developed by Zhan and Dai (19), which has been used for decades with
satisfactory clinical effects. In a previous clinical study, YQ was
demonstrated to reduce proteinuria, elevate serum albumin, decrease
serum cholesterol, and ameliorate certain symptoms associated with
chronic nephritis (19).
Furthermore, a previous animal study in an adriamycin rat model
suggested that YQ may be able to reduce glomerular mesangial
collagen type IV, fibronectin and laminin content (20). Our recent study revealed that YQ was
able to attenuate podocyte injury and inhibit vascular endothelial
growth factor overexpression in a puromycin aminonucleoside rat
model (21). However, the mechanisms
underlying the effects of YQ on MC proliferation and glomerular
sclerosis remain to be elucidated. The present study aimed to
investigate the expression of Wnt pathway-associated molecules and
TGF-β1 in LPS-stimulated MCs, and to determine the effects of YQ on
this pathway, in order to provide evidence for the role of YQ in
the prevention and treatment of glomerulosclerosis.
Materials and methods
Animals
A total of 40 healthy male Wistar rats (age, 7–8
weeks), weighing 250±20 g, were purchased from Beijing China Fukang
Biological Technology Co., Ltd. (no. SCXK 2009-0007; Beijing,
China). Rats were housed in the specific pathogen-free animal
facility at the China-Japan Friendship Hospital (Beijing, China),
with free access to water and standard rat chow. This study was
approved by the Ethics Committee of the China-Japan Friendship
Hospital and conducted in accordance with the Guiding Principles
for the Care and Use of Laboratory Animals (no. 2010-A10).
Cell line
A rat mesangial cell line was purchased from Peking
Union Medical College (no. HBZY-1; Beijing, China).
Reagents and instruments
Minimum essential medium (MEM), streptomycin and
ampicillin, phosphate-buffered saline (PBS) and trypsin were all
purchased from GE Healthcare (Logan, UT, USA). In addition, fetal
bovine serum (FBS) was obtained from Gibco Life Technologies
(Carlsbad, CA, USA); lipopolysaccharide (LPS) was purchased from
Sigma-Aldrich (St. Louis, MO, USA); MTT
[3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide] was
from Enzo Life Sciences, Inc. (Farmingdale, NY, USA); TRIzol
reagent was from Invitrogen Life Technologies (Carlsbad, CA, USA);
RevertAid™ First Strand cDNA Synthesis kit was from Fermentas,
Thermo Fisher Scientific (Vilnius, Lithuania); Wnt4 goat polyclonal
antibody (cat. no. sc-5214) was from Santa Cruz Biotechnology, Inc.
(Dallas, TX, USA); mouse TGF-β1 monoclonal antibody (cat. no.
ab64715) was from Abcam (Cambridge, UK); rabbit β-catenin
monoclonal antibody (cat. no. 8480S) was from Cell Signaling
Technology, Inc. (Danvers, MA, USA); goat anti-mouse IgG and goat
anti-rabbit IgG antibodies were from Beijing Zhongshanjinqiao
Biotechnology Co., Ltd (Beijing, China). The instruments used in
the present study were as follows: ABI 2720 thermal cycler (Applied
Biosystems Life Technologies, Foster City, CA, USA); AlphaImager
TM2200 gel imaging system (Alpha Innotech Corporation, San Leandro,
CA, USA); 1X51 inverted microscope (Olympus Corporation, Tokyo,
Japan); and NAPCO CO2 incubator (Thermo Fisher
Scientific, Inc., San Jose, CA, USA).
Experimental drugs
YQ was obtained from Guang'Anmen Hospital (Beijing,
China; certificate no. 98; Beijing Health & Drug no. 058). The
mixture included: 72 g Radix Astragali Mongolici (Huang Qi),
54 g Rhizoma Atractylodis Macrocephalae (Bai Zhu), 36 g
Radix Ledebouriellae (Fang Feng), 72 g Flos Lonicerae
(Jin Yin Hua), 72 g Fructus Forsythiae Suspensae (Lian
Qiao), 54 g Herba Duchesneae Indicae (She Mei), 180 g
Herba Hedyotis Diffusae (Bai Hua She She Cao), 72 g Poria
Cocos (Fu Ling), 125 g Rhizoma Alismatis (Ze Xie), 180 g
Herba Leonuri (Yi Mu Cao), 180 g Rhizoma Imperatae
(Bai Mao Gen) and 70 g Rhizoma Dioscoreae Nippponicae (Chuan
Shan Long). Herbal medicines were prepared as follows: Water
decoction for 30 min, followed by concentration of the supernatant
under ordinary pressure, centrifugation at 2,000 × g for 15 min,
and sterilization by filtration. The final standardized product
consisted of 4.5 g crude medicine per milliliter. fosinopril
tablets, containing fosinopril sodium, were provided by
Sino-American Shanghai Squibb Pharmaceutical Ltd. (Shanghai,
China).
Preparation of drug-containing
sera
After three days of acclimation, 40 Wistar rats were
divided at random into three groups, including the YQ (n=10),
fosinopril (n=10) and normal (n=20) groups. Rats received an
intragastric dose of YQ (5.7 g/kg, which corresponds to 20 times
the adult clinical dose), fosinopril (1.67 mg/kg, which corresponds
to 10 times the adult clinical dose), or an equal volume of
distilled water, twice a day for seven days. Abdominal aortic blood
(5–10 ml) was collected following the final drug administration.
Blood samples were allowed to stand at 4°C for 4 h, followed by
centrifugation at 900 × g for 10 min at 4°C. Sera were carefully
extracted by suction and then incubated in a 56°C water bath for 30
min to inactivate complements and antibodies present in the sera.
Subsequent to 0.22 µm filter-sterilization, the sera were stored in
sterile centrifuge tubes at −20°C.
Recovery and passage of cells
Frozen rat MCs (HBZY-1) were removed from liquid
nitrogen, and rapidly transferred to a 37°C water bath for ~1 min
until dissolved into a mixture of ice and water. MCs were
subsequently transferred to a 25 cm2 culture flask with
5 ml MEM containing 10% FBS, and 100 IU/ml streptomycin and
ampicillin, and then placed in a 5% CO2 incubator at
37°C. Following overnight culture until the cells became adherent,
the medium was changed. The medium was discarded when the MCs were
in the logarithmic phase, and the cells were washed twice with PBS,
followed by the addition of 1 ml trypsin (0.25%). When the cells
exhibited a round shape, culture medium was immediately added to
terminate the digestion. Subsequently, the cell suspension was
transferred to a new culture flask containing 10% FBS-containing
MEM, and placed in an incubator with 5% CO2 at 37°C for
3–5 days until they reached 80–100% confluence. The 5th generation
of MCs was used for subsequent experimentation.
Experimental design
Cells of the 5th generation were transferred into
96-well plates (3×103/well), and incubated with MEM
supplemented with 5% FBS and 20% normal rat serum for 24 h.
Subsequently, the cells were incubated with MEM containing 2% FBS
for 24 h, in order to be synchronized at G0 phase. The
cells were divided into: Control group (incubated with MEM + 5% FBS
+ 20% normal rat serum), the LPS-stimulated (LPS) group (incubated
with MEM + 5% FBS + 20% normal rat serum + 5 µg/ml LPS), the YQ
containing serum (YQ-S) group (incubated with MEM + 5% FBS + 20%
YQ-S + 5 µg/ml LPS), the fosinopril containing serum (For-S) group
(incubated with MEM + 5% FBS + 20% For-S + 5 µg/ml LPS). There were
6 wells for each group.
Effect of LPS and drug-containing
serum on rat MC proliferation
Once the cells were synchronized at G0
phase after 24 h, the supernatant was removed and 200 µl medium
supplemented with 5% FBS and various concentrations of LPS (0.0,
0.5, 1.0, 5.0 and 10.0 µg/ml), and YQ-S or For-S (5, 10 and 20%)
were added to each well (n=3, triplicate wells). The cells were
further incubated for 48 h, and subsequently 150 µl MTT (5 mg/ml)
was added to each well. Following incubation at 37°C for 4 h, the
supernatant was discarded carefully, and 150 µl DMSO was added to
each well. Following shaking at room temperature for 10 min, the
optical density values were measured at 492 nm using a microplate
absorbance reader (Model 680; Bio-Rad Laboratories, Inc., Hercules,
CA, USA). Each experiment was repeated three times.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) for the detection of Wnt4,
β-catenin and TGF-β1 mRNA expression levels
MCs in the logarithmic growth phase were digested
and suspended, and 1×105 cells were seeded in a 100 mm
culture dish and incubated for 24 h in order to reach adherence.
After 48 h of stimulation according to the various groups, the
cells were lysed with TRIzol to extract the total RNA, which was
then reverse transcribed into cDNA. PCR amplification primers were
synthesized by Beijing Qing Ke New Industrial Biotechnology Co.,
Ltd. (Beijing, China). Primer sequences and reaction conditions are
presented in Table I. PCR was
performed in triplicate in a final volume of 12.5 µl: 6.25 µl PCR
mix (Beijing ComWin Biotech Co., Ltd., Beijing, China), 2 µl
diluted cDNA products, 1 µl of each paired primer, and 3.25 µl
deionized water. PCR was carried out using an ABI 2720 thermal
cycler (Applied Biosystems Life Technologies). Cycling conditions
were as follows: Initial denaturation for 2 min at 94°C, followed
by 29 cycles (for cyclophilin B and TGF-β1), 30 cycles (for
β-catenin) or 34 cycles (for Wnt4) of denaturation for 30 sec at
94°C, extension for 1 min at 72°C, and a final elongation for 10
min at 72°C. PCR products were loaded onto a 1.2% agarose gel and
electrophoresis was performed at 100 V constant voltage for 30 min.
Gel images were analyzed using ImageJ 1.40g software (National
Institutes of Health, Bethesda, MD, USA), which was used to
determine each gray value of the target band. Relative quantities
of each target gene were represented as the ratio of the gray
values of target to that of cyclophilin B.
 | Table I.Polymerase chain reaction primers and
reaction parameters. |
Table I.
Polymerase chain reaction primers and
reaction parameters.
| Primer | Primer
sequence | Fragment length
(bp) | Annealing
temperature (°C) | Cycles |
|---|
| Wnt4 | F
5′-CGGGAAGGTGGTGACACAAG-3′ | 375 | 58 | 34 |
|
| R
5′-GCTCGCCAGCATGTCTTTAC-3′ |
|
|
|
| β-catenin | F
5′-AATGGCTTGGAATGAGACTG-3′ | 198 | 56 | 30 |
|
| R
5′-AGCCCATCAACTGGATAGTC-3′ |
|
|
|
| TGF-β1 | F
5′-TACCATGCCAACTTCTGTCTG-3′ | 204 | 58 | 29 |
|
| R
5′-CACGATCATGTTGGACAACTG-3′ |
|
|
|
| Cyclophilin B | F
5′-GTGGTTTTCGGCAAAGTTCTG-3′ | 147 | 56 | 29 |
|
| R
5′-GGCAAAGGGTTTCTCCACTTC-3′ |
|
|
|
Western blot analysis of Wnt4,
β-catenin and TGF-β1 protein expression levels
Following 48 h of stimulation according to the
grouping aforementioned, the MCs were collected and lysed with cell
lysis buffer (150 mM NaCl, 1 mM EDTA, 20 mM Tris HCl, pH 7.4) and
1X protein inhibitor cocktail for extraction of total proteins. The
protein concentrations of the samples were determined by Bradford
assay (Beijing Solarbio Science & Technology Co., Ltd.,
Beijing, China). Samples containing 60 µg proteins were loaded onto
10% SDS-PAGE gel and separated by electrophoresis. The initial
electrophoresis with condensed gel was conducted at 80 V for 25
min, followed by electrophoresis with separation gel at 150 V for
50 min. The proteins were then electrotransferrd to a
polyvinylidene fluoride membrane (EMD Millipore, Billerica, MA,
USA), and the membranes were blocked with 5% skim milk in
Tris-buffered saline containing 0.1% Tween 20 (TBST) for 2 h. The
membrane was then incubated at 4°C overnight with the primary
antibodies against Wnt4 (1:1,000), β-catenin (1:1,000) and TGF-β1
(1:2,000), and was subsequently washed three times with TBST for 10
min. Horseradish peroxidase-conjugated second antibodies (1:3,000)
were then added. Following 1 h incubation with agitation, the
membranes were washed three times with TBST for 10 min. Following
visualization in the dark for 5 min in a Tanon 4500 chemical
imaging system, the images were analyzed using ImageJ 1.40g
software. Relative expression levels of the target proteins were
quantified as the ratio of the target bands to that of β-actin.
Statistical analysis
Experimental data were analyzed using SPSS
statistical software, version 17.0 (SPSS, Inc., Chicago, IL, USA).
Measurement data are expressed as the mean ± standard deviation.
Measurement data among different groups were compared using
single-factor analysis of variance. Groups were compared using the
least significant difference test and P<0.05 was considered to
indicate a statistically significant difference.
Results
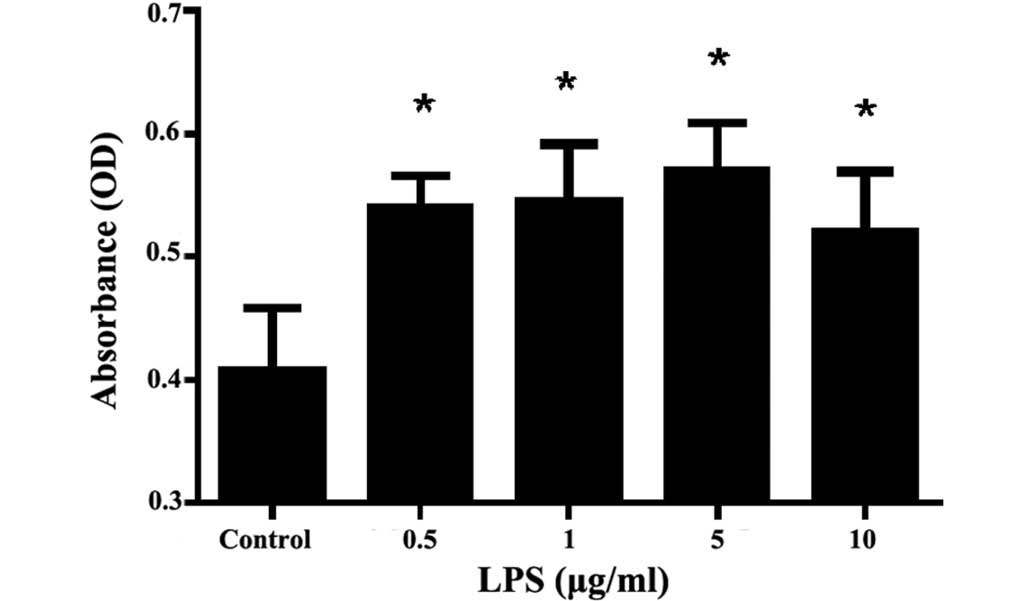
Effect of LPS on rat MC
proliferation
Compared with the control group, different
concentrations of LPS (0.5, 1.0, 5.0 and 10 µg/ml) were able to
stimulate rat MC proliferation to various degrees (P<0.01),
among which the effect of 5.0 µg/ml LPS was the most evident.
Combined with the results of our previous study (22), LPS 5.0 µg/ml was selected as the
stimulation dose for the subsequent experiments (Fig. 1).
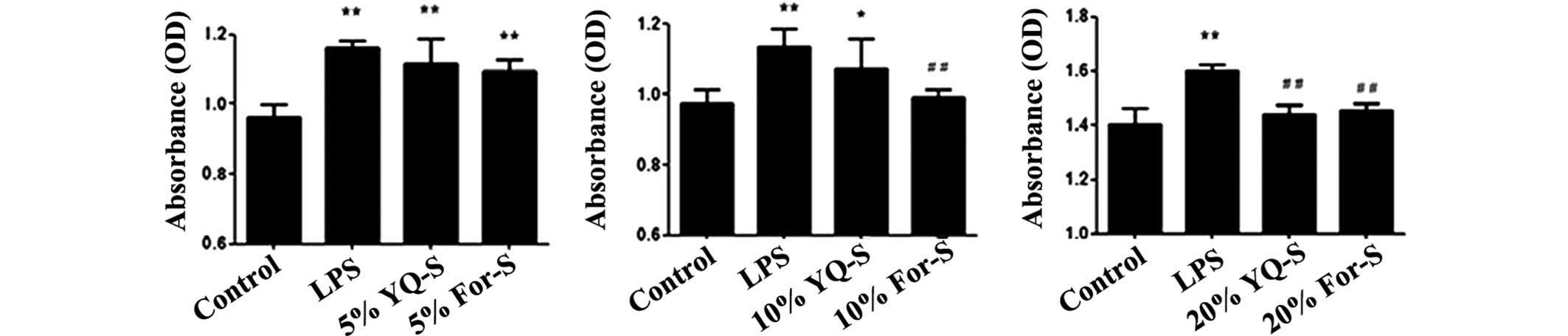
Effect of YQ-S on rat MC
proliferation
The MTT assay results indicated that, compared with
the LPS group, 5% YQ-S and 5% For-S exerted no significant
inhibition on MC proliferation. Following treatment of the MCs with
10% For-S or 10% YQ-S, only For-S exerted a significant inhibitory
effect on LPS-induced MC proliferation (P<0.01). In the MCs that
received a 20% serum containing intervention, both YQ-S and For-S
significantly inhibited MC proliferation (P<0.01). Therefore,
20% serum was selected as the appropriate dose for the subsequent
intervention experiments (Fig.
2).
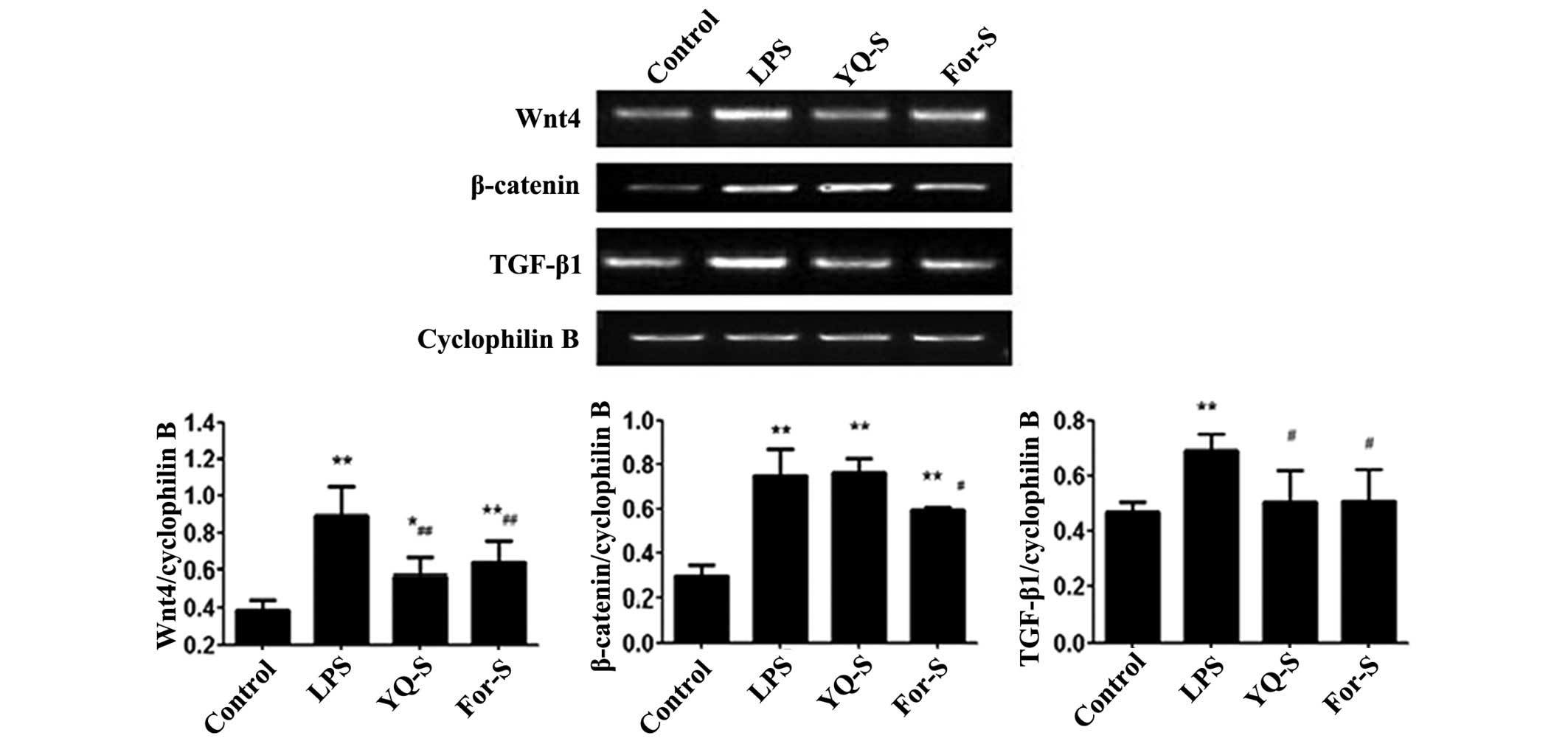
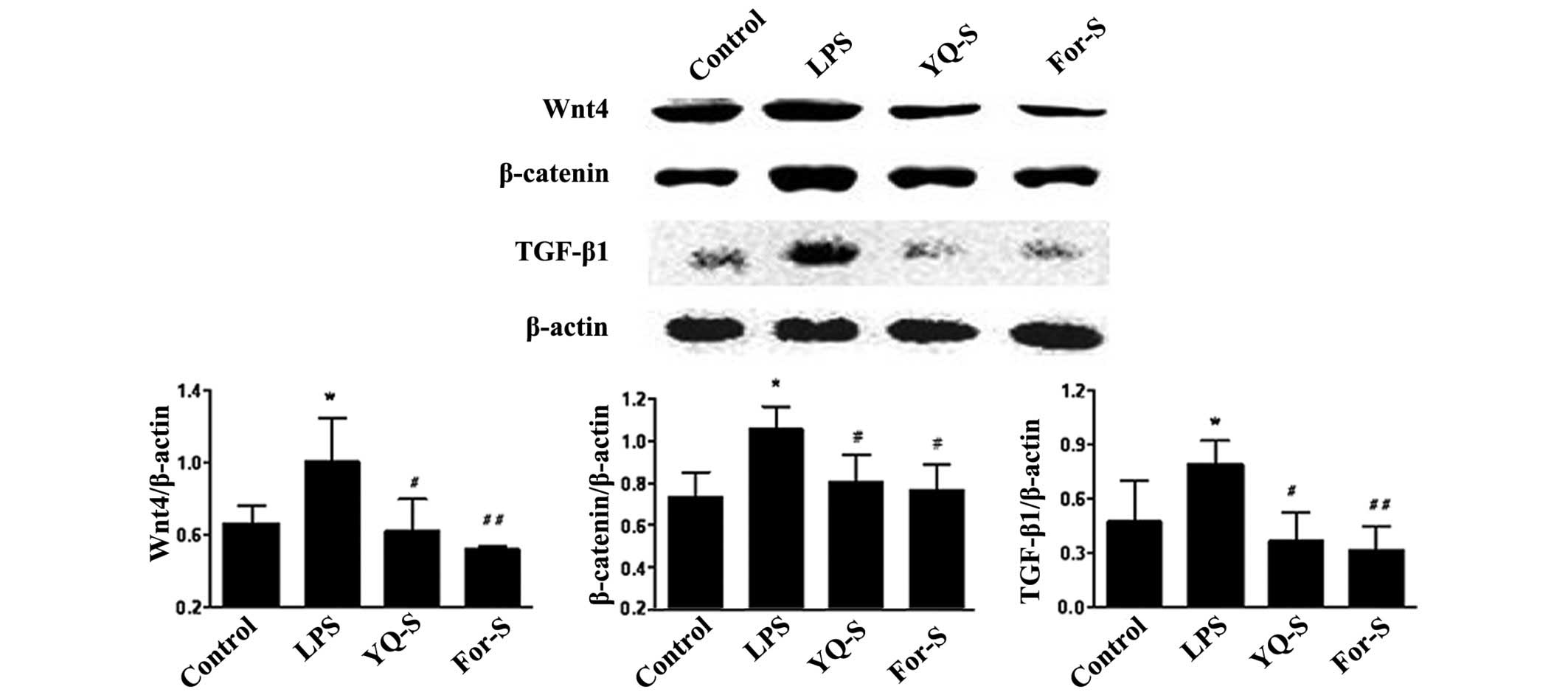
Effect of YQ-S on LPS-stimulated Wnt4,
β-catenin and TGF-β1 expression
After LPS stimulation for 48 h, the MCs were
collected for RT-qPCR and western blot analysis. The results
indicated that, compared with control group, the expression levels
of Wnt4, β-catenin and TGF-β1 were significantly increased
(P<0.01 or P<0.05). Following stimulation of the MCs with the
drug-containing serum for 48 h, RT-qPCR results indicated that,
compared with LPS group, the Wnt4 and TGF-β1 mRNA expression levels
were significantly reduced in the YQ-S and For-S groups (P<0.01
or P<0.05), while β-catenin mRNA expression was significantly
reduced in the For-S group (P<0.05). Western blot analysis
revealed that, compared with the LPS stimulation group, Wnt4,
β-catenin and TGF-β1 protein expression levels were significantly
reduced in the YQ-S and For-S groups (P<0.01 or P<0.05;
Figs. 3 and 4).
Discussion
MCs are located in the central stalk of glomeruli
and have various fundamental roles, including maintaining the
structural backbone of the glomerular tuft, regulating capillary
blood flow and thus glomerular filtration rate, generation of the
mesangial matrix, and manipulation of immune complexes (5). Injuries to MCs, predominantly cell
proliferation, hypertrophy, apoptosis, and ECM deposition, are
pathological hallmarks of a wide spectrum of glomerular diseases,
which lead directly to progressive renal failure (23). The mechanisms associated with MC
injury have recently garnered extensive attention. The Wnt
signaling pathway is an evolutionarily conserved pathway across
species, which is closely associated with numerous kidney diseases,
including ischemic renal injury, acute kidney injury (AKI),
diabetic nephropathy, renal interstitial fibrosis and renal tumors.
In ischemia-reperfusion injury of AKI, β-catenin ameliorates
tubular epithelial cell apoptosis via activation of the Akt
pathway, induction of survival and suppression of p53 (24). Activation of the canonical Wnt
pathway contributes to obstructive renal injury, and the Wnt
antagonist Dickkopf-1 (DKK-1) protects against renal fibrosis by
inhibiting Wnts (10). In podocyte
injury and proteinuric kidney diseases, the Wnt pathways are
essential regulators and potential therapeutic targets (13,14).
Studies regarding the role of the Wnt signaling pathway in MCs have
mainly focused on hyperglycemic conditions (15,16,25,26). Lin
et al (15) observed that in
diabetic nephropathy, the Wnt/β-catenin signaling pathway serves a
crucial role in the regulation of MC proliferation. In
vitro, high glucose-induced apoptosis of MCs is accompanied by
reduced expression of Wnt4 and Wnt5a. In addition, transfection of
MCs with Wnt4, Wnt5a or persistent activation of β-catenin, was
able to inhibit high glucose-induced mesangial apoptosis.
Furthermore, treatment with simvastatin restored Wnt4, Wnt5a and
β-catenin expression in MCs and attenuated diabetic kidney injuries
both in vivo and in vitro (16). In addition, it has been demonstrated
that abrogation of oxidative stress may restore Wnt5a/β-catenin
signaling and attenuate high glucose-induced MC apoptosis (25). DKK-1 expression was also increased in
these same conditions, and mediated c-Jun, TGF-β1, and fibronectin
expression, whereas stable β-catenin expression alleviated
DKK-1-induced profibrotic factors, indicating that β-catenin may be
a potential therapeutic target of MC dysfunction of diabetic
nephropathy (26). In
immune-mediated MC injury, the Wnt pathway has been demonstrated to
be involved in the anti-Thy-1.1 rat model of glomerulonephritis
(27), and development of lupus
nephritis (28). However, there is
currently scarce in vitro evidence regarding Wnts and
β-catenin expression in LPS-stimulated MCs. As demonstrated in the
present study, following stimulation of MCs with LPS for 48 h, the
expression levels of Wnt4 and β-catenin were significantly
elevated, indicating that both the canonical and non-canonical Wnt
pathways were activated, and treatment with YQ-S was able to
attenuate MC proliferation by inhibiting the Wnt signaling
pathways. Further studies are required to explore the mechanisms
underlying this phenomenon. Previous studies have indicated that
TGF-β1 is a key mediator of glomerular sclerosis and MC injury
(4,29,30).
Crosstalk between TGF-β1 and the canonical Wnt signaling pathway
has recently garnered much attention. In cultured MCs, high glucose
levels increased TGF-β1 and fibronectin expression, and reduced
Wnt4, Wnt5a and β-catenin expression. Restoring Wnt4, Wnt5a and
cytosolic β-catenin significantly abrogated TGF-β1 expression, and
exogenous inhibitors of the Wnt pathway also significantly
alleviated TGF-β1-induced renal fibrosis, thus indicating that the
Wnt pathway, particularly the β-catenin-associated pathway, is an
important regulator of TGF-β1 expression in MCs (18). Furthermore, it has been demonstrated
that, in experimental fibrosis, TGF-β1 may decrease DKK-1 levels in
a p38-dependent manner, thus stimulating the canonical Wnt pathway
(17). Wnt11 was identified by a
gene screen study as the direct target of the stimulated
TGF-β1/Smad3 pathway in renal epithelial cells, and further
transferred its signals by the c-Jun N-terminal kinase (JNK)
pathway (31). Zu et al
previously demonstrated that LPS induced MC proliferation and
upregulated TGF-β1, c-jun, and c-fos (22). The present study demonstrated that
TGF-β1 expression was elevated after 48 h incubation with LPS, as
was Wnt4 and β-catenin, thus indicating that the
Wnt/β-catenin/TGF-β1 pathways are fundamental for LPS-induced MC
proliferation; however, whether Wnt/β-catenin or TGF-β1 is upstream
of this process requires further study.
The effectiveness of YQ for treating chronic
nephritis has been indicated by previous studies in animal models
and patients (19–21). The pharmacological mechanisms
underlying the effects of this mixture have yet to be elucidated.
There are 12 components of YQ, and many of these have previously
been shown to exert regulatory effects on the Wnt pathway. Radix
Astragali Mongolici (Huang Qi) is the main component of YQ, and
Astragaloside IV (AS-IV) is the major active ingredient. In the
unilateral ureteral occlusion (UUO) model of renal interstitial
fibrosis, AS-IV was able to attenuate renal fibrosis and restore
renal function by inhibiting TGF-β1, connective tissue growth
factor and α-smooth muscle actin (SMA) expression, Smad2/3
phosphorylation, and collagen matrix expression, and upregulating
Smad7. Similar results have been detected in TGF-β1-stimulated rat
renal NRK-49F fibroblasts. Knockdown of Smad7 significantly
abrogated these effects, indicating that the renal protective
effects of AS-IV occur predominantly via Smad7 and therefore the
TGF-β1/Smads pathway (32). Previous
studies have demonstrated that there are synergistic effects of
AS-IV and ferulic acid on TGF-β1, α-SMA, and p-JNK expression in
the same rat and cell models of renal fibrosis (33). Astragaloside has also been shown to
exert inhibitory effects on TGF-β1, Wnt4, Wnt5a, Frizzled-2, −3, −6
and β-catenin expression in a cholestatic liver fibrosis rat model
(34). Flos Lonicerae (Jin
Yin Hua) together with Fructus Forsythiae Suspensae (Lian
Qiao) have anti-inflammatory effects in rat models of chronic
obstructive pulmonary disease, via inhibition of tumor necrosis
factor-α, TGF-β1 and interleukin-1β expression, and there were
synergistic effects of those two compounds with Radix
Platycodon (35). In the UUO rat
model, leonurine, the active component of Herba Leonuri (Yi
Mu Cao) ameliorated renal tubulointerstitial fibrosis and
inflammation via the TGF-β/Smad3 and nuclear factor-κB pathway,
indicating the potential renoprotective effect of leonurine
(36). The regulatory effects of YQ
in LPS-stimulated MCs via inhibition of the Wnt signaling pathway
and TGF-β1 expression may be due to the aforementioned mechanisms
of the active components in YQ.
In conclusion, the results of the present study
demonstrated that LPS is able to promote the proliferation of MCs
and induce the increased expression of Wnt4, β-catenin and TGF-β1.
In addition, a positive correlation was detected between Wnt and
TGF-β1. Furthermore, YQ-S and For-S treatments were shown to reduce
the mRNA and protein expression levels of Wnt4, β-catenin and
TGF-β1. Future studies are required in order to investigate the
mechanisms underlying the effects of YQ-S and its component herbs
on the Wnt and TGF-β1 signaling pathways.
Acknowledgements
The present study was supported by the National
Nature Science Foundation of China (grant nos. 81102588 and
81473614).
References
|
1
|
Martín-Cleary C and Ortiz A: CKD hotspots
around the world: Where, why and what the lessons are. A CKJ review
series. Clin Kidney J. 7:519–523. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Liyanage T, Ninomiya T, Jha V, Neal B,
Patrice HM, Okpechi I, Zhao MH, Lv J, Garg AX, Knight J, et al:
Worldwide access to treatment for end-stage kidney disease: A
systematic review. Lancet. 385:1975–1982. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Duffield JS: Cellular and molecular
mechanisms in kidney fibrosis. J Clin Invest. 124:2299–22306. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Aihara K, Ikeda Y, Yagi S, Akaike M and
Matsumoto T: Transforming growth factor-β1 as a common target
molecule for development of cardiovascular diseases, renal
insufficiency and metabolic syndrome. Cardiol Res Pract.
2011:1753812010.PubMed/NCBI
|
|
5
|
Herrera GA, Turbat-Herrera EA and Teng J:
Mesangial homeostasis and pathobiology: Their role in health and
disease. Contrib Nephrol. 169:6–22. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kawakami T, Ren S and Duffield JS: Wnt
signalling in kidney diseases: Dual roles in renal injury and
repair. J Pathol. 229:221–231. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Barker N: The canonical Wnt/beta-catenin
signaling pathway. Methods Mol Biol. 468:5–15. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kikuchi A, Yamamoto H, Sato A and
Matsumoto S: New insights into the mechanism of Wnt signaling
pathway activation. Int Rev Cell Mol Biol. 291:21–71. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Macdonald BT, Semenov MV and He X:
SnapShot: Wnt/beta-catenin signaling. Cell. 131:12042007.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
He W, Dai C, Li Y, Zeng G, Monga SP and
Liu Y: Wnt/beta-catenin signaling promotes renal interstitial
fibrosis. J Am Soc Nephrol. 20:765–776. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
He W, Tan RJ, Li Y, Wang D, Nie J, Hou FF
and Liu Y: Matrix metalloproteinase-7 as a surrogate marker
predicts renal Wnt/β-catenin activity in CKD. J Am Soc Nephrol.
23:294–304. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Li Z, Xu J, Xu P, Liu S and Yang Z:
Wnt/β-catenin signalling pathway mediates high glucose induced cell
injury through activation of TRPC6 in podocytes. Cell Prolif.
46:76–85. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Dai C, Stolz DB, Kiss LP, Monga SP,
Holzman LB and Liu Y: Wnt/beta-catenin signaling promotes podocyte
dysfunction and albuminuria. J Am Soc Nephrol. 20:1997–2008. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Heikkilä E, Juhila J, Lassila M, Messing
M, Perälä N, Lehtonen E, Lehtonen S, Verbeek Sjef J and Holthofer
H: beta-Catenin mediates adriamycin-induced albuminuria and
podocyte injury in adult mouse kidneys. Nephrol Dial Transplant.
25:2437–2446. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lin CL, Wang JY, Huang YT, Kuo YH,
Surendran K and Wang FS: Wnt/beta-catenin signaling modulates
survival of high glucose-stressed mesangial cells. J Am Soc
Nephrol. 17:2812–2820. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lin CL, Cheng H, Tung CW, Huang WJ, Chang
PJ, Yang JT and Wang JY: Simvastatin reverses high glucose-induced
apoptosis of mesangial cells via modulation of Wnt signaling
pathway. Am J Nephrol. 28:290–297. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Akhmetshina A, Palumbo K, Dees C, Bergmann
C, Venalis P, Zerr P, Horn A, Kireva T, Beyer C, Zwerina J, et al:
Activation of canonical Wnt signalling is required for
TGF-β-mediated fibrosis. Nat Commun. 3:7352012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ho C, Lee PH, Hsu YC, Wang FS, Huang YT
and Lin CL: Sustained Wnt/β-catenin signaling rescues high glucose
induction of transforming growth factor-β1-mediated renal fibrosis.
Am J Med Sci. 344:374–382. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhan YL and Dai XW: Treatment of 30 cases
with chronic nephritis with Yiqihuoxuejiedu formula. Zhong Yi Yao
Tong Bao. 44:922 9242003.(In Chinese).
|
|
20
|
Zhan YL, Dai XW, Li XY, Li S and Rao XR:
Renal protective effect of Yiqiqingre extract on adriamycin induced
nephropathy. Chin West Med. 4:135 1382003.(In Chinese).
|
|
21
|
Zhan Y, Yang L, Wen Y, Liu H, Zhang H, Zhu
B, Han W, Gu Y, Sun X, Dong X, et al: Yi qi qing re gao attenuates
podocyte injury and inhibits vascular endothelial growth factor
overexpression in puromycin aminonucleoside rat model. Evid Based
Complement Alternat Med. 2014:3759862014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zu N, Li P, Li N, Choy P and Gong Y:
Mechanism of saikosaponin-d in the regulation of rat mesangial cell
proliferation and synthesis of extracellular matrix proteins.
Biochem Cell Biol. 85:169–174. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
23
|
Migliorini A, Ebid R, Scherbaum CR and
Anders HJ: The danger control concept in kidney disease: Mesangial
cells. J Nephrol. 26:437–449. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhou D, Li Y, Lin L, Zhou L, Igarashi P
and Liu Y: Tubule-specific ablation of endogenous β-catenin
aggravates acute kidney injury in mice. Kidney Int. 82:537–547.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lin CL, Wang JY, Ko JY, Surendran K, Huang
YT, Kuo YH and Wang FS: Superoxide destabilization of beta-catenin
augments apoptosis of high-glucose-stressed mesangial cells.
Endocrinology. 149:2934–2942. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lin CL, Wang JY, Ko JY, Huang YT, Kuo YH
and Wang FS: Dickkopf-1 promotes hyperglycemia-induced accumulation
of mesangial matrix and renal dysfunction. J Am Soc Nephrol.
21:124–135. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Villa L, Boor P, Konieczny A, Kunter U,
van Roeyen CR, Denecke B, Gan L, Kupper MB, Hoffmann K, Eitner F,
et al: Effects and mechanisms of angiotensin II receptor blockade
with telmisartan in a normotensive model of mesangioproliferative
nephritis. Nephrol Dial Transplant. 26:3131–3143. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Tveita AA and Rekvig OP: Alterations in
Wnt pathway activity in mouse serum and kidneys during lupus
development. Arthritis Rheum. 63:513–522. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
López-Hernández FJ and López-Novoa JM:
Role of TGF-β in chronic kidney disease: An integration of tubular,
glomerular and vascular effects. Cell Tissue Res. 347:141–154.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lee HS and Song CY: Differential role of
mesangial cells and podocytes in TGF-beta-induced mesangial matrix
synthesis in chronic glomerular disease. Histol Histopathol.
24:901–908. 2009.PubMed/NCBI
|
|
31
|
Zhang P, Cai Y, Soofi A and Dressler GR:
Activation of Wnt11 by transforming growth factor-β drives
mesenchymal gene expression through non-canonical Wnt protein
signaling in renal epithelial cells. J Biol Chem. 287:21290–21302.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wang L, Chi YF, Yuan ZT, Zhou WC, Yin PH,
Zhang XM, Peng W and Cai H: Astragaloside IV inhibits renal
tubulointerstitial fibrosis by blocking TGF-β/Smad signaling
pathway in vivo and in vitro. Exp Biol Med (Maywood).
239:1310–1324. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Meng LQ, Tang JW, Wang Y, Zhao JR, Shang
MY, Zhang M, Liu SY, Qu L, Cai SQ and Li XM: Astragaloside IV
synergizes with ferulic acid to inhibit renal tubulointerstitial
fibrosis in rats with obstructive nephropathy. Br J Pharmacol.
162:1805–1818. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Yongping M, Zhang X, Xuewei L, Fan W, Chen
J, Zhang H, Chen G, Liu C and Liu P: Astragaloside prevents
BDL-induced liver fibrosis through inhibition of notch signaling
activation. J Ethnopharmacol. 169:200–209. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Li YH, Zheng FJ, Huang Y, Zhong XG and Guo
MZ: Synergistic anti-inflammatory effect of Radix Platycodon
in combination with herbs for cleaning-heat and detoxification and
its mechanism. Chin J Integr Med. 19:29–35. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Cheng H, Bo Y, Shen W, Tan J, Jia Z, Xu C
and Li F: Leonurine ameliorates kidney fibrosis via suppressing
TGF-β and NF-κB signaling pathway in UUO mice. Int Imunopharmacol.
25:406–415. 2015. View Article : Google Scholar
|