Introduction
Nasopharyngeal carcinoma (NPC), which has an
incidence of 2.8/100,000 per year in men and 1.9/100,000 per year
in women, is the most commonly diagnosed head and neck cancer in
Southern China (1). NPC has
previously been associated with Epstein-Barr virus infection,
tobacco consumption and various genetic alterations (2,3).
Furthermore, it has been suggested that microRNAs (miRNAs), which
are a class of non-coding RNA molecules, 18–25 nucleotides in
length, may have important roles in the carcinogenesis of NPC
(4). In previous studies, the
expression of several miRNAs, including miR-29c, miR-372, miR-214
and the let-7 family, has been detected in NPC samples and their
roles elucidated (5–8). Therefore, regulation of miRNAs may be
considered a potential novel strategy for the diagnosis and
treatment of patients with NPC.
miR-133b has been identified as a tumor suppressor
in numerous types of human cancer (9); miR-133b is often downregulated in
gastric cancer and its overexpression has been shown to reduce the
metastatic potential of gastric cancer cells (10,11). In
addition, miR-133b has been shown to be downregulated in colorectal
cancer tissues, as compared with adjacent tissues (12), and has been found to inhibit the
proliferation of colon cells by suppressing the TATA box-binding
protein-like protein 1 (TBPL1) (12). Furthermore, miR-133b has been shown
to inhibit the proliferation, migration and invasion of
osteosarcoma cells, and promote their apoptosis (13). In a previous study, serum levels of
miR-133b were able to predict the prognosis of patients with
osteosarcoma (14). However, to the
best of our knowledge, the expression status and clinical
significance of miR-133b in NPC have yet to be investigated. The
present study aimed to investigate the expression levels of
miR-133b in NPC tissue samples, as compared with adjacent normal
tissues. Furthermore, its roles and underlying mechanisms,
including its effects on cell proliferation, were investigated.
Materials and methods
Tissue samples
A total of 23 paired NPC and adjacent normal
specimens were collected from 23 male patients undergoing routine
therapeutic surgery at the Huaihe Hospital (Kaifeng, China) between
October 2012 and October 2014. The age range of the patients was 15
to 28-years-old. Written informed consent was obtained from all
patients. All tissue samples were flash-frozen in liquid nitrogen
immediately following collection and stored at −80°C until
experimentation. The present study was approved by the
institutional review board of Huaihe Hospital.
Cell culture
The NPC cell lines, CNE2 and HONE1, were obtained
from the Chinese Academy of Sciences Cell Bank (Shanghai, China).
The cells were cultured in RPMI-1640 medium (Sigma-Aldrich, St.
Louis, MO, USA), supplemented with 10% fetal bovine serum (Merck
Millipore, Wanchai, Hong Kong, China). The cultures were maintained
at 37°C in a humidified atmosphere containing 5%
CO2.
Transient transfection
The CNE2 and HONE1 cells were transfected with
miR-133b mimics, miR-133b antisense or negative control (NC; all
Genepharm, Inc., Sunnyvale, CA, USA) for 24 h at room temperature
using Lipofactamine 2000 (Invitrogen; Thermo Fisher Scientific,
Inc., Inc., Waltham, MA, USA), according to the manufacturer's
protocol.
RNA isolation and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA from the patient tissues and CNE2 and
HONE1 cell lines was extracted using TRIzol® reagent (Invitrogen;
Thermo Fisher Scientific), according to the manufacturer's
protocol. The RNA was purified by treatment with PureLink® DNase
Set (Thermo Fisher Scientific) and 20 µl RNA was reverse
transcribed into cDNA using the Reverse Transcription System
(Promega Corporation, Madison, WI, USA). The expression levels of
hsa-miR-133b were determined using the TaqMan® MicroRNA assay
(Applied Biosystems; Thermo Fisher Scientific, Inc.) and the
LightCycler® 480 instrument (Roche Diagnostics, Basel,
Switzerland). The primer sequences were as follows: miR-133b
forward, 5′-AAAGGACCCCAACAACCAGCAA-3′ and reverse,
5′-TTGCTGGTTGTTGGGGTCCTTT-3′; and U6 small nuclear (sn)RNA forward,
5′-CTCGCTTCGGCAGCACATATACT-3′ and reverse,
5′-ACGCTTCACGAATTTGCGTGTC-3′ (Biosune Biotechnology Co., Shanghai,
China). U6 snRNA was used as an internal control for normalization
and quantification of the miR-133b expression levels using the
2−ΔΔCq method (15).
In order to determine the mRNA expression levels of
B-cell lymphoma-2 (Bcl-2), myeloid cell leukemia-1 (Mcl-1) and
cellular inhibitor of apoptosis-2 (c-IAP2), qPCR was performed
using the SYBR Green Master Mix (Thermo Fisher Scientific) and the
LightCycler® 480 instrument, according to the manufacturer's
protocols. The PCR cycling conditions included an initial holding
period at 95°C for 5 min, followed by 45 cycles of 94°C for 5 sec
and 60°C for 35 sec. The primer sequences were as follows: Bcl-2
forward, 5′-GGTGGGGTCATGTGTGTGG-3′ and reverse,
5′-CGGTTCAGGTACTCAGTCATCC-3′; Mcl-1 forward,
5′-TGCTTCGGAAACTGGACATCA-3′ and reverse,
5′-TAGCCACAAAGGCACCAAAAG-3′; c-IAP2 forward,
5′-AAGCTACCTCTCAGCCTACTTT-3′ and reverse,
5′-CCACTGTTTTCTGTACCCGGA-3′; and β-actin forward,
5′-CATGTACGTTGCTATCCAGGC-3′ and reverse,
5′-CTCCTTAATGTCACGCACGAT-3′ (Biosune Biotechnology Co.). The
β-actin gene was used as an internal control for normalization and
quantification of the relative mRNA expression levels using the
2−ΔΔCq method (15). The
RT-qPCR data were analyzed using the LightCycler® 480 software,
version 1.5.0 (Roche Diagnostics).
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
assay
Cell growth was determined using an MTT assay
(Sigma-Aldrich). At 24 h following transfection of the NPC cell
lines with miR-133b mimics, miR-133b antisense or NC, the cells
were seeded into 96-well plates (2×103 cells/well), and
cell proliferation was recorded every 24 h for 4 days. The number
of viable cells was assessed by measuring the absorbance at 450 nm
using a microplate reader (Model 3550 Microplate Reader; Bio-Rad
Laboratories, Inc., Hercules, CA, USA).
Bromodeoxyuridine (BrdU) assay
A cell proliferation enzyme-linked immunosorbent
assay (BrdU kit; Beyotime Institute of Biotechnology, Haimen,
China) was used to analyze the incorporation of BrdU into the NPC
cell lines during DNA synthesis, according to the manufacturer's
protocol. All experiments were performed in triplicate. Absorbance
was measured at 450 nm using the SpectraMax 190 Microplate Reader
(Molecular Devices, LLC, Sunnyvale, CA, USA).
miR-133b target predictions
The putative targets of miR-133b were predicted
using miRWalk software, release 2.0 (http://www.umm.uni-heidelberg.de/apps/zmf/mirwalk/).
The algorithm produced a list of target genes for miR-133b by
searching for the presence of conserved 7-mer and 8-mer sites
matching the seed region of miR-133b.
Western blotting
The NPC cells were harvested and lysed using
ice-cold lysis buffer [50 mM Tris-HCl, pH 6.8, 5%
2-mercaptoethanol, 2% w/v sodium dodecyl sulfate (SDS), 10%
glycerol; Biosune Biotechnology Co.]. Following centrifugation at
20,000 × g for 10 min at 4°C, the proteins in the supernatants were
quantified using a Bicinchoninic Acid Assay kit (Pierce
Biotechnology Inc., Rockford, IL, USA). The proteins were separated
by 10% SDS-polyacrylamide gel electrophoresis, and transferred to a
nitrocellulose membrane (GE Healthcare Life Sciences, Chalfont,
UK). After blocking the membrane with 10% non-fat milk in
phosphate-buffered saline, the membranes were incubated overnight
at 4°C with the following primary antibodies: Rabbit anti-human
sphingosine-1-phosphate receptor 1 (S1PR1) polyclonal antibody
(1:1,000; sc-25489; Santa Cruz Biotechnology, Inc., Dallas, TX,
USA), goat anti-human phosphorylated signal transducer and
activator of transcription 3 (p-STAT3) polyclonal antibody (1:500;
sc-21876; Santa Cruz Biotechnology), rabbit anti-human total STAT3
polyclonal antibody (1:2,000; sc-482; Santa Cruz Biotechnology) and
mouse anti-human glyceraldehyde-3-phosphate dehydrogenase (GAPDH)
monoclonal antibody (1:2,000; sc-365062; Santa Cruz Biotechnology).
Subsequently, the membrane was washed three times for 5 min each
with Tris-buffered saline containing Tween-20 (Beyotime Institute
of Biotechnology), prior to incubation with horseradish
peroxidase-conjugated mouse anti-rabbit IgG (1:2,000; sc-2491;
Santa Cruz Biotechnology), Anti-Mouse IgG1 VHH Single Domain
Antibody (1:1,000; ab193651; Abcam, Cambridge, UK) and mouse
anti-goat IgG (1:2,000; sc-2489; Santa Cruz Biotechnology) for 2 h
at 25°C. The antibody complexes were detected using the SuperSignal
West Pico Chemiluminescent Substrate kit (Pierce Biotechnology),
according to manufacturer's protocol. The protein expression levels
of S1PR1 and STAT3 were normalized to those of GAPDH, using
Quantity One software, version 4.62 (Bio-Rad Laboratories,
Inc.).
Luciferase reporter assays
Total cDNA from CNE2 cells was used to amplify the
3′-untranslated region (UTR) of S1PR1 by PCR. The primers used were
as follows: Forward, 5′-CTCTGTATGACTCTAATA-3′ and reverse,
5′-GACCGTCGATCGACCT-3′. The S1PR1 3′-UTR was cloned into the Ambion
pMIR-REPORT® miRNA Expression Reporter Vector (Thermo Fisher
Scientific, Inc.), to yield pMIR-REPORT-S1PR1. Mutations were
introduced in potential miR-133b binding sites using the QuikChange
II Site-Directed Mutagenesis kit (Agilent Technologies, Inc., Santa
Clara, CA, USA). Subsequently, the CNE2 cells were transfected with
the pMIR-REPORT vectors containing either the wild-type or mutant
S1PR1 3′-UTR, and with either the miR-133b mimic or NC plasmids,
using Lipofectamine 2000, according to the manufacturer's protocol.
The pRL-TK vector (Promega Corporation), encoding the
Renilla luciferase gene, was used as an internal control to
normalize the transfection efficiency. Luciferase values were
determined using the Dual-Luciferase® Reporter Assay system
(Promega Corporation).
Statistical analysis
Data are presented as the mean ± standard error of
the mean of ≥3 separate experiments. Statistical analyses were
conducted using SAS software, version 9.2 (SAS Institute Inc.,
Cary, NC, USA). Differences between groups were analyzed using the
Student's t-test. P<0.05 was considered to indicate a
statistically significant difference.
Results
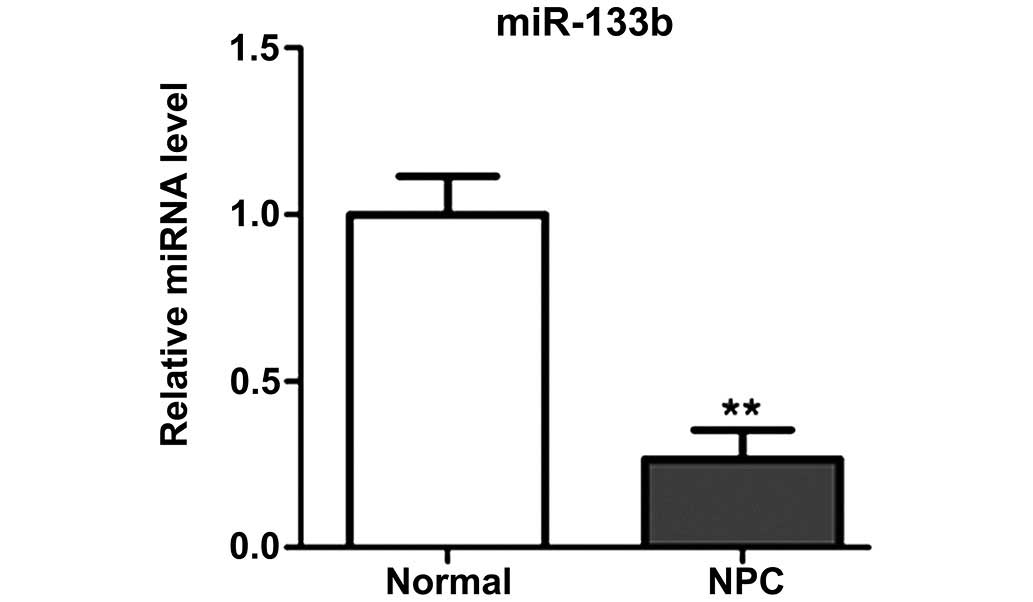
miR-133b expression levels are reduced
in NPC tissues
In order to determine the expression levels of
miR-133b, RT-qPCR was performed using 23 paired NPC tissues and
adjacent normal tissues. The results demonstrated that miR-133b was
significantly downregulated in NPC tissues, as compared with
adjacent normal tissues (P<0.01; Fig.
1).
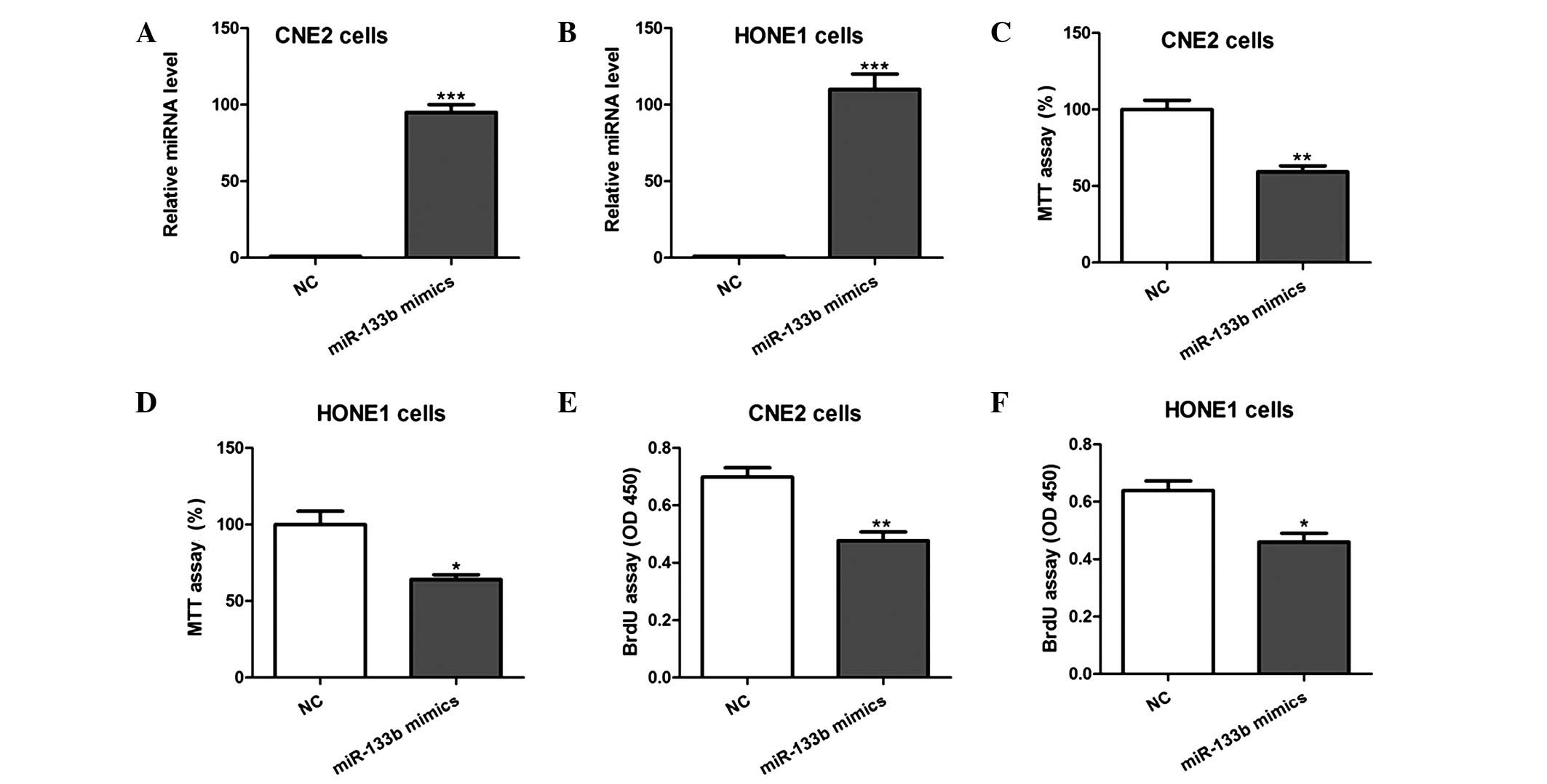
miR-133b overexpression inhibits NPC
cell proliferation
In order to evaluate the biological function of
miR-133b in NPC cell proliferation, miR-133b mimics or NC were
transfected into CNE2 and HONE1 cells, and MTT and BrdU
incorporation assays were conducted. Overexpression of miR-133b in
the two cell lines was confirmed using RT-qPCR (Fig. 2A and B). MTT assays demonstrated that
cell growth was significantly inhibited by miR-133b overexpression
(Fig. 2C and D). Consistent with
this, BrdU assays showed that cell proliferation was markedly
reduced in cells overexpressing miR-133b, as compared with those
transfected with the NC (Fig. 2E and
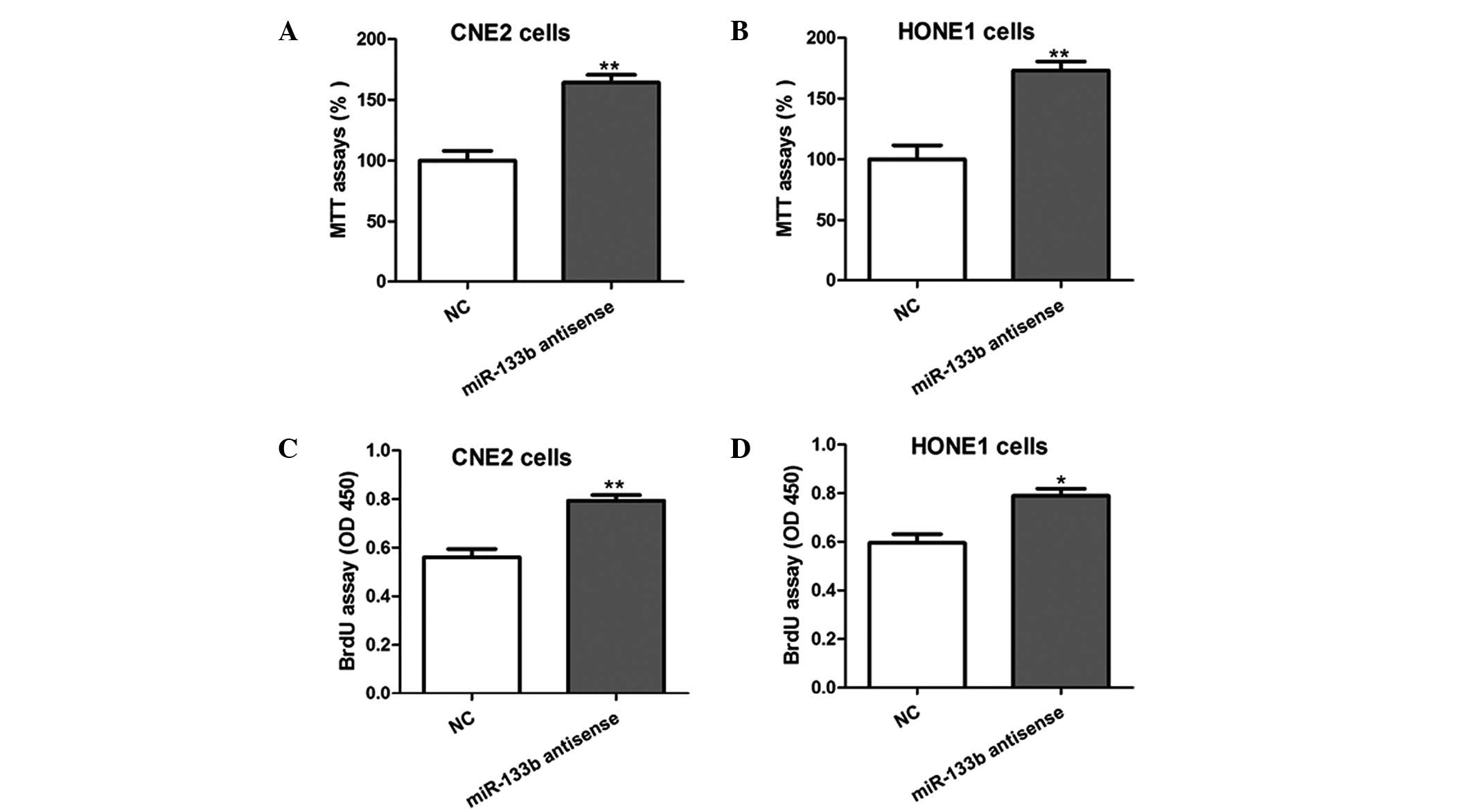
F). Furthermore, the inhibition of endogenous miR-133b by
transfection of the CNE2 and HONE1 cells with antisense
oligonucleotides was shown to promote the growth and proliferation
of the cells (Fig. 3). These results
indicate that miR-133b is able to inhibit the proliferation of NPC
cells in vitro.
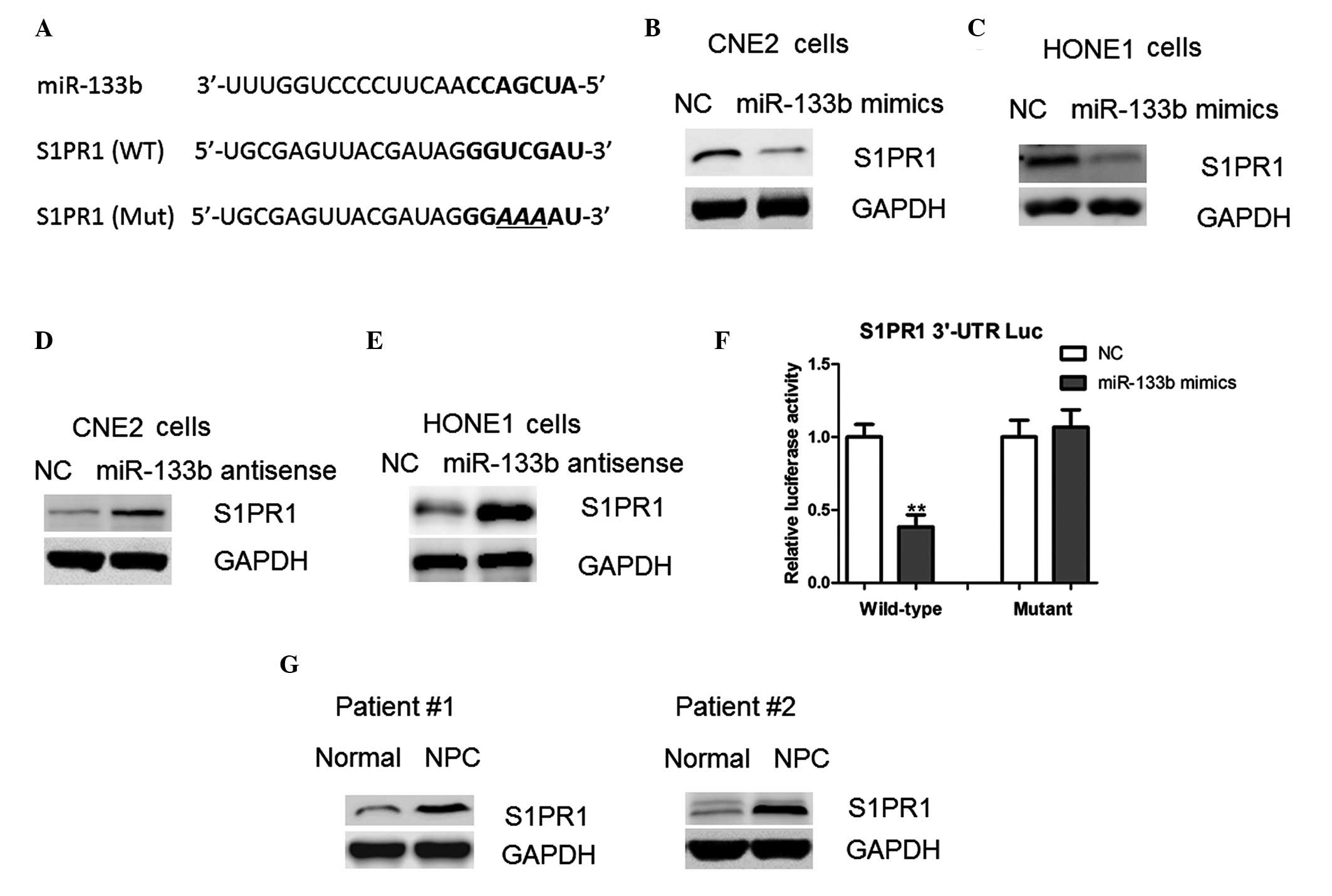
miR-133b inhibits the proliferation of
NPC cells via downregulation of S1PR1
Potential direct targets of miR-133b were predicted
using miWalk software, which revealed that S1PR1 harbors a
potential miR-133b binding site (Fig.
4A). Therefore, RT-qPCR and western blotting assays were
conducted in order to investigate the effect of miR-133b on the
protein expression levels of S1PR1. Notably, the protein expression
levels of S1PR1 were reduced in the cells overexpressing miR-133b
(Fig. 4B and C). In addition, the
protein expression levels of S1PR1 were markedly increased
following inhibition of miR-133b with the antisense
oligonucleotides (Fig. 4D and E).
These results suggest that miR-133b may regulate S1PR1 expression
at the translational level.
In order to confirm this, the full-length 3′-UTR of
the human S1PR1 gene was cloned into the pMIR-REPORT vector
downstream of the luciferase gene. Overexpression of the miR-133b
mimics led to a significant reduction in luciferase activity when
the reporter construct contained the S1PR1 3′-UTR (Fig. 4F). However, mutation of the miR-133b
binding site within the S1PR1 3′-UTR abolished this effect of
miR-133b (Fig. 4F). Consistent with
this, it was observed that protein expression levels of S1PR1 were
upregulated in the NPC tissues, as compared with the adjacent
normal tissues (Fig. 4G); thus
suggesting that S1PR1 is a direct target of miR-133b in NPC
cells.
miR-133b regulates STAT3 signaling in
NPC cells
It has previously been shown that S1PR1 is crucial
for persistent STAT3 activation in cancer cells (16). Therefore, the present study examined
STAT3 signaling pathways in the NPC cells following the
overexpression or depletion of miR-133b. The protein expression
levels of phosphorylated STAT3 were markedly reduced by miR-133b
overexpression (Fig. 5A).
Concordantly, the mRNA expression levels of B-cell lymphoma-2,
myeloid cell leukemia-1 and cellular inhibitor of apoptosis-2,
which are downstream targets of STAT3 signaling, were also
inhibited by miR-133b (Fig. 5B).
Conversely increased activation of these STAT3 signaling-associated
proteins was observed in the cells following the depletion of
miR-133b (Fig. 5C and D). These
results suggest that miR-133b may regulate the S1PR1-mediated
activation of STAT3 signaling.
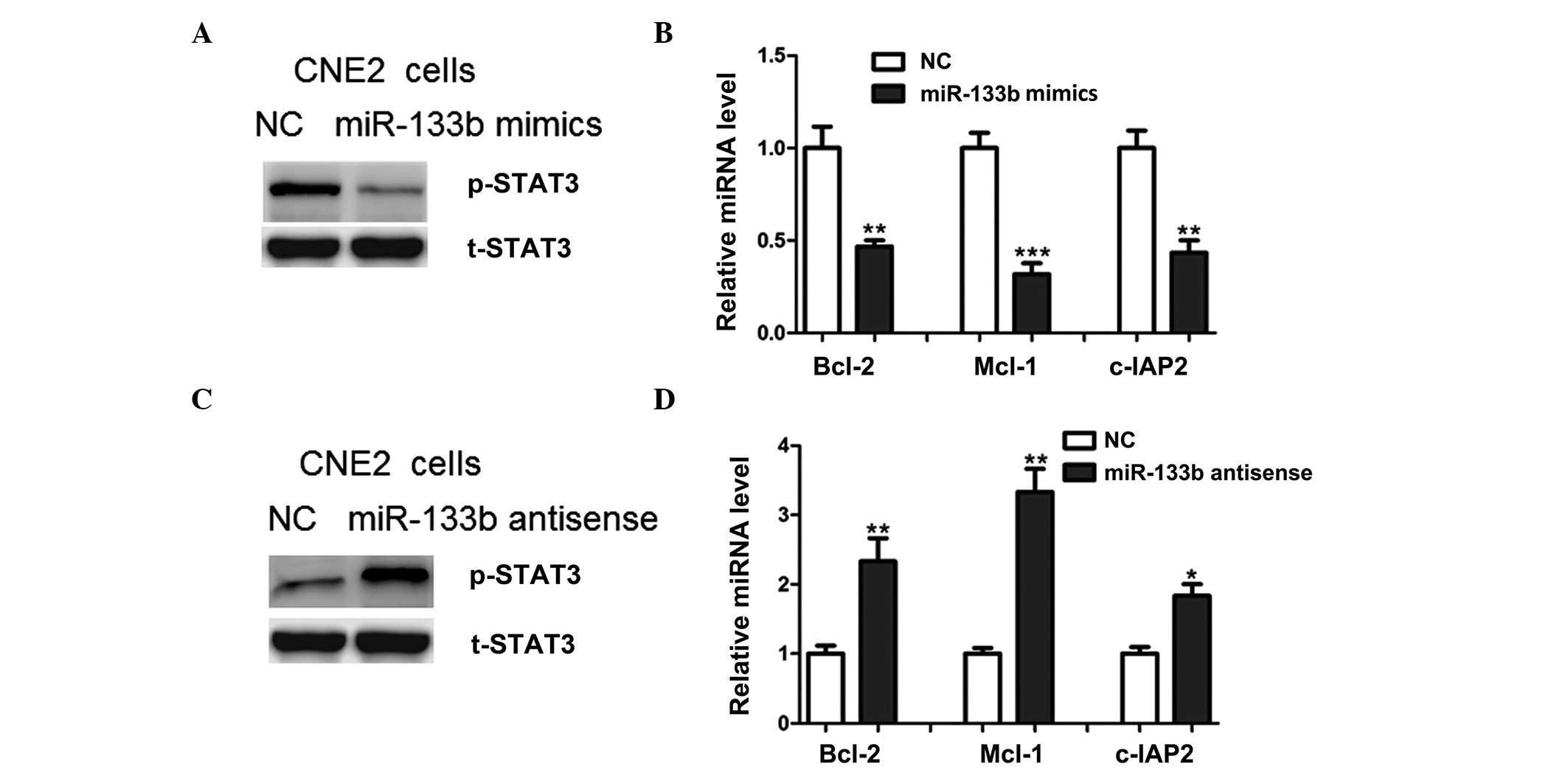 | Figure 5.miR-133b regulated STAT3 signaling in
NPC cells. (A) Relative protein expression levels of p-STAT3 in
CNE2 NPC cells transfected with miR-133b mimics or the NC; t-STAT3
was used as a loading control. (B) mRNA expression levels of Bcl-2,
Mcl-1 and c-IAP2 in CNE2 cells transfected with miR-133b mimics or
the NC. (C) Relative protein expression levels of p-STAT3 in CNE2
cells transfected with miR-133b antisense oligonucleotides or the
NC. (D) mRNA expression levels of Bcl-2, Mcl-1 and c-IAP2 in CNE2
cells transfected with miR-133b antisense oligonucleotides or the
NC. *P<0.05, **P<0.01 and ***P<0.001 vs. the NC. miR,
microRNA; STAT3, signal transducer and activator of transcription
3; NPC, nasopharyngeal carcinoma; p-STAT3, phosphorylated-STAT3;
t-STAT3, total STAT3; Bcl-2, B-cell lymphoma-2; Mcl-1, myeloid cell
leukemia-1; c-IAP2, cellular inhibitor of apoptosis-2; NC, normal
control. |
Discussion
In the present study, miR-133b was shown to be
expressed at significantly lower levels in NPC samples, as compared
with adjacent normal tissue samples. However, the molecular events
underlying the downregulation of miR-133b remain unclear.
Niederwieser et al (17)
reported that high expression levels of DNA
(cytosine-5-)-methyltransferase 3β were associated with
differentially expressed miR-133b in older adults with primary,
cytogenetically normal acute myeloid leukemia. Therefore, the
present study hypothesized that epigenetic factors may contribute
to the downregulation of miR-133b, although this requires further
investigation in future studies.
It is well known that each miRNA may regulate
multiple gene targets. Previous studies have identified numerous
target genes of miR-133b in a variety of tumor types; these genes
include TBPL1, fibroblast growth factor receptor-1, specificity
protein-1 and epidermal growth factor receptor (11,12,18–20);
thus suggesting that the roles of miR-133b in tumorigenesis may be
cell- or tissue-specific. The present study demonstrated that
miR-133b overexpression inhibited, whereas its suppression
increased, endogenous S1PR1 protein expression in NPC cells.
Furthermore, luciferase reporter assays further identified S1PR1 as
a direct target of miR-133b in NPC cells. Therefore, the results of
the present study suggested that miR-133b may inhibit NPC cell
proliferation, at least in part, by repressing S1PR1
expression.
Previous studies have reported upregulation of
S1PR1, a G protein-coupled receptor for lysophospholipid
sphingosine-1-phosphate (S1P), in STAT3-positive tumors (21–23).
S1PR1-mediated STAT3 activation has been shown to occur in part by
upregulation of the Janus Kinase 2 tyrosine kinase activity,
whereas ablation of S1PR1 in tumor cells or immune cells inhibited
tumor STAT3 activity (23). Given
that STAT3 has a crucial role in promoting the progression of human
cancers (23,24), molecules or chemical compounds that
target S1P/S1PR1 may be effective novel therapeutic agents for the
prevention of malignant progression. Notably, in the present study,
miR-133b was demonstrated to regulate STAT3 activation in NPC
cells, further confirming a tumor suppressive role for
miR-133b.
In conclusion, the present study demonstrated that
miR-133b expression was downregulated in NPC tissues. Furthermore,
miR-133b overexpression was able to inhibit the cell proliferation
of NPC by reducing the protein expression levels of S1PR1. The
results of the present study highlight an important role for
miR-133b in NPC progression, which may aid the development of novel
therapeutics for the treatment of patients with NPC.
References
|
1
|
Cao SM, Simons MJ and Qian CN: The
prevalence and prevention of nasopharyngeal carcinoma in China.
Chin J Cancer. 30:114–119. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Coghill AE and Hildesheim A: Epstein-Barr
virus antibodies and the risk of associated malignancies: Review of
the literature. Am J Epidemiol. 180:687–695. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Stoker SD, van Diessen JN, de Boer JP,
Karakullukcu B, Leemans CR and Tan IB: Current treatment options
for local residual nasopharyngeal carcinoma. Curr Treat Options
Oncol. 14:475–491. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
He ML, Luo MX, Lin MC and Kung HF:
MicroRNAs: Potential diagnostic markers and therapeutic targets for
EBV-associated nasopharyngeal carcinoma. Biochim Biophys Acta.
1825:1–10. 2012.PubMed/NCBI
|
|
5
|
Liu N, Tang LL, Sun Y, Cui RX, Wang HY,
Huang BJ, He QM, Jiang W and Ma J: MiR-29c suppresses invasion and
metastasis by targeting TIAM1 in nasopharyngeal carcinoma. Cancer
Lett. 329:181–188. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tan JK, Tan EL and Gan SY: Elucidating the
roles of miR-372 in cell proliferation and apoptosis of
nasopharyngeal carcinoma TW01 cells. Exp Oncol. 36:170–173.
2014.PubMed/NCBI
|
|
7
|
Zhang ZC, Li YY, Wang HY, Fu S, Wang XP,
Zeng MS, Zeng YX and Shao JY: Knockdown of miR-214 promotes
apoptosis and inhibits cell proliferation in nasopharyngeal
carcinoma. PLoS One. 9:e861492014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wong TS, Man OY, Tsang CM, Tsao SW, Tsang
RK, Chan JY, Ho WK, Wei WI and To VS: MicroRNA let-7 suppresses
nasopharyngeal carcinoma cells proliferation through downregulating
c-Myc expression. J Cancer Res Clin Oncol. 137:415–422. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Nohata N, Hanazawa T, Enokida H and Seki
N: MicroRNA-1/133a and microRNA-206/133b clusters: Dysregulation
and functional roles in human cancers. Oncotarget. 3:9–21. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhao Y, Huang J, Zhang L, Qu Y, Li J, Yu
B, Yan M, Yu Y, Liu B and Zhu Z: MiR-133b is frequently decreased
in gastric cancer and its overexpression reduces the metastatic
potential of gastric cancer cells. BMC Cancer. 14:342014.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wen D, Li S, Ji F, Cao H, Jiang W, Zhu J
and Fang X: MiR-133b acts as a tumor suppressor and negatively
regulates FGFR1 in gastric cancer. Tumour Biol. 34:793–803. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Xiang KM and Li XR: MiR-133b acts as a
tumor suppressor and negatively regulates TBPL1 in colorectal
cancer cells. Asian Pac J Cancer Prev. 15:3767–3772. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhao H, Li M, Li L, Yang X, Lan G and
Zhang Y: MiR-133b is down-regulated in human osteosarcoma and
inhibits osteosarcoma cells proliferation, migration and invasion
and promotes apoptosis. PLoS One. 8:e835712013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhang C, Yao C, Li H, Wang G and He X:
Serum levels of microRNA-133b and microRNA-206 expression predict
prognosis in patients with osteosarcoma. Int J Clin Exp Pathol.
7:4194–4203. 2014.PubMed/NCBI
|
|
15
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2-ΔΔCt method. Methods. 25:402–408. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Priceman SJ, Shen S, Wang L, Deng J, Yue
C, Kujawski M and Yu H: S1PR1 is crucial for accumulation of
regulatory T cells in tumors via STAT3. Cell Rep. 6:992–999. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Niederwieser C, Kohlschmidt J, Volinia S,
Whitman SP, Metzeler KH, Eisfeld AK, Maharry K, Yan P, Frankhouser
D, Becker H, et al: Prognostic and biologic significance of DNMT3B
expression in older patients with cytogenetically normal primary
acute myeloid leukemia. Leukemia. 29:567–575. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Qiu T, Zhou X, Wang J, Du Y, Xu J, Huang
Z, Zhu W, Shu Y and Liu P: MiR-145, miR-133a and miR-133b inhibit
proliferation, migration, invasion and cell cycle progression via
targeting transcription factor Sp1 in gastric cancer. FEBS Lett.
588:1168–1177. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Liu L, Shao X, Gao W, Zhang Z, Liu P, Wang
R, Huang P, Yin Y and Shu Y: MicroRNA-133b inhibits the growth of
non-small-cell lung cancer by targeting the epidermal growth factor
receptor. FEBS J. 279:3800–3812. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tao J, Wu D, Xu B, Qian W, Li P, Lu Q, Yin
C and Zhang W: MicroRNA-133 inhibits cell proliferation, migration
and invasion in prostate cancer cells by targeting the epidermal
growth factor receptor. Oncol Rep. 27:1967–1975. 2012.PubMed/NCBI
|
|
21
|
Liang J, Nagahashi M, Kim EY, Harikumar
KB, Yamada A, Huang WC, Hait NC, Allegood JC, Price MM, Avni D, et
al: Sphingosine-1-phosphate links persistent STAT3 activation,
chronic intestinal inflammation, and development of
colitis-associated cancer. Cancer Cell. 23:107–120. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Deng J, Liu Y, Lee H, Herrmann A, Zhang W,
Zhang C, Shen S, Priceman SJ, Kujawski M, Pal SK, et al:
S1PR1-STAT3 signaling is crucial for myeloid cell colonization at
future metastatic sites. Cancer Cell. 21:642–654. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lee H, Deng J, Kujawski M, Yang C, Liu Y,
Herrmann A, Kortylewski M, Horne D, Somlo G, Forman S, et al:
STAT3-induced S1PR1 expression is crucial for persistent STAT3
activation in tumors. Nat Med. 16:1421–1428. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yu H, Lee H, Herrmann A, Buettner R and
Jove R: Revisiting STAT3 signalling in cancer: New and unexpected
biological functions. Nat Rev Cancer. 14:736–746. 2014. View Article : Google Scholar : PubMed/NCBI
|