Introduction
Circulating microparticles (MPs) released by various
cells upon activation or apoptosis have been reported to be
associated with cardiovascular events, which are characterized by
endothelial dysfunction, abnormal hemostasis/thrombosis and/or a
pro-inflammatory state (1). It has
previously been reported that increased circulating MP levels
indicated a poor prognosis in cardiovascular patients (2). Thus, MPs are emerging as novel
biomarkers of acute myocardial infarction (AMI); however, further
detailed evidence is required (2).
Inflammation in the coronary artery and inflammatory
cytokines, such as C-reactive protein (CRP), have an important role
in the pathogenesis of AMI (3). CRP
has previously been identified as an important prognostic marker of
unstable angina and myocardial infarction (4,5). MPs are
important cytokine transporters (6),
and their association with CRP has been investigated in several
studies (7,8). However, the association between CRP and
leukocyte-derived MPs (LMPs) has yet to be elucidated.
ST-segment elevation myocardial infarction (STEMI)
results from ischemic injury due to rupture of unstable
atherosclerotic plaque in a coronary artery (9). It has previously been observed that the
number of total circulating MPs in STEMI patients was significantly
higher than in patients with stable angina and controls (10). Upon reperfusion following
percutaneous transluminal coronary intervention (PCI), large
amounts of plaque-derived MPs were released from plaque in the
crime vessel, and thus may influence the composition of circulating
MPs. However, the time course of changes in the composition of MPs
originating from different cells has yet to be determined.
In the present study, the concentrations of MPs of
differing cell origin were measured in the circulation of STEMI
patients undergoing PCI. Subsequently, the present study assessed
whether there was a correlation between LMP levels and traditional
serum markers for acute myocardial infarction, including cardiac
troponin T (TnT) and high-sensitivity (hs)-CRP. Furthermore, the
correlation between PMPs and coronary angiographic Gensini scores
was examined.
Materials and methods
Study population
In total, 24 patients diagnosed with STEMI in the
Department of Cardiology, Peking University Third Hospital
(Beijing, China) between 1st of January 2012 and 1st of June 2013
were recruited into the present study. STEMI was diagnosed and
treated according to the 2004 American College of
Cardiology/American Heart Association guidelines (11). All patients underwent primary PCI
within 12 h after the onset of symptoms, and the Thrombolysis In
Myocardial Infarction (TIMI) flow grade (12) was ≥2 subsequent to PCI. Exclusion
criteria included patients with an age of >80 years, cardiogenic
shock at admission, TIMI flow grade <2 following PCI, previous
history of myocardial infarction, significant valvular heart
disease, peripheral vascular disease, chronic heart failure,
chronic inflammatory diseases, significant kidney or hepatic
diseases, cancer and administration of glycoprotein IIb/IIIa
inhibitors. The present study was approved by the ethics review
boards of Peking University Health Science Center (Beijing, China).
All patients provided written informed consent for participation in
the study. The Gensini Score, identifying the severity of coronary
lesions, was calculated based on the angiographic results,
according to a previously described method (13). A higher Gensini Score indicates more
severe coronary lesions.
Treatment and procedures
STEMI patients were treated with a loading dose of
aspirin (300 mg; 100 mg daily; Bayer AG, Leverkusen, Germany) and
clopidogrel (600 mg; 75 mg daily; Sanofi, Paris, France) at
admission, and a bolus of 100 IU/kg heparin (Sanofi) prior to PCI.
The PCI procedure was performed according to ACC/AHA/SCAI
guidelines (14), and involved the
implantation of drug-eluted stents. Following PCI, the patients
received standard therapy including aspirin (Bayer), clopidogrel,
statins (Pfizer, Inc., New York, NY, USA), β-blockers
(Astra-Zeneca, London, UK) and angiotensin-converting enzyme
inhibitors (Astra-Zeneca, London, UK)/angiotensin II receptor
blockers (if there were no contraindications; Sanofi). Serum TnT,
creatine kinase-MB and hs-CRP levels were detected in blood samples
immediately prior to PCI, immediately after PCI, and at 4, 24 and
48 h post-PCI, by the Department of Clinical Laboratory of Peking
University Third Hospital. The measurement methods were as previous
described (15).
MP separation
Blood samples were collected at multiple time points
prior to and after PCI: Immediately prior to PCI, immediately after
PCI, and at 4, 24 and 48 h post-PCI. The blood samples (3 ml) were
collected from the right radial artery during PCI for the first two
time-points, while samples from subsequent time-points were
collected by standard vein puncture by trained nurses. Vacuum blood
collection tubes with sodium citrate as a anticoagulation agent (BD
Vacutainer Citrate tubes; Becton Dickinson, Franklin Lakes, NJ,
USA) were used. Platelet-free plasma was immediately separated by
1409 × g centrifugation for 15 min, followed by 13,000 × g
centrifugation for 2 min at room temperature. Platelet-free plasma
was stored at −80℃ for MP detection.
MP detection
For the detection of MPs, a Beckman Coulter Gallios
flow cytometer (Beckman Coulter, Inc. Brea, CA, USA) was used to
ensure the accurate enumeration and characterization of MPs of
different origin. Megamix beads (0.5, 0.9 and 3 µm) were purchased
from Biocytex (Marseille, France) and were used according to the
manufacturer's instructions and as previously described (16). Endothelial MPs (EMPs) were identified
using mouse monoclonal Phycoerythrin-CD144 antibodies (1:50;
358505; Biolegend, London, UK), platelet MPs (PMPs) were identified
using mouse monoclonal fluorescein isothiocyanate-CD41 antibodies
(1:100; ab19708; Abcam, Cambridge, UK), and LMPs were identified
using mouse monoclonal PerCP/CY5.5-CD45 antibodies (1:50; 368503;
Biolegend). EMPs were identified by dual staining using
Phycoerythrin CD144 and Annexin V, PMPs were identified by dual
staining using fluorescein isothiocyanate CD41 and Annexin V, and
LMPs were identified by dual staining using PerCP/CY5.5 CD45 and
Annexin V. All fluorescence stains were purchased from Nanjing
KeyGen Biotech. Co., Ltd. (Nanjing, China) and staining was
performed according to the manufacturer's instructions.
Statistical analysis
Results are presented as the mean ± standard error
of the mean. MP levels at different time points were compared using
Student's t-tests, while binary logistic regression analysis was
performed to identify an interaction between the Gensini score and
MP levels. Two-tailed tests of significance are reported. For all
comparisons, P<0.05 was considered to indicate a statistically
significant difference. Statistical analysis was performed using
SPSS version 19.0 (IBM SPSS, Armonk, NY, USA).
Results
Patient characteristics
A total of 24 patients diagnosed with STEMI were
recruited. MP subpopulations in the blood samples from all 24
patients were obtained. The clinical characteristics and medication
administered to the participants of the present study are displayed
in Table I, and were comparable with
the patient population recruited in previous studies (17,18).
 | Table I.Patients' characteristics and
therapies administered. |
Table I.
Patients' characteristics and
therapies administered.
| Characteristic | Value |
|---|
| Age, years | 75–40 (58±4) |
| Male, % | 62.5 |
| Smoking, % | 37.5 |
| Diabetes, % | 70.8 |
| Hypertension, % | 66.7 |
| Body-Mass Index,
kg/m2 | 18.4–29.4
(23.7±1.5) |
| Total cholesterol,
mmol/l | 3.3–5.8
(4.6±0.3) |
| HDL-C, mmol/l | 0.6–1.1
(0.8±0.04) |
| LDL-C, mmol/l | 1.9–4.6
(3.2±0.3) |
| Medication |
|
| Statin,
%a | 100 |
| ACEI/ARB,
%a | 79.2 |
|
β-blocker, %a | 100 |
|
Asprin+clopidogrel,
%b | 100 |
MP detection
In the present study, a Beckman Coulter Gallios flow
cytometer (Beckman Coulter, Inc.) was used, which is a
high-sensitivity cytometer with superior reproducibility for MP
measurement (19,20). Megamix containing 0.5, 0.9 and 3 µm
fluorescent beads was applied to ensure accurate identification of
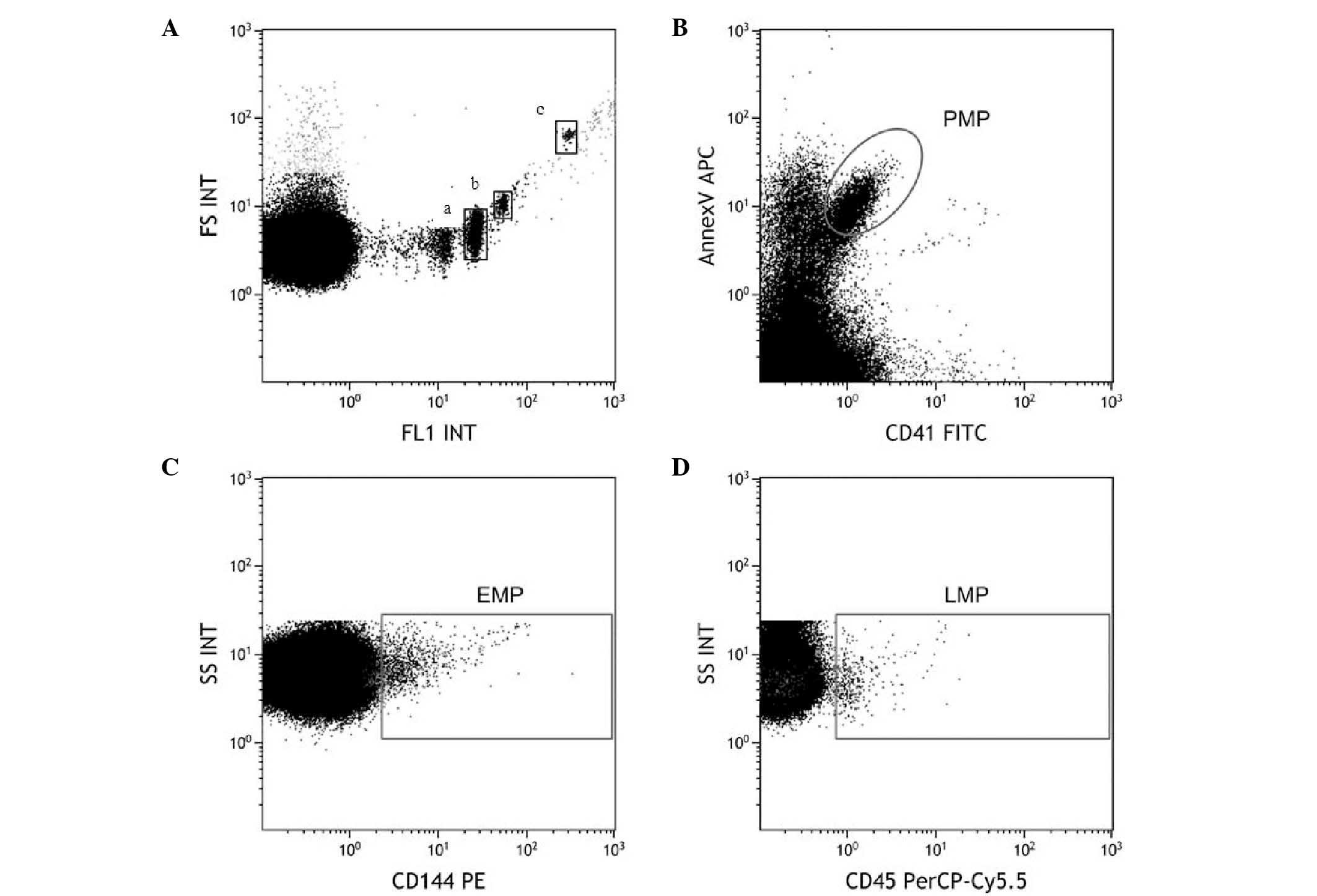
MPs in the flow cytometer (Fig. 1A).
PMPs were identified as CD41+/Annexin V+. EMPs and LMPs were
characterized by CD144+ and CD45+, respectively (Fig. 1B–D).
Time course of MPs
To the best of our knowledge, no previous study has
examined the time course of MPs originating from different cells
STEMI patients during PCI. In the present study, the levels of
PMPs, EMPs and LMPs were measured at five time-points: Immediately
prior to PCI, immediately after PCI, and 4, 24 and 48 h post-PCI
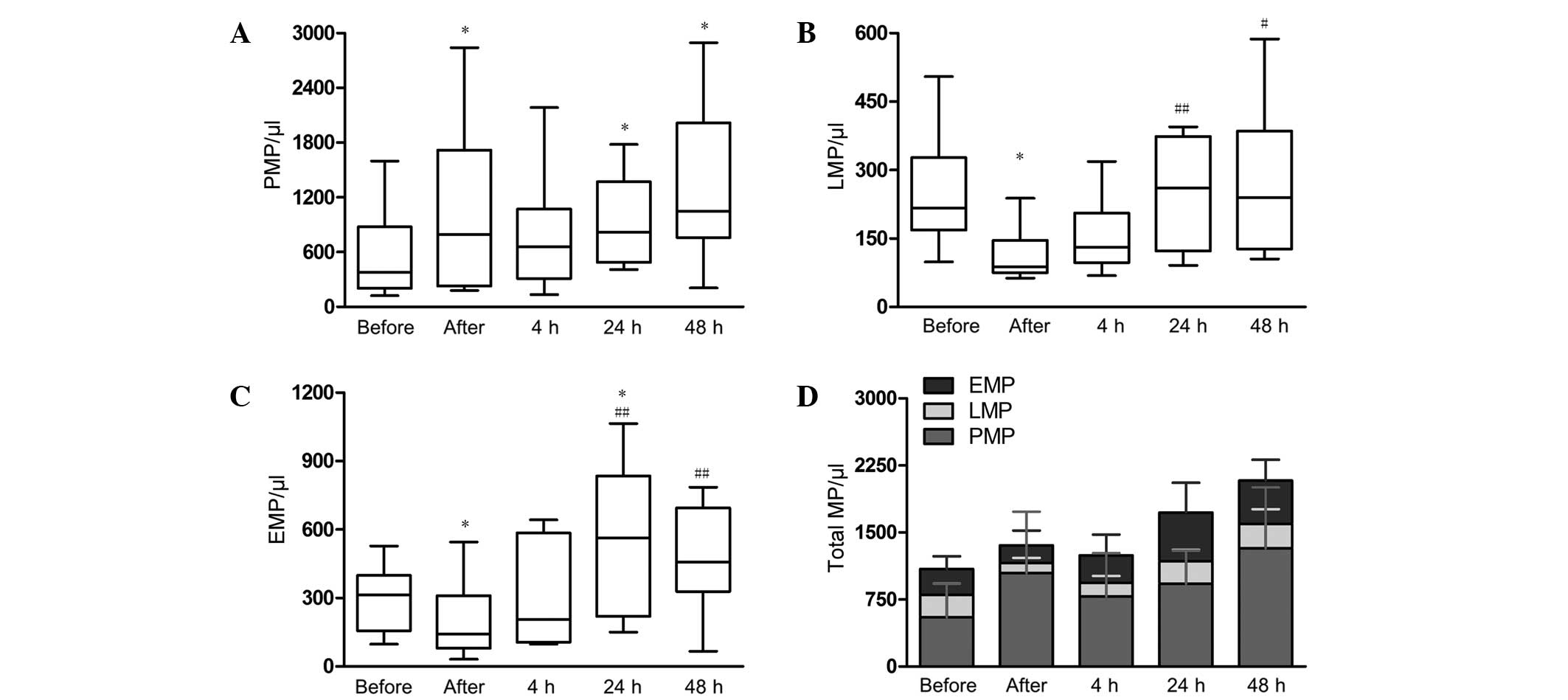
(Fig. 2). It was revealed that the
level of PMPs was evidently elevated immediately after PCI
(1045±895/µl; P<0.05), and reached a maximum level at 48 h
post-PCI (1325±882/µl; Fig. 2A). In
addition, the levels of LMPs and EMPs decreased significantly
immediately after PCI (LMPs: 250±126/µl vs. 114±59/µl, P=0.01;
EMPs: 289±143/µl vs. 198±165/µl, P=0.04), and then increased
gradually with time (Fig. 2B and C).
EMPs reached peak levels at 24 h post-PCI, which is significantly
higher compared with baseline levels (546±330/µl vs. 289±143,
respectively; P=0.04). LMPs reached peak levels 48 h post-PCI.
However, there was no significant difference compared with the
baseline level (272±164 vs. 250±126, respectively; P=0.63). The
total amount of MPs increased gradually 48 h after PCI (Fig. 2D).
Correlation between PMP levels and
Gensini scores
PMPs have previously been reported to increase in
patients with acute coronary syndrome (21), and may act as a marker of coagulation
(22). Thus, the present study aimed
to identify whether PMPs were correlated with the severity of
coronary disease. Linear regression between coronary angiographic
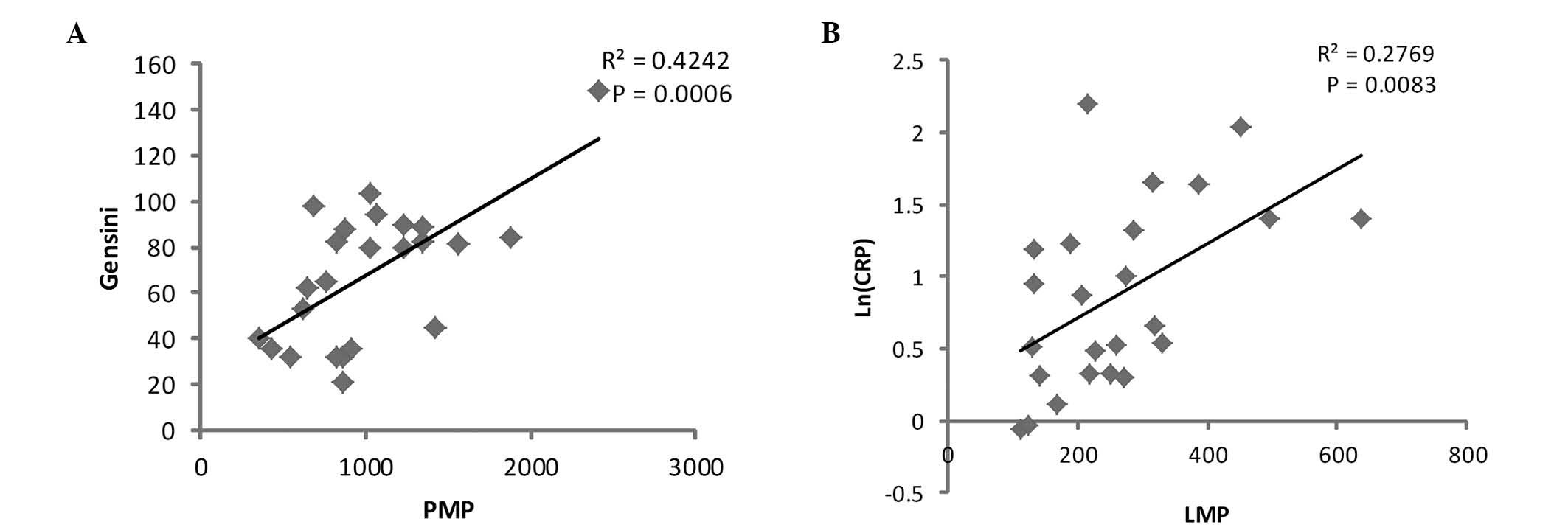
Gensini scores and PMP level prior to PCI was performed. The
results identified that the Gensini score was significantly
positively correlated with the level of PMPs prior to PCI (r2=0.42;
P=0.0006; Fig. 3A). However, no
significant correlation was detected between EMPs and LMPs with the
Gensini score (data not shown).
Correlation between LMP levels and
CRP
LMPs have previously been identified to play an
important role in atherosclerosis by promoting inflammation
(23). CRP is also a
well-established inflammatory marker, often used in patients with
STEMI (5). Thus, linear regression
analysis was performed between log-normalized hs-CRP [ln (hs-CRP)]
and LMP levels prior to PCI, and a significant correlation was
identified (r2=0.86, P<0.01, Fig.
3B). However, PMPs and EMPs displayed no statistical
correlation with hs-CRP (data not shown). In addition, no
significant correlation was observed between MPs and TnT, or
between MPs and CK-MB.
Discussion
Previous studies have investigated the changes in
MPs of different cell origin in STEMI patients and alterations in
MP levels at early (immediately after PCI) or late time-points (24
and 48 h after PCI) (7,17,21).
However, to the best of our knowledge, there are no reports in the
literature examining the detailed time course changes in MPs of
different cell origin during PCI.
In the present study, different cell origin MPs were
identified using flow cytometry, and the dynamic changes in MPs of
different cell origin were elucidated. It was identified that LMP
and EMP circulating levels decreased following successful
reperfusion, which may result from the recovery of pump function
and effective clearance of MPs. In the present study, circulating
procoagulant PMPs increased significantly following the surgery,
possibly due to the reperfusion of the occluded coronary artery
containing procoagulant substance, as well as a marked amount of
PMPs, which enter the circulation immediately after PCI (24). A second possible explanation may be
the direct injury of vessels caused by PCI, which may result in the
accumulation of PMPs. Subsequent to PCI, LMP and EMP levels also
increased. Recently, EMPs and LMPs have been reported to be a cause
of fibrinolysis (25,26). Thus, the increase of EMPs and LMPs
following PCI may act as an antagonistic response to the elevation
of procoagulant PMPs.
Clinical trails concerning MPs have shown a great
variation in results throughout the literature (27–29). A
previous study indicated that PMPs only experience a slight
increase following PCI surgery (7).
This observation may be a result of the use of a different MP
detection method. Compared with ELISA, flow cytometry is a more
commonly used high-throughput technique for the enumeration and
characterization of the cellular origin of MPs (20). Robert et al (16) established a standard flow cytometry
protocol for the measurement of MPs based on Megamix beads and the
results were reported in a multicenter study (19). The aforementioned protocol was
utilized in the present study, along with the Beckman Coulter
Gallios flow cytometer, which is a recent flow cytometer model that
provides more accurate results.
The association between MPs and coronary heart
disease has attracted increasing attention. Based on angiographic
results, the Gensini score is a well established scoring system
used to evaluate the severity of coronary disease (30). The association between Gensini score
and serum procoagulant factors, including fibrinogen and
glycoproteins, has been reported in previous studies (31,32). The
present study revealed that Gensini score is significantly
positively correlated with PMP levels prior to PCI, and thus
procoagulant PMPs may reflect the high plaque burden in patients
with higher Gensini scores. Furthermore, the present study examined
the time course of LMPs during PCI surgery for the first time and
determined the association between LMPs and hs-CRP. Previous
studies have revealed the association between PMPs and hs-CRP;
however, only a weak correlation was observed (7,8). Since
hs-CRP is predominately a symbol of acute inflammation, the present
study observed that LMPs, but not PMPs, correlated significantly
with hs-CRP. In addition, it is noteworthy that the distribution of
hs-CRP was not found to be a normal distribution (33), but a log-normal distribution
(34). In the present study, an
improved correlation was observed subsequent to logarithmic
transformations of hs-CRP data.
In conclusion, the present study aimed to provide a
detailed description of the time course of changes in circulating
MPs of different cell origin in STEMI patients who underwent PCI,
and provided information regarding the possible functions of
different MPs in STEMI. Future research may focus on the functional
importance of MPs of different cell origin in STEMI patients.
Acknowledgements
This present study was supported by grants from the
National Natural Science Foundation of China (no. 81300076) and the
Beijing Natural Science Foundation (no. 7132195).
References
|
1
|
György B, Szabó TG, Pásztói M, Pál Z,
Misják P, Aradi B, László V, Pállinger E, Pap E, Kittel A, et al:
Membrane vesicles, current state-of-the-art: Emerging role of
extracellular vesicles. Cell Mol Life Sci. 68:2667–2688. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Nozaki T, Sugiyama S, Koga H, Sugamura K,
Ohba K, Matsuzawa Y, Sumida H, Matsui K, Jinnouchi H and Ogawa H:
Significance of a multiple biomarkers strategy including
endothelial dysfunction to improve risk stratification for
cardiovascular events in patients at high risk for coronary heart
disease. J Am Coll Cardiol. 54:601–608. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hansson GK: Inflammation, atherosclerosis
and coronary artery disease. N Engl J Med. 352:1685–1695. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Liuzzo G, Biasucci LM, Gallimore JR,
Grillo RL, Rebuzzi AG, Pepys MB and Maseri A: The prognostic value
of C-reactive protein and serum amyloid a protein in severe
unstable angina. N Engl J Med. 331:417–424. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lindahl B, Toss H, Siegbahn A, Venge P and
Wallentin L: Markers of myocardial damage and inflammation in
relation to long-term mortality in unstable coronary artery
disease. FRISC Study Group. Fragmin during Instability in Coronary
Artery Disease. N Engl J Med. 343:1139–1147. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Habersberger J, Strang F, Scheichl A, Htun
N, Bassler N, Merivirta RM, Diehl P, Krippner G, Meikle P,
Eisenhardt SU, et al: Circulating microparticles generate and
transport monomeric C-reactive protein in patients with myocardial
infarction. Cardiovasc Res. 96:64–72. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Inoue T, Komoda H, Kotooka N, Morooka T,
Fujimatsu D, Hikichi Y, Soma R, Uchida T and Node K: Increased
circulating platelet-derived microparticles are associated with
stent-induced vascular inflammation. Atherosclerosis. 196:469–476.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Biasucci LM, Porto I, Di Vito L, De Maria
GL, Leone AM, Tinelli G, Tritarelli A, Di Rocco G, Snider F,
Capogrossi MC and Crea F: Differences in microparticle release in
patients with acute coronary syndrome and stable angina. Circ J.
76:2174–2182. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Leroyer AS, Rautou PE, Silvestre JS,
Castier Y, Lesèche G, Devue C, Duriez M, Brandes RP, Lutgens E,
Tedgui A and Boulanger CM: CD40 ligand+ microparticles from human
atherosclerotic plaques stimulate endothelial proliferation and
angiogenesis a potential mechanism for intraplaque
neovascularization. J Am Coll Cardiol. 52:1302–1311. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Stępień E, Stankiewicz E, Zalewski J,
Godlewski J, Zmudka K and Wybrańska I: Number of microparticles
generated during acute myocardial infarction and stable angina
correlates with platelet activation. Arch Med Res. 43:31–35. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Pollack CV Jr, Diercks DB, Roe MT and
Peterson ED: American College of Cardiology; American Heart
Association: 2004 American college of cardiology/American Heart
Association guidelines for the management of patients with
ST-elevation myocardial infarction: Implications for emergency
department practice. Ann Emerg Med. 45:363–376. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Stringer KA: TIMI grade flow, mortality,
and the GUSTO-III trial. Pharmacotherapy. 18:699–705.
1998.PubMed/NCBI
|
|
13
|
Gensini GG: A more meaningful scoring
system for determining the severity of coronary heart disease. Am J
Cardiol. 51:6061983. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Levine GN, Bates ER, Blankenship JC,
Bailey SR, Bittl JA, Cercek B, Chambers CE, Ellis SG, Guyton RA,
Hollenberg SM, et al: 2011 ACCF/AHA/SCAI Guideline for percutaneous
coronary intervention. A report of the American college of
cardiology foundation/American heart association task force on
practice guidelines and the society for cardiovascular angiography
and interventions. J Am Coll Cardiol. 58:e44–e122. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chen S, Guo L, Cui M, Sun L and Mi L:
Dynamic changes in serum angiopoietin-1, angiopoietin-2 and
angiopoietin-2/angiopoietin-1 ratio in acute myocardial infarction
patients treated with primary percutaneous coronary intervention.
Biomarkers. 17:441–446. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Robert S, Poncelet P, Lacroix R, Arnaud L,
Giraudo L, Hauchard A, Sampol J and Dignat-George F:
Standardization of platelet-derived microparticle counting using
calibrated beads and a Cytomics FC500 routine flow cytometer: A
first step towards multicenter studies? J Thromb Haemost.
7:190–197. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Empana JP, Boulanger CM, Tafflet M, Renard
JM, Leroyer AS, Varenne O, Prugger C, Silvain J, Tedgui A, Cariou
A, et al: Microparticles and sudden cardiac death due to coronary
occlusion. The TIDE (Thrombus and Inflammation in sudden DEath)
study. Eur Heart J Acute Cardiovasc Care. 4:28–36. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang K, Zuo G, Zheng L, Zhang C, Wang D,
Cao Z, Hu S and Du X: Effects of tirofiban on platelet activation
and endothelial function in patients with ST-elevation myocardial
infarction undergoing primary percutaneous coronary intervention.
Cell Biochem Biophys. 71:135–142. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lacroix R, Robert S, Poncelet P, Kasthuri
RS, Key NS and Dignat-George F: ISTH SSC Workshop: Standardization
of platelet-derived microparticle enumeration by flow cytometry
with calibrated beads: Results of the international society on
thrombosis and haemostasis SSC collaborative workshop. J Thromb
Haemost. 8:2571–2574. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Robert S, Lacroix R, Poncelet P, Harhouri
K, Bouriche T, Judicone C, Wischhusen J, Arnaud L and Dignat-George
F: High-sensitivity flow cytometry provides access to standardized
measurement of small-size microparticles-brief report. Arterioscler
Thromb Vasc Biol. 32:1054–1058. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Biasucci LM, Porto I, Di Vito L, De Maria
GL, Leone AM, Tinelli G, Tritarelli A, Di Rocco G, Snider F,
Capogrossi MC and Crea F: Differences in microparticle release in
patients with acute coronary syndrome and stable angina. Circ J.
76:2174–2182. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sinauridze EI, Kireev DA, Popenko NY,
Pichugin AV, Panteleev MA, Krymskaya OV and Ataullakhanov FI:
Platelet microparticle membranes have 50- to 100-fold higher
specific procoagulant activity than activated platelets. Thromb
Haemost. 97:425–434. 2007.PubMed/NCBI
|
|
23
|
Angelillo-Scherrer A: Leukocyte-derived
microparticles in vascular homeostasis. Circ Res. 110:356–369.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Vidal C, Spaulding C, Picard F, Schaison
F, Melle J, Weber S and Fontenay-Roupie M: Flow cytometry detection
of platelet procoagulation activity and microparticles in patients
with unstable angina treated by percutaneous coronary angioplasty
and stent implantation. Thromb Haemost. 86:784–790. 2001.PubMed/NCBI
|
|
25
|
Lacroix R and Dignat-George F:
Microparticles as a circulating source of procoagulant and
fibrinolytic activities in the circulation. Thromb Res. 129(Suppl
2): S27–S29. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lacroix R, Plawinski L, Robert S, Doeuvre
L, Sabatier F, de Lizarrondo Martinez S, Mezzapesa A, Anfosso F,
Leroyer AS, Poullin P, et al: Leukocyte- and endothelial-derived
microparticles: A circulating source for fibrinolysis.
Haematologica. 97:1864–1872. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Tan KT, Tayebjee MH, Lim HS and Lip GY:
Clinically apparent atherosclerotic disease in diabetes is
associated with an increase in platelet microparticle levels.
Diabet Med. 22:1657–1662. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hron G, Kollars M, Weber H, Sagaster V,
Quehenberger P, Eichinger S, Kyrle PA and Weltermann A: Tissue
factor-positive microparticles: Cellular origin and association
with coagulation activation in patients with colorectal cancer.
Thromb Haemost. 97:119–123. 2007.PubMed/NCBI
|
|
29
|
Pereira J, Alfaro G, Goycoolea M, Quiroga
T, Ocqueteau M, Massardo L, Pérez C, Sáez C, Panes O, Matus V and
Mezzano D: Circulating platelet-derived microparticles in systemic
lupus erythematosus. Association with increased thrombin generation
and procoagulant state. Thromb Haemost. 95:94–99. 2006.PubMed/NCBI
|
|
30
|
Chen J, Zhang Y, Liu J, Chen MH, Guo YL,
Zhu CG, Xu RX, Dong Q and Li JJ: Role of lipoprotein(a) in
predicting the severity of new on-set coronary artery disease in
type 2 diabetics: A Gensini score evaluation. Diab Vasc Dis Res.
12:258–264. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Mori T, Sasaki J, Kawaguchi H, Handa K,
Takada Y, Matsunaga A, Kono S and Arakawa K: Serum glycoproteins
and severity of coronary atherosclerosis. Am Heart J. 129:234–238.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Handa K, Kono S, Saku K, Sasaki J, Kawano
T, Sasaki Y, Hiroki T and Arakawa K: Plasma fibrinogen levels as an
independent indicator of severity of coronary atherosclerosis.
Atherosclerosis. 77:209–213. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Woloshin S and Schwartz LM: Distribution
of C-reactive protein values in the United States. N Engl J Med.
352:1611–1613. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Rohde LE, Hennekens CH and Ridker PM:
Survey of C-reactive protein and cardiovascular risk factors in
apparently healthy men. Am J Cardiol. 84:1018–1022. 1999.
View Article : Google Scholar : PubMed/NCBI
|