Introduction
The prevalence of diabetic nephropathy (DN) is
increasing markedly worldwide, and DN is currently the most common
cause of end-stage renal failure requiring renal replacement
therapy (1,2). The pathological features of DN include
mesangial expansion, caused by the proliferation of mesangial cells
and the excessive accumulation of extracellular matrix (ECM)
(3,4). Hyperglycemia has been demonstrated to
be the main initiation factor in the etiology of DN (5). Hyperglycemia can activate multiple
intracellular signaling factors, resulting in the accumulation of
ECM (6–8). Among these factors, transforming growth
factor (TGF)-β1 and its downstream mediator connective
tissue growth factor (CTGF) are recognized as fibrogenic cytokines
and play a critical role in the kidney pathophysiology of DN
(9,10). Mesangial cells (MCs) are recognized
as the major ECM-secreting cells (11,12). The
inhibition of MC proliferation and ECM accumulation should be
beneficial in DN. The major ECM proteins include collagen IV
(colIV) and fibronectin (FN) (13).
Therefore, changes in the concentrations of colIV and FN can be
used to study the pathological changes of DN (14,15).
However, at present there is no treatment available
that is able to arrest the progression of DN to end-stage renal
failure, and new therapeutic strategies for the management of DN
are therefore required (16).
Chinese medical herbs are considered to be a promising source of
potential treatments due to their variety of species and
applications. Radix Astragali, the root of Astragalus
membranaceus Bunge, is widely used in traditional Chinese
medicine for its anti-inflammatory, anti-oxidative,
immune-regulatory and neuroprotective activities (17,18). The
principle bioactive components extracted from Radix Astragali are
known as astragalosides. A clinical trial has shown that
astragalosides have a potential role in the treatment of DN
(19). Moreover, the results of a
recent study suggested that the intravenous infusion of
astragalosides in healthy Chinese volunteers was safe and well
tolerated (20), and astragalosides
appear to be efficient and safe for use in the therapy of DN.
Therefore, the aim of the present study was to investigate the
mechanism underlying the effects of astragalosides in the treatment
of DN.
Materials and methods
Materials
Astragalosides were purchased from Shanghai Yuanye
Biotechnology Co., Ltd (Shanghai, China). Rat MCs (No. HBZT-1) were
obtained from Wuhan Boster Biological Technology, Ltd. (Wuhan,
China). 5-Bromo-2′-deoxyuridine (BrdU) kit was purchased from
Beyotime Institute of Biotechnology (Shanghai, China). The TRIzol
kit and cDNA reverse transcription kit were from Takara Bio, Inc.
(Otsu, Japan). The rat TGF-β1, CTGF, FN and colIV enzyme-linked
immunosorbent assay (ELISA) kits were purchased from R&D
Systems, Inc. (Minneapolis, MN, USA).
Cell culture and treatment
MCs were cultured in Gibco Dulbecco's modified
Eagle's medium (DMEM; Thermo Fisher Scientific, Inc., Waltham, MA,
USA) that was supplemented with 100 µg/ml streptomycin, 100 U/ml
penicillin (both purchased from Beyotime Institute of
Biotechnology) and 10% (v/v) fetal bovine serum (Hangzhou Sijiqing
Biological Engineering Materials Co., Ltd., Hangzhou, China). The
cells were incubated at 37°C in a humidified atmosphere of 5%
CO2. During the experiments, the cells were first
exposed to a normal concentration of glucose [5.56 mmol/l; normal
glucose (NG) group] for 4 h, and then treated with high glucose (HG
group; 30 mmol/l glucose), high glucose with 50 µg/ml
astragalosides (ATSL group), high glucose with 100 µg/ml
astragalosides (ATSM group), and high glucose with 200 µg/ml
astragalosides (ATSH group). Mannitol (MA group) was used as a
control to rule out the effect of osmotic pressure. The cells were
harvested for analysis after 24 and 48 h of treatment.
Cell proliferation assay
Cell proliferation was examined by BrdU assay. MCs
were seeded into 96-well plates at a density of 1.0×106
per well. After 24 and 48 h, BrdU solution was added and the MCs
continued to be cultured for another 2 h. Absorbance was read at
450 nm by visible spectrometry (CliniBio 128C; Biochrom, Ltd.,
Cambridge, UK).
Reverse transcription-polymerase chain
reaction (RT-PCR)
An RT-PCR procedure was performed to determine the
relative mRNA quantities of TGF-β1, CTGF, ColIV and FN in MCs.
Total RNA was extracted from the MCs with TRIzol reagent according
to the manufacturer's protocol. The total RNA (0.5 µg) obtained was
converted into cDNA using the RevertAid™ First Strand cDNA
Synthesis kit with Random Hexamer primers (Thermo Fisher
Scientific, Inc.). The upstream and downstream primers (Shanghai
Sangon Biotech Co., Ltd., China) of these genes are shown in
Table I. The reaction mixture was
incubated at 48°C for 45 min for reverse transcription, and then
underwent cycling. The cycling conditions of these genes were:
Initial denaturation for 5 min at 94°C, 30 cycles at 94°C for 30
sec, 60°C for 30 sec, 72°C for 1 min, and final elongation at 72°C
for 7 min. The RT-PCR products were separated by 2% agarose
electrophoresis, and the band densities were analyzed using laser
densitometry (Tanon-1600R; Tanon Science & Technology Co.,
Ltd., Shanghai, China). The relative quantities of mRNA of these
four genes in the MCs were represented by the ratio of the band
density of objective gene relative to that of β-actin.
 | Table I.Primer sequences for reverse
transcription-polymerase chain reaction. |
Table I.
Primer sequences for reverse
transcription-polymerase chain reaction.
| Gene name | Forward sequence (5′
to 3′) | Reverse sequence (5′
to 3′) |
|---|
|
TGF-β1 |
ATGTGCAGGATAATTGCTGCC |
TGGTGTTGTACAGGCTGAGG |
| CTGF |
GCTAAGACCTGTGGAATGGGC |
CTCAAAGATGTCATTGCCCCC |
| ColIV |
TCGGCTATTCCTTCGTGATG |
TCTCGCTTCTCTCTATGGTG |
| FN |
GCGACTCTGACTGGCCTTAC′ |
CCGTGTAAGGGTCAAAGCAT |
| β-actin |
TCAGGTCATCACTATCGGCAAT |
AAAGAAAGGGTGTAAAACGCA |
ELISA
Levels of TGF-β1, CTGF, ColIV and FN in the
supernatants of the MCs were determined by ELISA. The MC culture
medium was collected and centrifuged at 13,000 × g for 15 min to
pelletize the debris. The levels of these four proteins were
determined according to the kit manufacturer's protocol. The
colorimetric reaction was measured at 450 nm.
Statistical analysis
Data are expressed as the mean ± standard deviation.
Statistical analyses were performed using the paired t-test for the
comparison of two groups and one-way analysis of variance with
Dunnett's test for the comparison of multiple groups. A value of
P<0.05 was considered statistically significant.
Results
Effects of AST on MC
proliferation
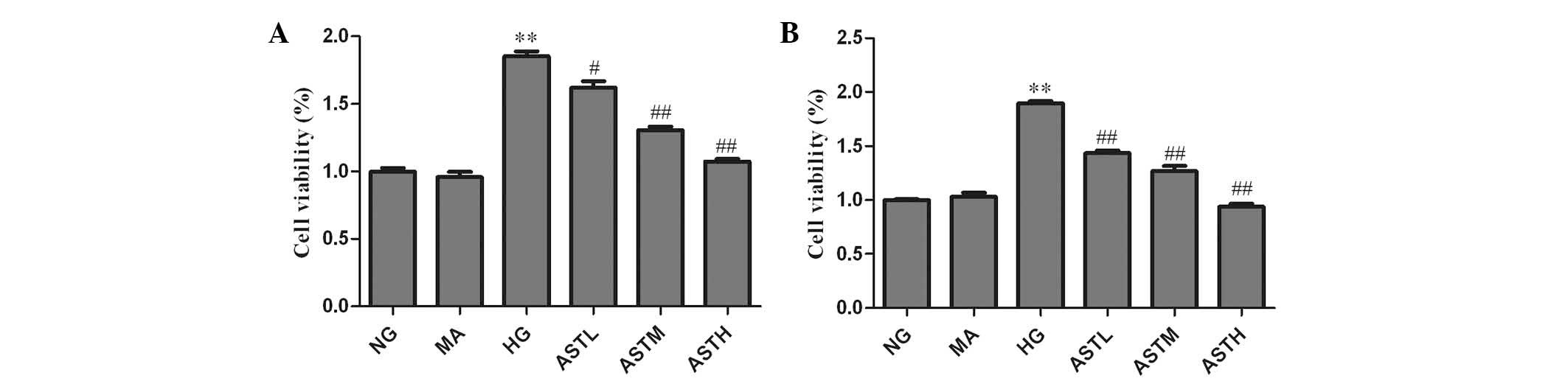
A BrdU assay was employed to evaluate the impact of
AST on MC proliferation. As shown in Fig. 1, the proliferation of MCs was
increased after 24 and 48 h in the presence of a high concentration
of glucose (HG group vs. NG group, P<0.01). The cell
proliferation rates in the ASTL, ASTM and ASTH groups were
significantly lower than those in the HG group (P<0.05 for ASTL;
P<0.01 for ASTM and ASTH). Cell viability in the MA group was
almost identical to that in the NG group, indicating no evident
impact of the osmotic pressure generated by high glucose. The above
results indicate that the administration of AST significantly
suppressed high glucose induced-MC proliferation.
Effects of AST on high glucose-induced
TGF-β1, CTGF, ColIV and FN mRNA expression
The RT-PCR detection results showed that the mRNA
levels of TGF-β1, CTGF, ColIV and FN in the HG group were elevated
compared with those of the NG group (P<0.01). At 24 h
(representative results, Fig. 2A),
the TGF-β1, CTGF and FN mRNA levels in the groups treated with 100
or 200 µg/ml AST were all lower than those of the HG group
(Fig. 2B, C and E). The ColIV mRNA
levels in the group treated with 50 µg/ml AST were also decreased
compared with those in the HG group (Fig. 2D). At 48 h, the four types of mRNA in
the ASTL, ASTM and ASTH groups were notably downregulated compared
with those in the HG group (Fig. 3).
No significant changes in the levels of TGF-β1, CTGF, ColIV and FN
mRNA were observed between the MA group and the NG group (Figs. 2 and 3). These results indicate that the
administration of AST significantly decreased the high
glucose-induced increases of TGF-β1, CTGF, ColIV and FN mRNA
levels.
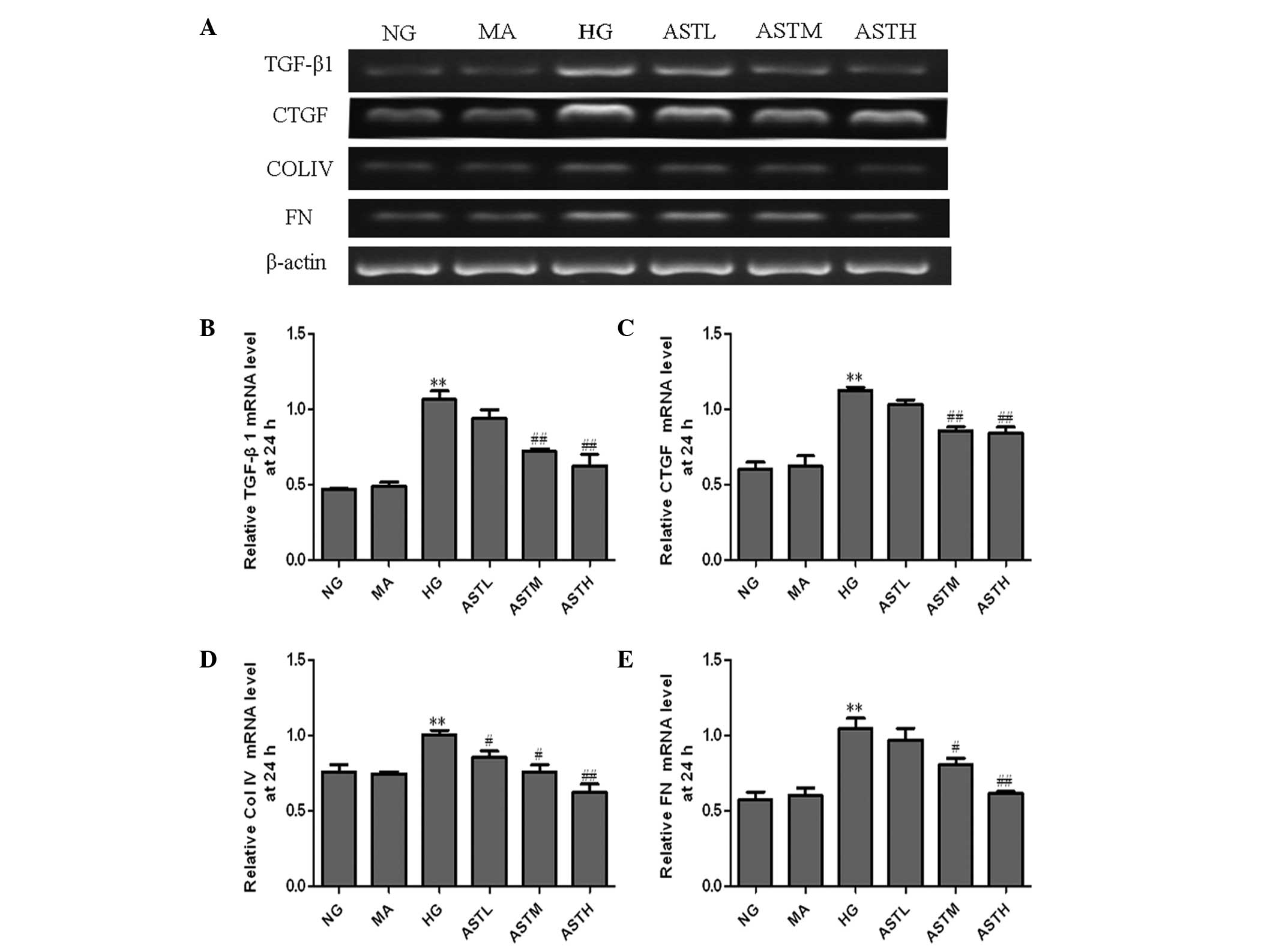 | Figure 2.mRNA levels of TGF-β1, CTGF, ColIV and
FN in the astragaloside (AST)-treated mesangial cells at 24 h. (A)
Agarose electrophoresis of reverse transcription polymerase chain
reaction (RT-PCR) products amplified from the total RNA extracts of
mesangial cells; β-actin was used as the internal standard in each
sample. RT-PCR data for the relative mRNA quantities of (B) TGF-β1,
(C) CTGF, (D) ColIV and (E) FN determined by densitometric
analysis. Values are presented as mean ± standard deviation (n=3).
**P<0.01 vs. the NG group; #P<0.05,
##P<0.01 vs. the HG group. TGF, transforming growth
factor; CTGF, connective tissue growth factor; ColIV, collagen IV;
FN, fibronectin; NG, normal glucose, MA, mannitol; HG, high
glucose; ASTL group, low-dose AST; ASTM, medium-dose AST; ASTH,
high-dose AST. |
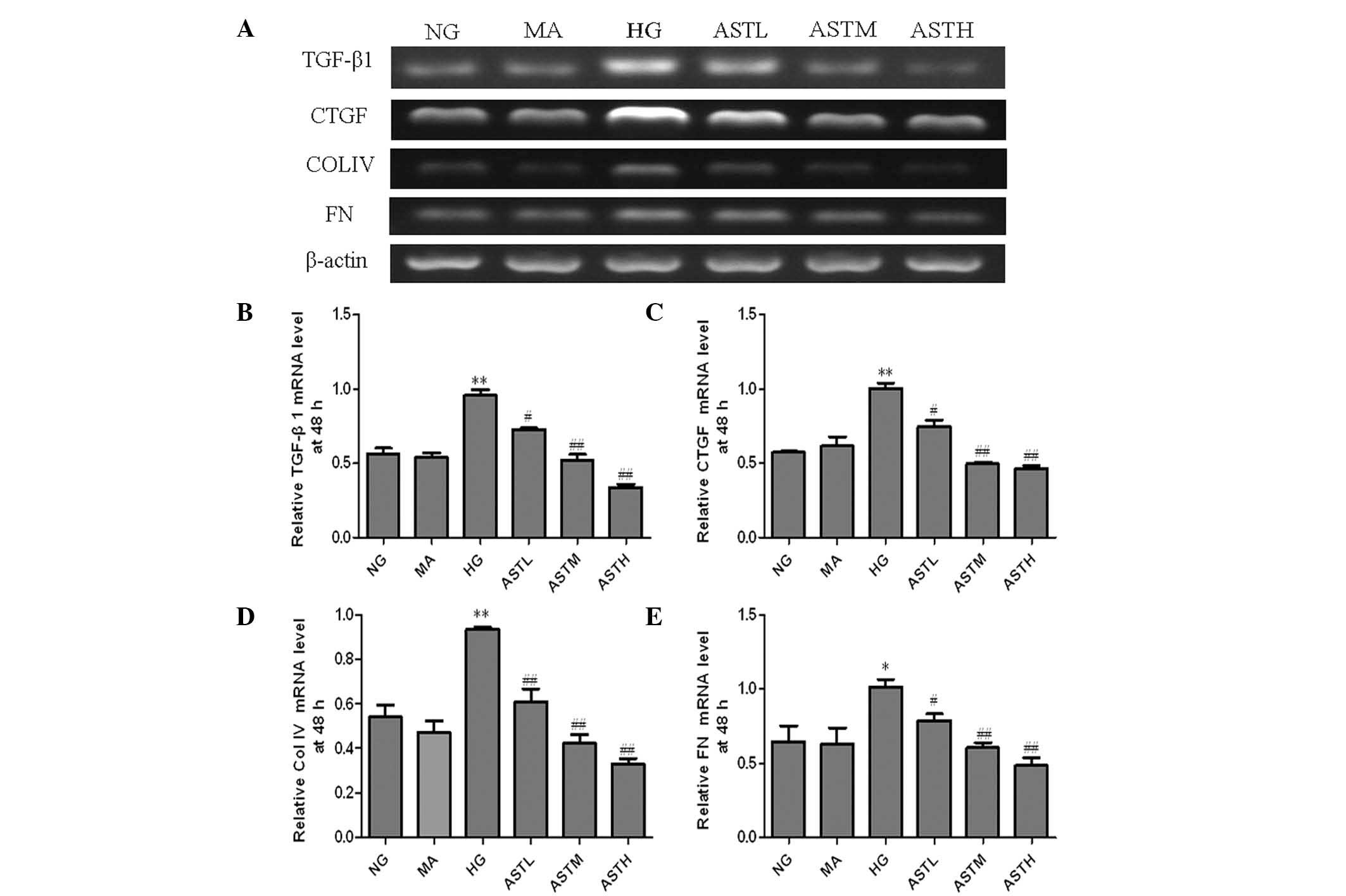 | Figure 3.mRNA levels of TGF-β1, CTGF, ColIV and
FN in the astragaloside (AST)-treated mesangial cells at 48 h. (A)
Agarose electrophoresis of reverse transcription polymerase chain
reaction (RT-PCR) products amplified from the total RNA extracts of
mesangial cells; β-actin was used as the internal standard in each
sample. RT-PCR data for the mRNA relative quantity of (B) TGF-β1,
(C) CTGF, (D) ColIV and (E) FN performed by densitometric analysis.
Values are presented as mean ± standard deviation, n=3. *P<0.05,
**P<0.01 vs. the NG group; #P<0.05,
##P<0.01 vs. the HG group. TGF, transforming growth
factor; CTGF, connective tissue growth factor; ColIV, collagen IV;
FN, fibronectin; FN, fibronectin; NG, normal glucose, MA, mannitol;
HG, high glucose; ASTL group, low-dose AST; ASTM, medium-dose AST;
ASTH, high-dose AST. |
Effect of AST on high glucose-induced
regulation of TGF-β1, CTGF, ColIV and FN
The levels of TGF-β1, CTGF, ColIV and FN in the cell
culture supernatants of the MCs in the HG group were markedly
upregulated compared with those of the NG group (P<0.01,
Figs. 4 and 5). No significant difference between the NG
group and the MA group was observed with regard to the secretion of
these four proteins (Figs. 4 and
5). These results indicate that the
administration of AST significantly reduced the high
glucose-induced secretion of TGF-β1, CTGF, ColIV and FN
proteins.
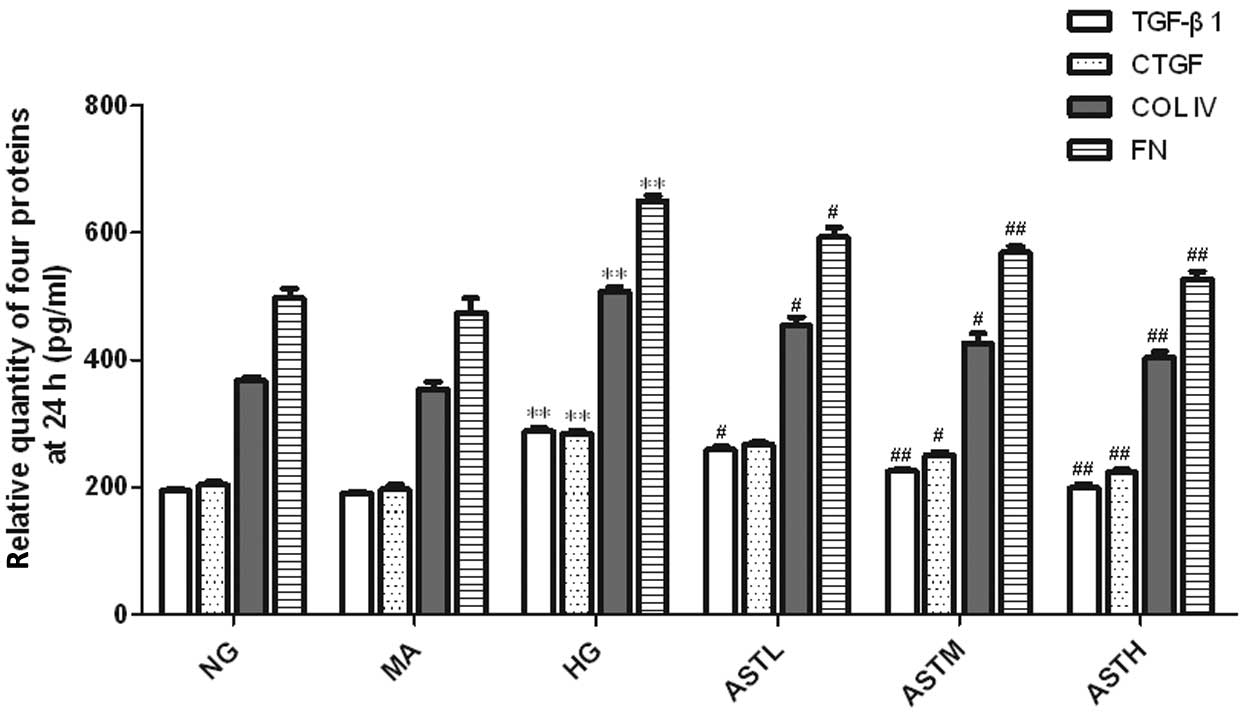 | Figure 4.Relative quantity of TGF-β1, CTGF,
ColIV and FN proteins in the supernatant of astragaloside
(AST)-treated mesangial cells at 24 h. Values are presented as mean
± standard deviation (n=3).**P<0.01 vs. the NG group;
#P<0.05, ##P<0.01 vs. the HG group.
TGF, transforming growth factor; CTGF, connective tissue growth
factor; ColIV, collagen IV; FN, fibronectin; FN, fibronectin; NG,
normal glucose, MA, mannitol; HG, high glucose; ASTL group,
low-dose AST; ASTM, medium-dose AST; ASTH, high-dose AST. |
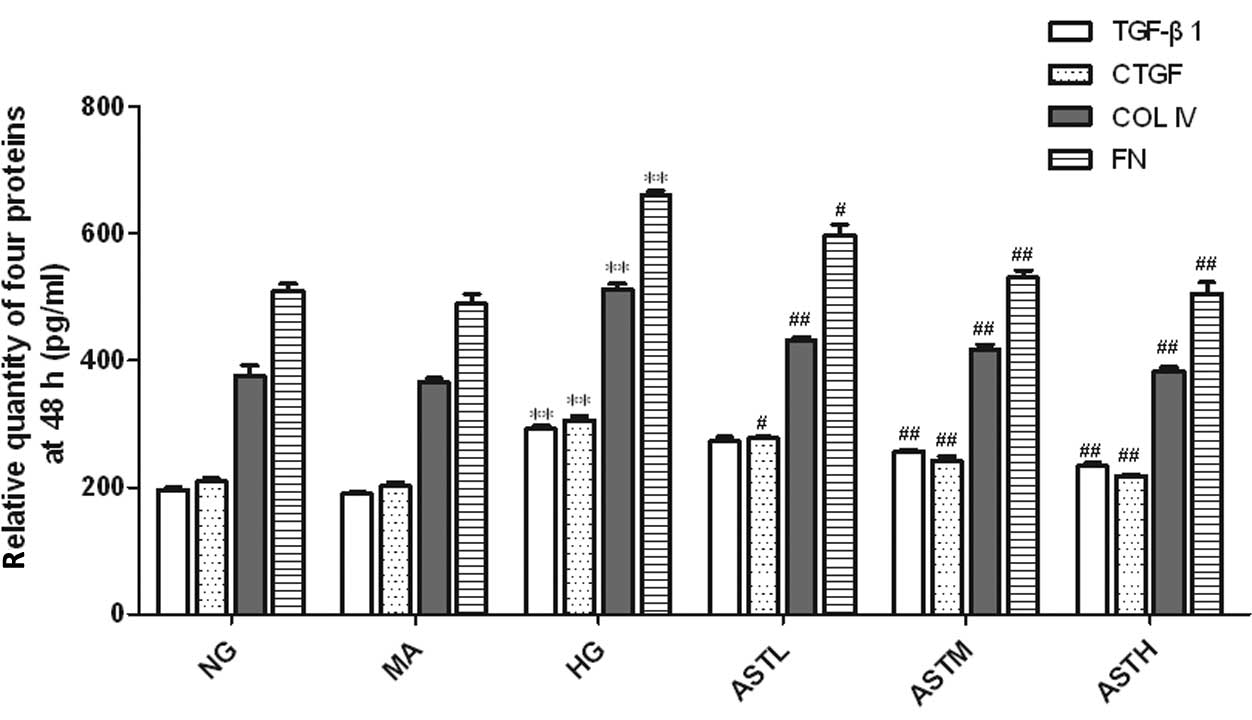 | Figure 5.Relative quantity of TGF-β1, CTGF,
ColIV and FN proteins in the supernatant of astragaloside
(AST)-treated mesangial cells at 48 h. Values are presented as mean
± standard deviation (n=3).**P<0.01 vs. the NG group;
#P<0.05, ##P<0.01 vs. the HG group.
TGF, transforming growth factor; CTGF, connective tissue growth
factor; ColIV, collagen IV; FN, fibronectin; FN, fibronectin; NG,
normal glucose, MA, mannitol; HG, high glucose; ASTL group,
low-dose AST; ASTM, medium-dose AST; ASTH, high-dose AST. |
Discussion
The pathological features of advanced DN include
expansion of the mesangial ECM and thickening of the glomerular
basement membrane (21). These
pathological changes are caused by accumulation of ECM proteins and
changes in the composition of these proteins (22). The accumulation of ECM in the
mesangial area is generally associated with the deposition of FN
and colIV (16). Therefore,
reversing changes in FN and colIV synthesis might play a key role
in delaying the progression of DN.
TGF-β is a potent fibrogenic factor (23,24).
TGF-β1 is highly expressed in the kidneys of diabetic
animals (25) and humans (26) and contributes to the accumulation of
ECM. CTGF, acting downstream of TGF-β1, has been shown
to mediate the expression of ECM proteins in response to various
external perturbations (27,28). Moreover, CTGF facilitates
TGF-β1 signaling and consequently promotes renal
fibrosis (29). The coordinated
expression of TGF-β1 and CTGF appears to be crucial for
the induction of ECM proteins and thus, for the development of DN
(29).
The degree of ECM accumulation has been found to
correlate with the extent of renal insufficiency and proteinuria
(16), and MCs are recognized as
being the major cells in the secretion ECM (11,12).
Therefore, the inhibition of MC proliferation and ECM accumulation
should be beneficial in DN. In the present study it was found that
astragalosides significantly suppressed high glucose induced-MC
proliferation and decreased the high glucose-induced secretion of
ColIV and FN proteins in vitro. Additionally, it was also
found that astragalosides significantly suppressed the increases in
the mRNA and protein expression levels of TGF-β1 and
CTGF that were induced by a high concentration of glucose.
In summary, astragalosides inhibited the
accumulation of ColIV and FN, possibly through the downregulation
of TGF-β1-CTGF signaling, which provides a theoretical
basis for their use in the treatment of DN.
Acknowledgements
This study was funded by the National Innovative
Practice Training Program for Students of Higher Education
Institutions (no. 201410313024) and the Innovative Practice
Training Program for Students of Jiangsu Higher Education
Institutions (no. 201410313024Z). This study was also funded by
School of Pharmacy, Xuzhou Medical College Innovative Practice
Training Program for Graduates (no. 2014YKYCX013).
References
|
1
|
Ritz E and Orth SR: Nephropathy in
patients with type 2 diabetes mellitus. N Engl J Med.
341:1127–1133. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Remuzzi G, Schieppati A and Ruggenenti P:
Clinical practice: Nephropathy in patients with type 2 diabetes. N
Engl J Med. 346:1145–1151. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kolset SO, Reinholt FP and Jenssen T:
Diabetic nephropathy and extracellular matrix. J Histochem
Cytochem. 60:976–986. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Tervaert TW, Mooyaart AL, Amann K, Cohen
AH, Cook HT, Tervaert TW, Mooyaart AL, Amann K, Cohen AH, Cook HT,
Drachenberg CB, Ferrario F, Fogo AB, Haas M, de Heer E, et al:
Pathologic classification of diabetic nephropathy. J Am Soc
Nephrol. 21:556–563. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Phillips CA and Molitch ME: The
relationship between glucose control and the development and
progression of diabetic nephropathy. Curr Diab Rep. 2:523–529.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Mishra R, Emancipator SN, Kern T and
Simonson MS: High glucose evokes an intrinsic proapoptotic
signaling pathway in mesangial cells. Kidney Int. 67:82–93. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Cooper ME: Interaction of metabolic and
haemodynamic factors in mediating experimental diabetic
nephropathy. Diabetologia. 44:1957–1972. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ha H and Lee HB: Reactive oxygen species
as glucose signaling molecules in mesangial cells cultured under
high glucose. Kidney Int Suppl. 77:S19–S25. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Gao Q, Qin WS, Jia ZH, Zheng JM, Zeng CH,
Li LS and Liu ZH: Rhein improves renal lesion and ameliorates
dyslipidemia in db/db mice with diabetic nephropathy. Planta Med.
76:27–33. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Brosius FC III: New insights into the
mechanisms of fibrosis and sclerosis in diabetic nephropathy. Rev
Endocr Metab Disord. 9:245–254. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang JY, Yin XX, Wu YM, Tang DQ, Gao YY,
Wan MR, Hou XY and Zhang B: Ginkgo biloba extract suppresses
hypertrophy and extracellular matrix accumulation in rat mesangial
cells. Acta Pharmcol Sin. 27:1222–1230. 2006. View Article : Google Scholar
|
|
12
|
Lal MA, Brismar H, Eklöf AC and Aperia A:
Role of oxidative stress in advanced glycation end product-induced
mesangial cells activation. Kidney Int. 61:2006–2014. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Dronavalli S, Duka I and Bakris GL: The
pathogenesis of diabetic nephropathy. Nat Clin Pract Endocrinol
Metab. 4:444–452. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wang JY, Yin XX, Wu YM, Tang DQ, Gao YY,
Wan MR, Hou XY and Zhang B: Ginkgo biloba extract suppresses
hypertrophy and extracellular matrix accumulation in rat mesangial
cells. Acta Pharmacol Sin. 27:1222–1230. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Xu W, Shao X, Tian L, Gu L, Zhang M, Wang
Q, Wu B, Wang L, Yao J, Xu X, et al: Astragaloside IV ameliorates
renal fibrosis via the inhibition of mitogen-activated protein
kinases and antiapoptosis in vivo and in vitro. J Pharmacol Exp
Ther. 350:552–562. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sato S, Kawamura H, Takemoto M, Maezawa Y,
Fujimoto M, Shimoyama T, Koshizaka M, Tsurutani Y, Watanabe A, Ueda
S, et al: Halofuginone prevents extracellular matrix deposition in
diabetic nephropathy. Biochem Biophys Res Commun. 379:411–416.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Rios JL and Waterman PG: A review of the
pharmacology and toxicology of Astragalus. Phytother Res.
11:411–418. 1997. View Article : Google Scholar
|
|
18
|
Liu G, Song J, Guo Y, Wang T and Zhou Z:
Astragalus injection protects cerebral ischemic injury by
inhibiting neuronal apoptosis and the expression of JNK3 after
cerebral ischemic reperfusion in rats. Behav Brain Funct. 9:362013.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wei XP, Wu ZL and Wang JT: Clinical
research on treating diabetes with Astragalus injection and
TMP injection. Zhongguo Lin Chuang Yan Jiu. 6:142014.(In
Chinese).
|
|
20
|
Xu M, Yin J, Xie L, Zhang J, Zou C, Zou J,
Liu F, Ju W and Li P: Pharmacokinetics and tolerance of total
astragalosides after intravenous infusion of astragalosides
injection in healthy Chinese volunteers. Phytomedicine.
20:1105–1111. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Mason RM and Wahab NA: Extracellular
matrix metabolism in diabetic nephropathy. J Am Soc Nephrol.
14:1358–1373. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Nakajima T, Haseqawa G, Kamiuchi K, Fukui
M, Yamasaki M, Tominaga M, Asano M, Hosoda H, Yoshikawa T and
Nakamura N: Differential regulation of intracellular redox state by
extracellular matrix proteins in glomerular mesangial cells:
Potential role in diabetic nephropathy. Redox Rep. 11:223–230.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sakharova OV, Taal MW and Brenner BM:
Pathogenesis of diabetic nephropathy: Focus on transforming growth
factor-beta and connective tissue growth factor. Curr Opin Nephrol
Hypertens. 10:727–738. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhao TT, Zhang HJ, Lu XG, Huang XR, Zhang
WK, Wang H, Lan HY and Li P: Chaihuang-Yishen granule inhibits
diabetic kidney disease in rats through blocking TGF-β/Smad3
signaling. PLoS One. 9:e908072014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sharma K and Ziyadeh FN: Renal hypertrophy
is associated with upregulation of TGF-beta 1 gene expression in
diabetic BB rat and NOD mouse. Am J Physiol. 267:F1094–F1101.
1994.PubMed/NCBI
|
|
26
|
Border WA and Noble NA: Evidence that
TGF-beta should be a therapeutic target in diabetic nephropathy.
Kidney Int. 54:1390–1391. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Liu X, Luo F, Pan K, Wu W and Chen H: High
glucose upregulates connective tissue growth factor expression in
human vascular smooth muscle cells. BMC Cell Biol. 8:12007.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Guha M, Xu ZG, Tung D, Lanting L and
Natarajan R: Specific down-regulation of connective tissue growth
factor attenuates progression of nephropathy in mouse models of
type 1 and type 2 diabetes. FASEB J. 21:3355–3368. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Qi W, Chen X, Poronnik P and Pollock CA:
Transforming growth factor-beta/connective tissue growth factor
axis in the kidney. Int J Biochem Cell Biol. 40:9–13. 2008.
View Article : Google Scholar : PubMed/NCBI
|