Introduction
Thrombotic thrombocytopenic purpura (TTP), which is
a rare life-threatening disorder characterized by thrombus
formation in small blood vessels, has an incidence of ~37,000 per
10 million people and is associated with a high mortality rate
(1). The signs and symptoms of TTP
are purple-colored bruises on the skin or mucous membranes,
small-sized red or purple dots on the skin, fatigue, fever,
increased heart rate or shortness of breath, headache, speech
changes, confusion, coma, stroke or seizure, and low levels of
protein in the urine. The main cause of this disease is low
activity levels of the ADAMTS13 enzyme and the low expression
levels of the ADAMTS13 gene, leading to blood clotting (2).
The most common treatment option is plasma exchange,
although other treatment options include medication and surgical
procedures, or occasionally both. The treatments are mostly carried
out in clinical settings. Plasma therapy is started immediately
following diagnosis of TTP. The frozen plasma is administered
through an intravenous injection in order for the inherited TTP to
replace the missing or altered ADAMTS13 enzyme. The plasma exchange
can also be used for acquired TTP as a life-saving procedure
(3). The plasma removes antibodies
from the blood that damage the ADAMTS13 enzyme or replaces the
ADAMTS13 enzyme. A cell separator is used, which removes plasma
from the blood. The non-plasma fraction of the blood is collected,
and the donated plasma is added. The blood is then placed into the
patient via intravenous administration. This procedure requires ~2
h to complete. The treatment is then continued until the signs and
symptoms of TTP improve. This may take days or weeks, depending on
the condition of the patient, and long-term hospitalization may be
required for complete recovery. Occasionally, a return of the
symptoms may occur either in hospital or when the patient has
returned home. In these cases plasma therapy is continued (4–7).
Alternative treatments are not always effective. For
acquired TTP, the medications used to treat TTP include
glucocorticoids, vincristine, rituximab and cyclosporine A.
Surgical removal of the spleen may occasionally be required as the
spleen is responsible for the formation of the antibodies that
inhibit ADAMTS13 enzyme activity (8).
Previous experimental studies and clinical
observations have implicated thrombin sensitive protein 1 (TSP1),
anti-endothelial cell antibodies (AECAs) and the excessive release
of von Willebrand factor (vWF) multimers in the pathogenesis of TTP
(8–11). Since the introduction of plasma
exchange (PE) in 1970s, the mortality rate associated with TTP has
gradually decreased from 90 to 10–20% (12). The present case reports describes two
patients with TTP who underwent PE in an intensive care unit (ICU)
in 2013 and recovered successfully.
Case reports
Case 1
A 56-year-old woman was admitted to the Liaocheng
People's Hospital (Liaocheng, China) on July 9, 2013 with fatigue,
a 7-day history of skin mucosal petechiae, and a 5-h history of
convulsions with delirium. A physical examination revealed that the
patient had a body temperature of 38.5°C, a heart rate of 108
beats/min, a respiratory rate of 23 breaths/min, and a blood
pressure of 117/96 mmHg. At the time of admission, the patient was
in a coma (Glasgow Coma Scale, 5) with multiple
ecchymoses/petechiae in the skin mucosal membrane over the entire
body. Blood tests on admission revealed the following: Hemoglobin
(Hb) level, 5.5 g/dl; platelet (PLT) count, 13×109/l;
total bilirubin, 46 µmol/l; and indirect bilirubin, 45 µmol/l. No
evident abnormality was observed by craniocerebral computed
tomography (CT; 64 slice CT scanner, Philips Healthcare, Andover,
MA, USA).
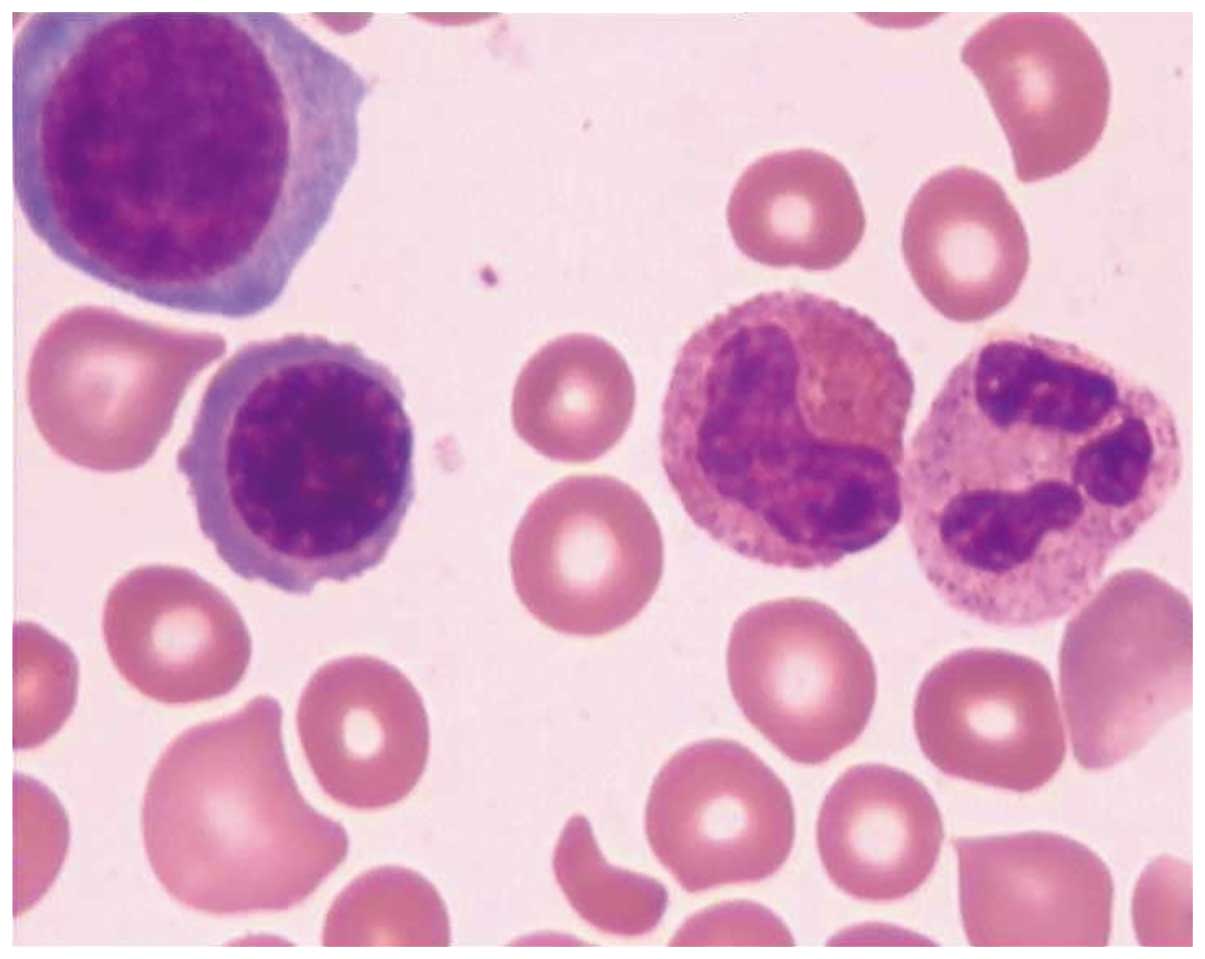
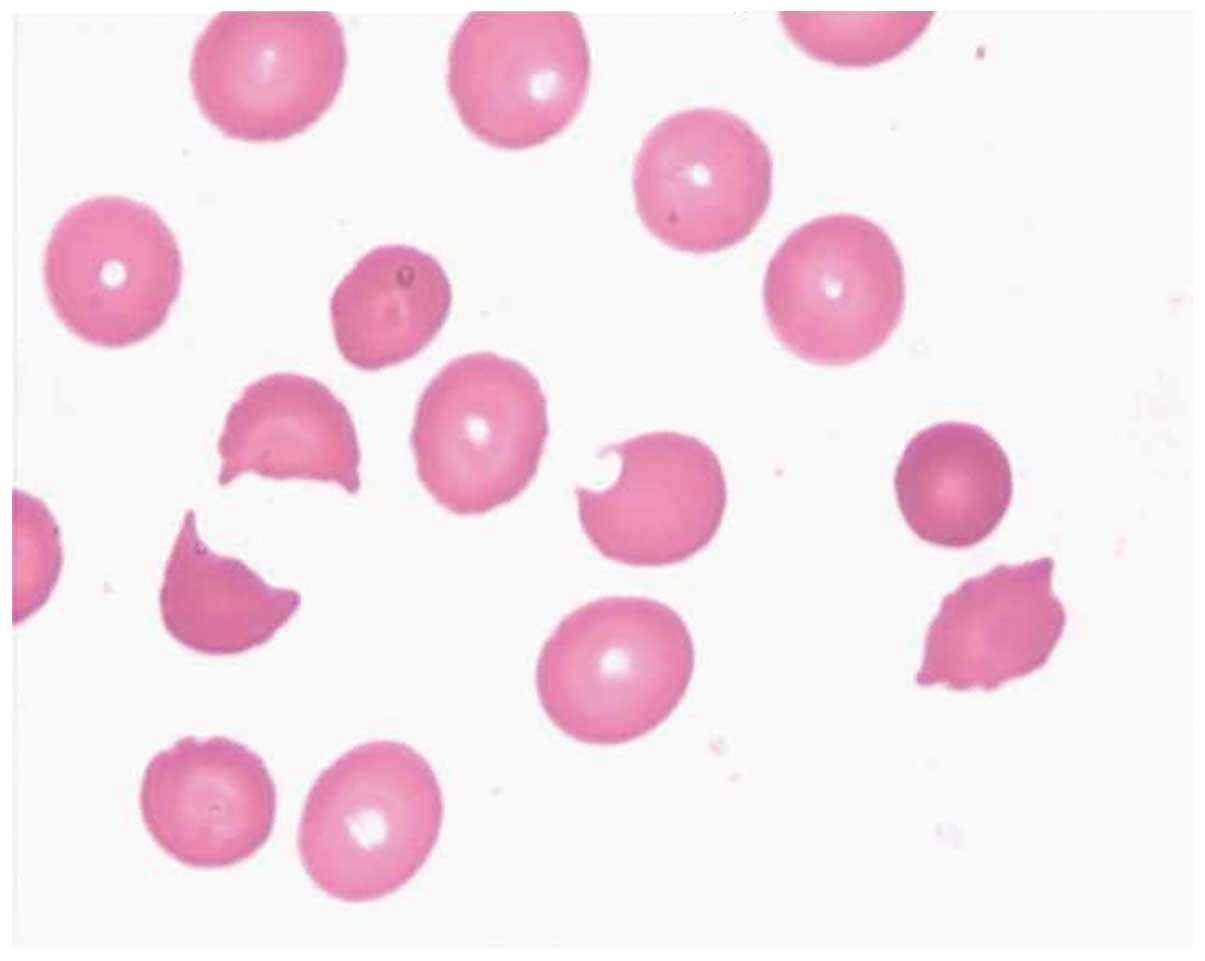
A bone marrow biopsy on July 10 indicated a
diagnosis of TTP and hemolytic-uremic syndrome (Figs. 1 and 2). The patient's urine tested positive for
protein, with 5% deformed red blood cells. The patient was
diagnosed with TTP and multiple organ dysfunction syndrome (MODS),
affecting the central nervous, respiratory and circulation systems.
Treatment of the patient with PE was recommended; however, it was
rejected by the family of the patient due to the high cost. On July
11, tracheal intubation and mechanical ventilation was administered
due to frequent convulsions and respiratory failure.
On July 15, the family provided consent and PE by
membrane plasma separation (MPS) was performed once daily with a
blood flow rate of 120 ml/min and a plasma extraction rate of 24
ml/min using Plasma Flux P2 dry plasma exchange filters (Fresenius
Medical Care Deutschland GmbH, Wendel, Germany). A total of 2,000
ml plasma was replaced. On July 16, the patient regained
consciousness and the mechanical ventilation was removed. Blood
tests revealed that the patient's Hb level was 5.9 g/dl and PLT
count was 10×109/l. On July 17, a B-mode ultrasound
system (Logiq E9; GE Heathcare Life Sciences, Shanghai, China)
detected thrombosis formation in the veins of the muscles in the
right lower extremity. On July 20, the coagulation mechanism of the
patient had been restored to normal with a Hb level of 6.4 g/dl and
a PLT count of 41×109/l.
Based on the relatively stable condition of the
patient, PE was performed daily in the hemodialysis room between
July 25 and August 7, after which PE treatment was ceased, since
the patient had regained a good mental condition and all indices
had been restored to within their normal ranges. The patient was
discharged from the hospital on August 27, 2013. At the follow-up
on September 30, 2013 the patient exhibited no reoccurrence with Hb
levels of 12.4 g/dl and PLT counts of 230×109/l.
Case 2
A 35-year-old woman was admitted to the Liaocheng
People's Hospital on September 18, 2013 with a 7 day history of
skin petechiae and ecchymoses. A physical examination revealed that
the patient had a body temperature of 37.5°C, a heart rate of 80
beats/min, a respiratory rate of 18 breaths/min and a blood
pressure of 120/80 mmHg. In addition, the patient exhibited clear
consciousness, appeared anemic and had light yellowish skin and
scattered petechiae and bleeding sites on the mucosal membrane. A
blood test on the date of admission recorded the following: Total
bilirubin, 74 µmol/l and indirect bilirubin, 69 µmol/l. On
September 19, 2003, the blood test showed a PLT count of
7×109/l, and the patient was intravenously treated with
16 U PLT.
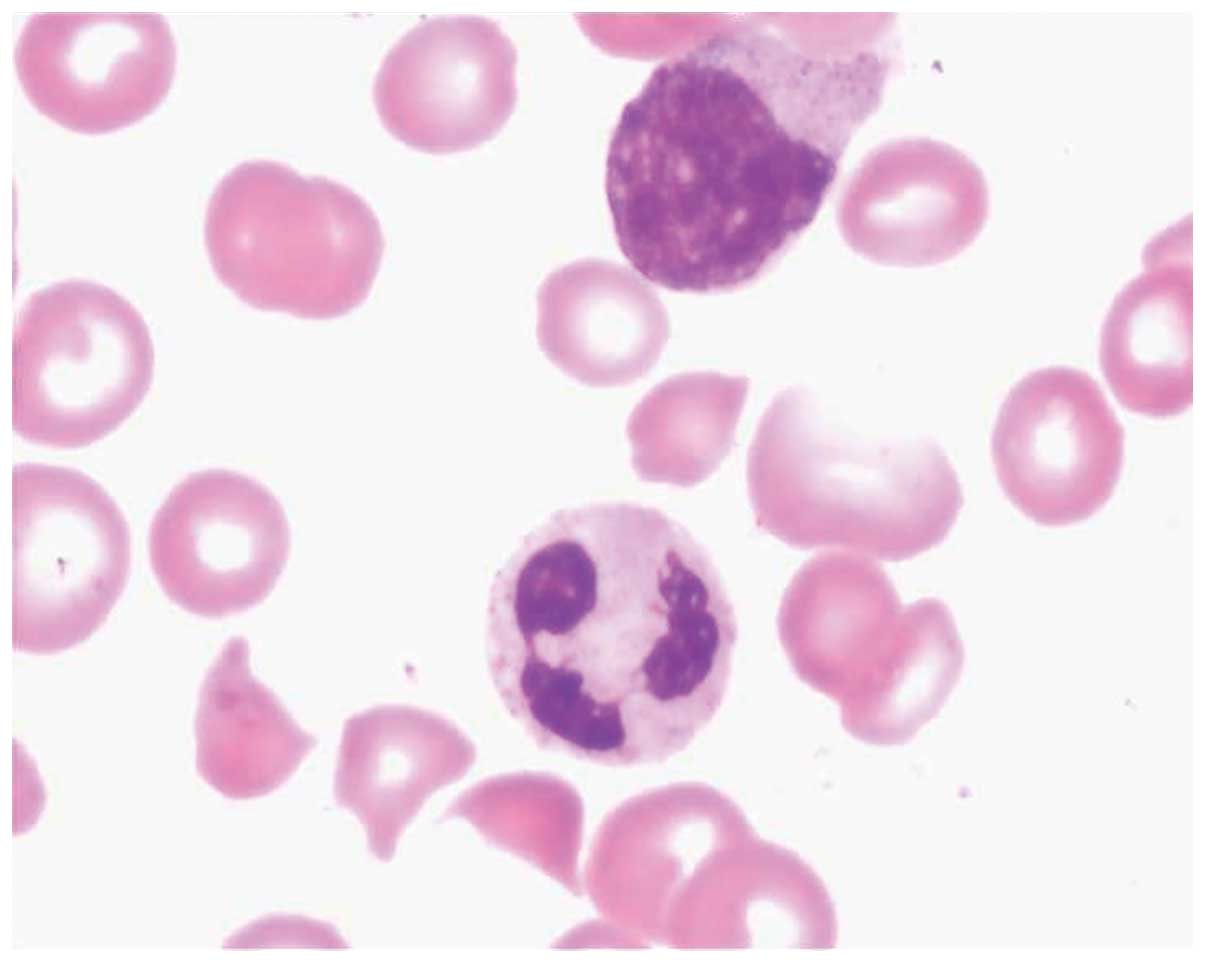
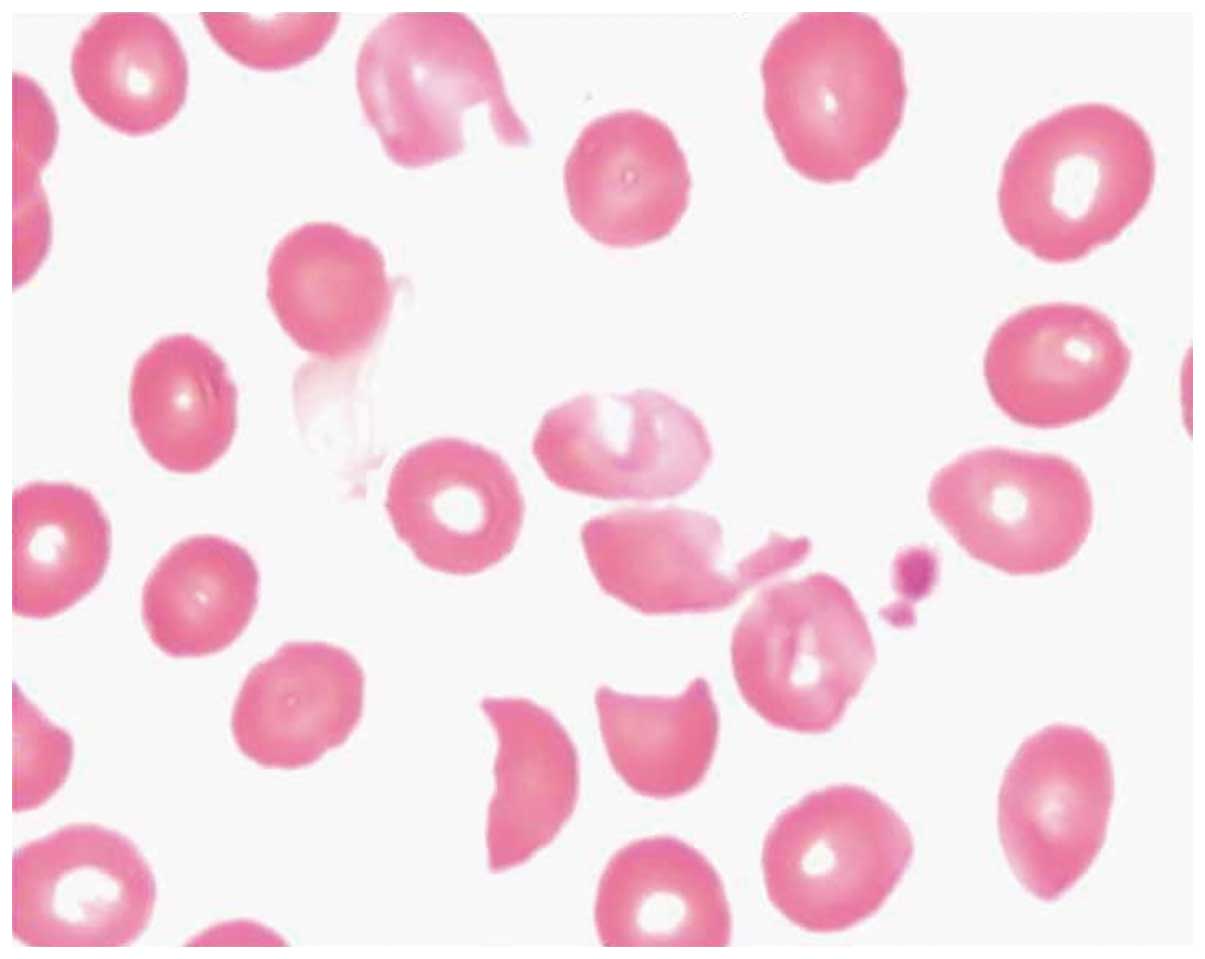
Cell morphological analyses of a bone marrow biopsy
taken on September 19, 2013 were conducted. The bone marrow was
aspirated from the posterior superior iliac spine, and 2 ml bone
marrow was obtained. Giemsa staining was performed on the bone
marrow, and detected abnormally formed erythrocytes, indicative of
TTP, reduced hyperplasia, two megakaryocytes, which were indicative
of normocytic anemia, and a reduced numbers of platelets (Figs. 3 and 4). The reticulocyte count was 10.99% and
the Coombs test was negative, indicating no autoimmune hemolysis.
An intravenous drip administering 200 mg methylprednisolone
anti-inflammatory treatment once daily was initiated on September
20 since the patient was positive for anti-Smith (++, diluted
>1:320) and anti-Sjögren's-syndrome-related antigen A
autoantibodies (+++, diluted >1:640) detected using an
IMTEC-ANA-LIA Profile (HUMAN Diagnostics, Wiesbaden, Germany), and
was suspected to have connective tissue disease, systemic lupus
erythematosus and possible secondary Evans syndrome.
The patient twice presented with paroxysmal numbness
and speech disorder on September 22. Although the condition eased
within 2 min without treatment, there was subsequent impairment in
cognition and speech. On September 23, 2003, the blood test showed
a PLT count of 8×109/l and Hb levels of 3.4 g/l, and the
patient was treated with 16 U PLT and 6 U washed red blood cells
via intravenous injection. The dose of methylprednisolone was
increased to 500 mg once daily, and 20% mannitol was administered
at 150 ml every 6 h in order to dehydrate the patient and reduce
intracranial pressure. On September 24, the patient exhibited
numbness in the left-side limbs, slow responses and drooping of the
mouth on the left side, with blood indices comprising an Hb level
of 54 g/l and PLT count of 10×109/l. However, acute
craniocerebral CT was unable to detect any apparent abnormality.
The patient was diagnosed with connective tissue disease and
systemic lupus erythematosus, with TTP as a complication.
Subsequently, the methylprednisolone dosage was reduced to 240 mg
once daily and the patient was transferred to the ICU for PE
treatment by MPS (blood flow, 120 ml/min; plasma extraction rate,
24 ml/min; 2,000 ml plasma was replaced) once daily, with the
consent of the family.
On September 26, the patient regained consciousness
but was exhausted. On September 27, the methylprednisolone dosage
was reduced to 120 mg once daily after a blood test detected a Hb
level of 3.4 g/dl and a PLT count of 33×109/l. On
October 1, blood tests revealed a Hb level of 9.7 g/dl and a PLT
count of 125×109/l. PE was performed once every 2 days
from October 2 and the methylprednisolone dosage was reduced to 60
mg once daily. On October 8, PE treatment was ceased and was
replaced with orally administered 30 mg prednisone three times a
day following a blood test that revealed a Hb level of 10.3 g/dl
and a PLT count of 138×109/l.
On October 14, a B-mode ultrasound indicated
thrombosis formation around the right femoral vein catheter. In
addition, the patient appeared to have recovered and was clearly
conscious, with liver function and blood coagulation functions
restored to within their normal ranges. The patient was discharged
on October 23, 2013. At the follow-up on November 25, 2013 the
patient exhibited no reoccurrence with Hb levels of 13 g/dl and PLT
counts of 183×109/l.
Discussion
At present, PE is a very important treatment for
TTP, and the mechanisms underlying its therapeutic effects include:
i) Scavenging abnormal vWF multimers released by endothelial cells,
ii) scavenging a disintegrin and metalloproteinase with
thrombospondin motifs-like 3 (ADAMTSl3) autoantibodies in the body,
iii) supplementing the vWF lyase ADAMTSl3, and restoring the normal
degradation of circulating vWF, iv) replenishing prostacyclin I in
plasma, v) scavenging abnormal antibodies in plasma, vi) scavenging
various cytokines that damage endothelial cells and activate
platelets, and vii) scavenging circulating inflammatory factors,
including tumor necrosis factor-α, interleukin (IL)-6 and IL-8 to
prevent MODS (13). Patients with
TTP rarely exhibit all the typical 5 symptoms of TTP, which
comprises fever, changes in the nervous system, kidney damage,
microvascular hemolytic anemia and consumptive reduction of
platelet aggregation (14).
Therefore, PE therapy should be administered as soon as possible
following the detection of significant reductions in platelet
numbers and microvascular hemolytic anemia, without any other clear
etiology. Due to financial constraints, the family of case 1 agreed
to PE therapy only on day 5 following hospital admission, on which
day the patient experienced continuous convulsions and was
comatose, requiring mechanical ventilation for respiratory failure.
Case 2 showed disturbances in consciousness on day 5 following
hospital admission, and underwent PE in the ICU after being
diagnosed with TTP on day 7. In these two patients, PE therapy was
initiated at a late stage; however, both regained consciousness
after three PE sessions. Mechanical ventilation and tracheal
intubation were removed from case 1 after two PE treatments, and
after seven PE sessions, the patient exhibited a markedly improved
mental status, was able to answer simple questions, and had an
increased PLT of 41×109/l. After 12 PE sessions, the
patient was stable and was transferred from the Hematology Unit to
the dialysis room to receive PE treatment once every 2 days for a
total of 23 PE treatments. Case 2 stopped having seizures after
regaining consciousness and her platelet counts were restored to
within the normal range after 10 PE sessions. Case 2 was
transferred from the Hematology Unit to the dialysis room to
receive PE treatment once every 2 days, prior to discharge from the
hospital. Both patients were in a serious condition upon admission
and thus their PE treatments were initiated in the ICU.
The ICU at the Liaocheng People's Hospital initially
performed blood purification procedures in 2004 to treat patients
with systemic inflammatory response syndrome, sepsis, all types of
intoxication and MODS. PE was performed for the first time in our
ICU for these two patients. It was found to be effective,
convenient and fast since the ICU staff were able to complete the
procedure independently without moving the patients. PE is a type
of blood purification technique, which involves extraction of
plasma using the MPS technique and its replacement with an equal
volume of fresh frozen plasma or human blood albumin, in order to
scavenge various metabolic toxins and pathogenic factors (15). PE filters differ from filters used in
other blood purification techniques; PE filters comprise hollow
fiber-type separators, prepared from cellulose acetate, poly(methyl
methacrylate) or polysulfone membranes (16). The present study employed a
hollow-fiber-type separator with a polysulfone membrane, and
achieved good efficacy in these two cases without reoccurrence
within 1 month of discharge from the hospital.
In conclusion, the present study successfully
treated two patients with severe TTP using PE, and in doing so
developed a strategy for the treatment of patients with severe
conditions who cannot be treated in the dialysis room. Furthermore,
this provides a novel concept for the treatment of patients with
TTP, rheumatic autoimmune disease, Guillain-Barre syndrome and
acute myelitis. However, special attention should be given to
complications associated with the long-term use of PE, including
systemic infection, partial or total blockage of catheters, low
blood pressure, venous thrombosis formation, and bleeding or
pneumothorax resulting from catheterization. The two patients in
the present study exhibited venous thrombosis in their lower limbs.
Case 1 showed venous thrombosis earlier and predominantly within
muscular veins instead of in the catheterized femoral vein;
therefore the venous thrombosis in the patient was likely
associated with the TTP. Conversely, case 2 exhibited venous
thrombosis 23 days following catheterization in the femoral vein
around the catheter. The patient was relatively stable at that time
with platelet counts within the normal range, thus suggesting that
the venous thrombosis was likely due to long-term
catheterization.
References
|
1
|
Allford SL, Hunt BJ, Rose P and Machin SJ:
Haemostasis and Thrombosis Task Force, British Committee for
Standards in Haematology: Guidelines on the diagnosis and
management of the thrombotic microangiopathic haemolytic anaemias.
Br J Haematol. 120:556–573. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Coppo P1, Wolf M, Veyradier A, Bussel A,
Malot S, Millot GA, Daubin C, Bordessoule D, Pène F, Mira JP, et
al: Prognostic value of inhibitory anti-ADAMTS13 antibodies in
adult-acquired thrombotic thrombocytopenic purpura. Br J Haematol.
132:66–74. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Rock GA: Management of thrombotic
thrombocytopenic purpura. Br J Haematol. 109:496–507. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
O'Connor NT, O'Shea MJ and Hill LF:
Vincristine for thrombotic thrombocytopenic purpura. Lancet.
340:4901992. View Article : Google Scholar
|
|
5
|
Durand JM, Lefevre P, Kaplanski G, Telle H
and Soubeyrand J: Vincristine for thrombotic thrombocytopenic
purpura. Lancet. 340:977–978. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Durand JM, Lefevre P, Kaplanski G and
Soubeyrand J: Ineffectiveness of high-dose intravenous
gammaglobulin infusion in thrombotic thrombocytopenic purpura. Am J
Hematol. 42:2341993. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Udvardy M and Rak K: Cyclophosphamide for
chronic relapsing thrombotic thrombocytopenic purpura. Lancet.
336:1508–1509. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Schneppenheim R and Budde U: von
Willebrand factor: The complex molecular genetics of a multidomain
and multifunctional protein. J Thromb Haemost. 9(Suppl 1): 209–215.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Motto DG, Chauhan AK, Zhu G, Homeister J,
Lamb CB, Desch KC, Zhang W, Tsai HM, Wagner DD and Ginsburg D:
Shigatoxin triggers thrombotic thrombocytopenic purpura in
genetically susceptible ADAMTS13-deficient mice. J Clin Invest.
115:2752–2761. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zheng XL, Kaufman RM, Goodnough LT and
Sadler JE: Effect of plasma exchange on plasma ADAMTS13
metalloprotease activity, inhibitor level and clinical outcome in
patients with idiopathic and nonidiopathic thrombotic
thrombocytopenic purpura. Blood. 103:4043–4049. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gadisseur A, Hermans C, Berneman Z,
Schroyens W, Deckmyn H and Michiels JJ: Laboratory diagnosis and
molecular classification of von Willebrand disease. Acta Haematol.
121:71–84. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bandarenko N and Brecher ME: United States
Thrombotic Thrombocytopenic Purpura Apheresis Study Group (US TTP
ASG): Multicenter survey and retrospective analysis of current
efficacy of therapeutic plasma exchange. J Clin Apher. 13:133–141.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yao LQ, Jin ZC, Ji MS, Xia CY, Yu ZX, Liu
J, Hu XL and Yan J: Effect of continuous renal replacement therapy
started at different time on patients with multiple organ
dysfunction syndrome. Zhonghua Yi Xue Za Zhi. 91:1663–1667.
2011.(In Chinese). PubMed/NCBI
|
|
14
|
Sadler JE, Moake JL, Miyata T and George
JN: Recent advances in thrombotic thrombocytopenic purpura.
Hematology Am Soc Hematol Educ Program. 2004.407–423. PubMed/NCBI
|
|
15
|
Paton E and Baldwin IC: Plasma exchange in
the intensive care unit: A 10 year retrospective audit. Aust Crit
Care. 27:139–44. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Maruyama Y, Yoshida H, Uchino S, Yokoyama
K, Yamamoto H, Takinami M and Hosoya T: Nafamostat mesilate as an
anticoagulant during continuous veno-venous hemodialysis: A
three-year retrospective cohort study. Int J Artif Organs.
34:571–576. 2011. View Article : Google Scholar : PubMed/NCBI
|