Introduction
High mobility group box 1 (HMGB1) protein is a
highly conserved DNA-binding protein that was named according to
its high gel mobility during electrophoresis (1,2). HMGB1
is a highly conserved DNA-binding protein that mediates cytokine
effects and, as a result, modulates gene transcription, stabilizes
nucleosomes, promotes inflammatory responses, and regulates neural
growth and tumor metastasis (3–6). In bone
fractures, HMGB1 was found to be released into the extracellular
environment by active secretion from stimulated cells as well as by
passive discharge from necrotic cells (7).
Mesenchymal stem cells (MSC) are multipotent stromal
cells that can be induced to differentiate into various types of
mesenchymal tissues in specific in vivo environments. MSCs
possess several stem cell characteristics, including self-renewal,
pluripotency and homing, therefore they are considered the
predominant source of stem cells for fracture restoration.
Migration and osteogenic differentiation of MSCs has a critical
role during the partial coalescence of fractures (8–10). It is
also widely known that MSCs are capable of secreting various types
of cytokines, including stem cell factor (SCF), thrombopoietin and
interleukin-6 (11,12). These cytokines regulate stem cell
differentiation, direct migration and mediate inflammatory
processes (13–15). Therefore, cytokines secreted from
MSCs may affect fracture coalescence. However, in an inflamed
environment, such as during partial coalescence of fractures, the
stimulation of inflammatory factors may alter the concentration and
type of cytokines secreted from MSCs (16). This phenomenon may directly affect
various cytokine-dependent biological processes and subsequently
modify the characteristics and functions of MSCs and related cells.
HMGB1 is ubiquitously present in the inflamed microenvironment of
fractures and is considered to be a pro-inflammatory cytokine
(17,18). Therefore, we hypothesized that,
consistent with other inflammatory factors, HMGB1 may affect
cytokine secretion from MSCs.
In the present study, antibody array assays were
performed to detect cytokine secretion from MSCs upon HMGB1
stimulation. As certain cytokines were differentially secreted from
MSCs upon the treatment with HMGB1, the roles of these cytokines
were analyzed in an attempt to elucidate the overall effects of
HMGB1 on the biological functions of MSCs. Although the promoting
actions of HMGB1 on the MSC osteogenic differentiation have
previously been reported (19), the
detailed mechanisms of these effects are yet to be investigated and
elucidated. Therefore, the aim of the present study was to
elucidate the mechanisms underlying the effects of HMGB1 on MSCs
using antibody array analysis. These results may provide a basis
for developing novel approaches in bone fracture-healing
therapy.
Materials and methods
Reagents
MSCs and basal culture medium were purchased from
Cyagen Biosciences, Inc., (Santa Clara, CA, USA). Recombinant human
HMGB1 protein and fetal bovine serum were purchased from
Sigma-Aldrich (St. Louis, MO, USA). Ras inhibitor (Selleckchem,
Houston, TX, USA), which was a transferase inhibitor for H-Ras and
K-Ras, was used at a concentration of 5 µM.
Isolation and culture-expansion of
human bone marrow MSCs
Adherent MSCs were trypsinized and passaged once
cell confluence reached ~80%. Cells at passage 3–5 were used in the
present experiments.
Assays for osteogenic
differentiation
To induce osteogenic differentiation, MSCs were
cultured in basal culture medium supplemented with fetal bovine
serum (FBS).
Total RNA extraction and reverse
transcriptase-quantitative polymerase chain reaction (RT-qPCR)
To observe MSC differentiation following exposure to
HMGB1, MSCs were cultured in basal culture medium or 25 ng/ml
HMGB-1 for 5 days. Total RNA was extracted with TRIzol reagent
(Thermo Fisher Scientific, Inc., Waltham, MA, USA) according to the
manufacturer's protocol. RT-qPCR was performed to observe the
expression of osteoblastic markers using a StepOne Plus Real-Time
PCR system with SYBR Green (Roche Diagnostics, Basel, Switzerland)
as a double-strand DNA-specific binding dye. Primer sequences were
as follows: Osteocalcin (OCN), forward 5′-AAGCAGGAGGGCAATAAGGT and
reverse, CAAGCAGGGTTAAGCTCACA; and GAPDH, forward
5′-CGTCCCGTAGACAAAATGGT and reverse 5′-GGCTGGTGGTCCAGGGGTCT (Sangon
Biotech Co., Ltd., Shanghai, China). According to the
manufacturer's protocol, DNA hydrolase was used to remove genomic
DNA. The reaction mixture included buffer (5 µl), dNTP (4 µl),
primer (4 µl), Taq (1 µl), sample (1 µl), SYBR Green (1 µl) and
ddH2O to make a total volume of 50 µl. Thermal cycling
conditions were as follows: 95°C for 30 sec and 40 cycles of 95°C
for 5 sec and 60°C for 35 sec. Relative target gene expression
levels were calculated according to the 2-ΔΔCq method
(20). All PCR reactions were
performed in triplicate. mRNA quantification of the target genes
and the GAPDH housekeeping gene was performed in separate
tubes.
Alkaline phosphatase (ALP) staining
and activity assay
MSCs (104 cells/cm2) were seeded into
24-well plates and cultured in basal culture medium. After one
week, ALP activity was assessed using a BCIP/NBT ALP color
development kit (Beyotime Institute of Biotechnology, Haimen,
China), according to the manufacturer's protocol. For the
quantitative determination of ALP activity, the MSCs were incubated
with p-nitrophenol phosphate (Beyotime Institute of Biotechnology)
as a substrate, washed with PBS buffer and lysed with 0.1% Triton
X-100 in 10 mM Tris HCL, (pH 9.0). Absorbance was determined at 405
nm using a microplate reader and compared with p-nitrophenol
standard titration curve. Data were presented as the mean ±
standard deviation (n=3).
Western blotting
Cells were harvested and lysed in RIPA buffer
(Cyagen Biosciences, Inc.) supplemented with protease inhibitors.
Following concentration determination (5.534 µg/µl), protein
samples (60 µg) were separated by 10% sodium dodecyl
sulfate-polyacrylamide gel electrophoresis and transferred to a
polyvinylidene difluoride membrane by western blotting. Membranes
were blocked in 5% skim milk for 1 h and incubated with antibodies
against GTP-Ras (cat. no. 16117; 1:500; Active Ras Detection kit;
Thermo Fisher Scientific, Inc.), Ras (cat. no. ab52939; Abcam,
Cambridge, UK), extracellular-signal-regulated kinases (ERK; cat.
no. ab196883; 1:500; Abcam), phosphorylated (p-)ERK (cat. no.
ab65142; 1:500; Abcam), p38 (cat. no. 9212; 1:1,000; Abcam), p-p38
(cat. no. 9215; 1:1,000; Abcam) and β-actin (cat. no. dc-130301;
1:2,000; Santa Cruz Biotechnology, Inc., Dallas, TX, USA),
respectively, at 4°C overnight. Following incubation with
horseradish peroxidase-conjugated goat anti-rabbit secondary
antibodies (cat. no. 31210; 1:5,000; Thermo Fisher Scientific,
Inc.), immunoreactive proteins were visualized using enhanced
chemiluminescence reagent (Thermo Fisher Scientific, Inc.).
Relative quantification of the bands was performed using ImageJ
software (version 5.0; National Institutes of Health, Bethesda, MA,
USA).
Antibody arrays
Soluble proteins in the medium of the stromal cell
lines were measured using the Human Cytokine Array G1000
(AAH-CYT-G1000; RayBiotech Inc., Norcross, GA, USA), according to
the manufacturer's protocol. These arrays can detect 120 proteins,
respectively. Stromal cells were plated n Dulbecco's modified Eagle
medium (DMEM) supplemented with 10% FBS 3 days prior to the
experiment and were 75–90% confluent when the media were collected
and filtered. DMEM containing 10% FBS was also hybridized to the
arrays and used for normalization. Ten technical and biological
replicates were performed, and both exhibited a high correlation
(correlation coefficient, >0.9) (data not shown). Hybridization
was performed overnight at 4°C. All slides were scanned using a
Gene Pix 4000B Microarray Scanner (Molecular Devices, LLC,
Sunnyvale, CA, USA) and analyzed using Gene Pix Pro 6.0 software.
The median signal score was averaged across the triplicates on each
array. Results were subsequently normalized using internal controls
by subtracting the values for the control DMEM supplemented with
10% FBS.
Statistical analysis
Statistical significance was determined using the
two-tailed Student's t-test, assuming equal variances. The
χ2 test was used to compare rates. SPSS version 17.0
(SPSS, Inc., Chicago, IL, USA) was used to analyze the data.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Effects of HMGB1 on cytokine secretion
from MSCs
To determine the influence of HMGB1 stimulation on
human MSCs at the protein level, antibody arrays were performed.
The same three groups were stimulated with 25 ng/ml HMGB1 and
compared with non-stimulated cultures (Fig. 1A). In line with the search criteria
for differential secretion, which were >1.5-fold induction or
repression and P<0.05, bioinformatic data analysis detected 10
cytokines which were differentially secreted between the groups.
Increased cytokine secretion was detected for insulin-like growth
factor binding protein 4, macrophage colony stimulating factor
(M-CSF), eotaxin-3, epidermal growth factor receptor (EGFR),
vascular endothelial growth factor (VEGF), placental growth factor,
angiopoetin-2, chemokine C-C motif ligand 5 (CCL-5), urokinase
plasminogen activator receptor and macrophage migration inhibitory
factor following induction with HMGB1 (Figs. 1B and C). These results suggest that
HMGB1 promotes the secretion of multiple cytokines from MSCs.
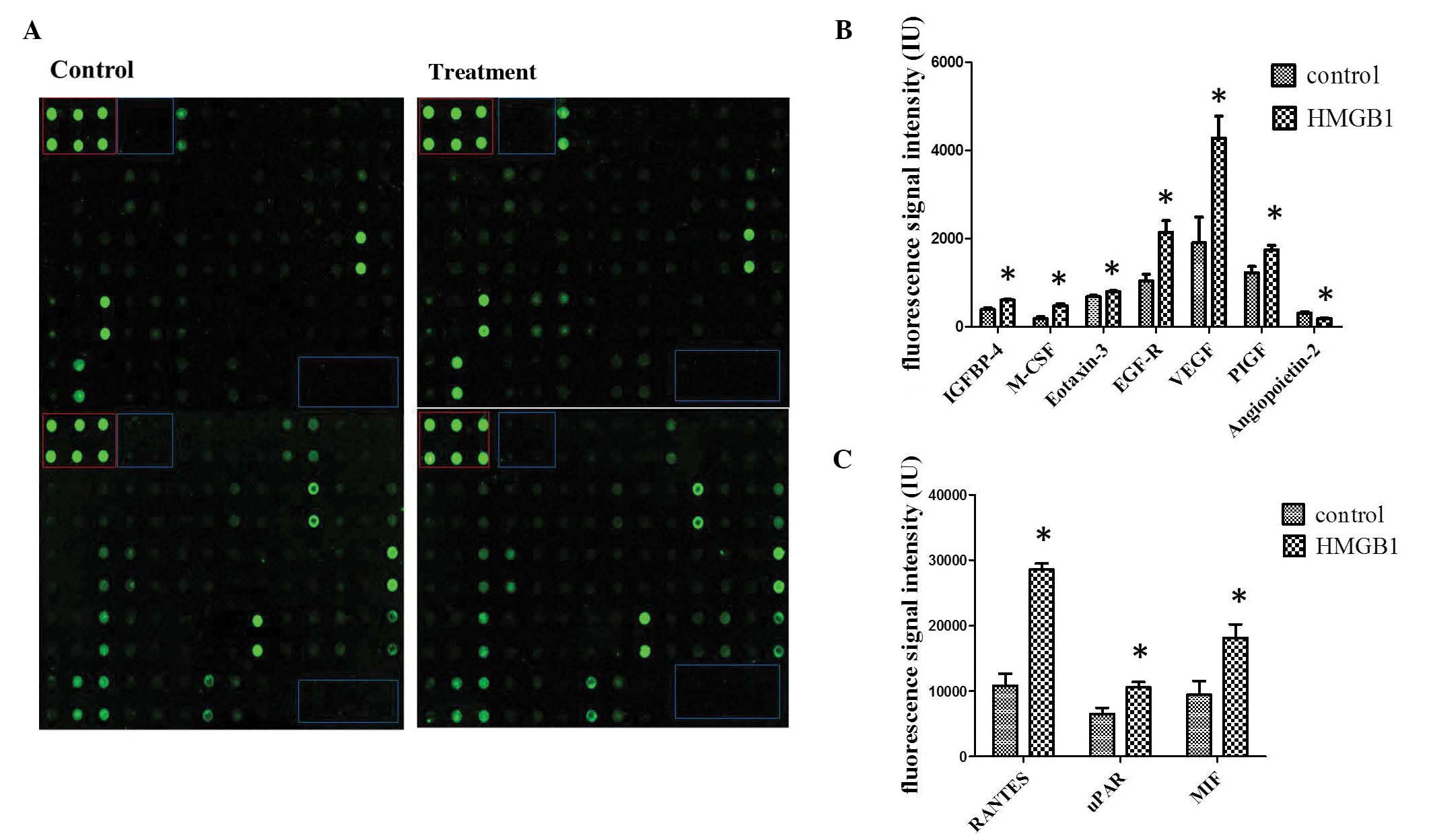 | Figure 1.HMGB1-induced cytokine secretion. (A)
Secretion of cytokines in cell culture supernatant was measured
using antibody array analysis. The blue box indicates the negative
controls, whereas the red box denotes the positive controls. Spots
were measured densitometrically. Following subtraction of the
negative control and normalization to the positive control, the
spots of stimulated MSCs were compared to the corresponding spots
of the unstimulated MSCs. (B and C) Quantification of antibody
array results for cytokines with increased secretion following
HMGB1 stimulation. *P<0.05 vs. the control. IGFBP, insulin-like
growth factor binding protein; M-CSF, macrophage colony stimulating
factor; EGFR, epidermal growth factor receptor; VEGF, vascular
endothelial growth factor; PIGF, placental growth factor; CCL-5,
chemokine C-C motif ligand 5; uPAR, urokinase plasminogen activator
receptor; MIF, macrophage migration inhibitory factor; HMGB1, high
mobility group box 1. |
Functions of the differentially
secreted cytokines from MSCs
Hierarchical clustering of these 10 cytokines was
performed with normalized cytokines secretion values for the two
distinct groups: the HMGB1-treated group and the non-stimulated
control group (Fig. 2). Six
differentially secreted cytokines were visualized as induced, as
indicated by red, and four cytokines were repressed, as indicated
by green.
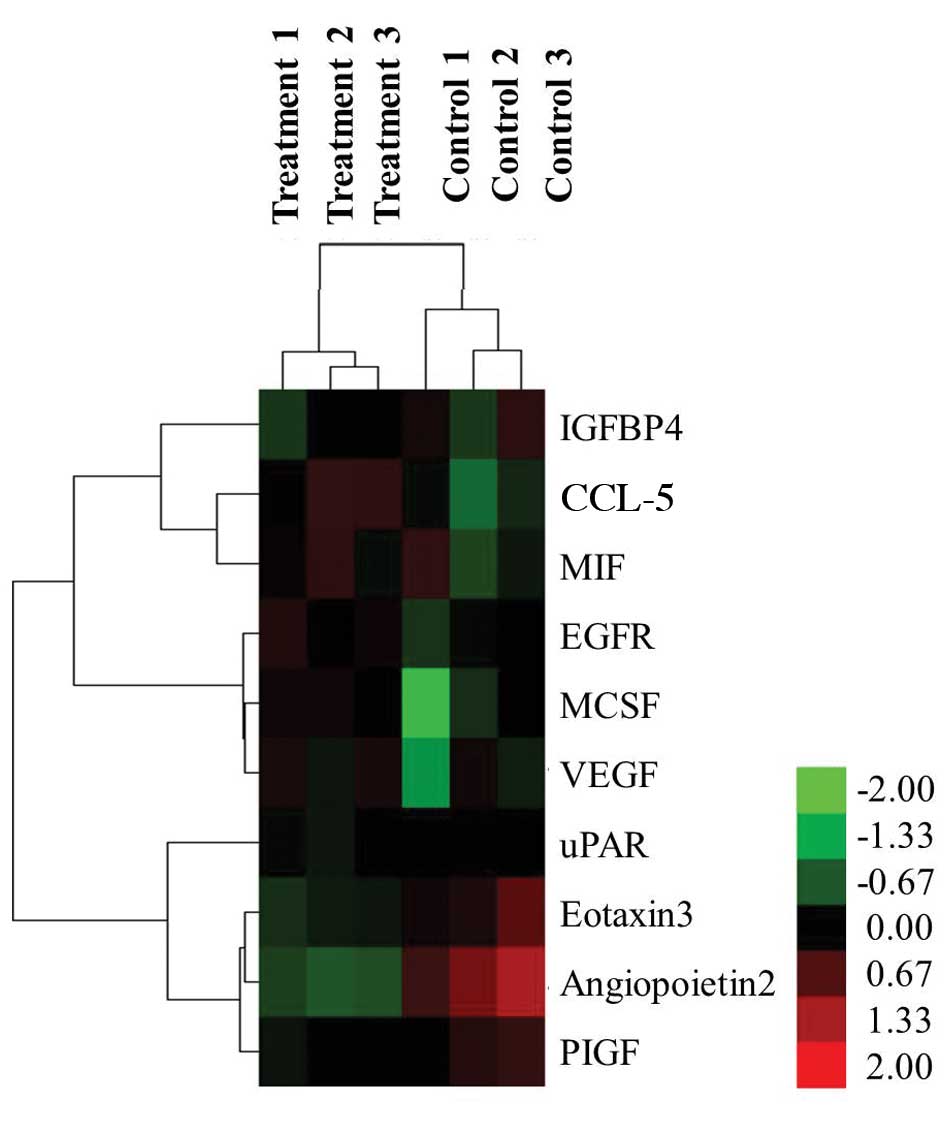 | Figure 2.Hierarchical cluster analysis.
Differentially secreted cytokines, which were defined as a
>1.5-fold induction from control, were clustered according to
their secretion (control or HMGB1-induced). Six HMGB1-induced
cytokines (red) and four repressed cytokines (green) were detected.
HMGB1, high mobility group box 1; IGFBP, insulin-like growth factor
binding protein; CCL-5, chemokine C-C motif ligand 5; MIF,
macrophage migration inhibitory factor; EGFR, epidermal growth
factor receptor; M-CSF, macrophage colony stimulating factor; VEGF,
vascular endothelial growth factor; uPAR, urokinase plasminogen
activator receptor; PIGF, placental growth factor. |
Notably, among the six induced cytokines, three
cytokines, EGFR, M-CSF and VEGF, were associated with Ras
signaling, and three cytokines were involved in signal
transduction: EGF-R, CCL-5 and VEGF. These 10 differentially
secreted cytokines have also been associated with cell development
(5), the regulation of growth
(5) and cell migration (8), response to stress (12) and immune system processes (9) according to the Gene Ontology database
(http://amigo.geneontology.org/amigo)
for annotation, visualization and integrated discovery. Various
differentially secreted cytokines were associated with more than
one biological process.
Some of the differentially secreted cytokines
detected in the present study also have a role in other molecular
and cellular functions, such as cellular growth (such as M-CSF) and
proliferation (such as macrophage migration inhibitory factor),
cell morphology (such as RANTES) and cellular development (such as
placental growth factor). Based on the 10 differentially secreted
cytokines, the cytokine-cytokine receptor interaction pathway
(0460hsa in KEGG) was demonstrated to be the dominant pathway that
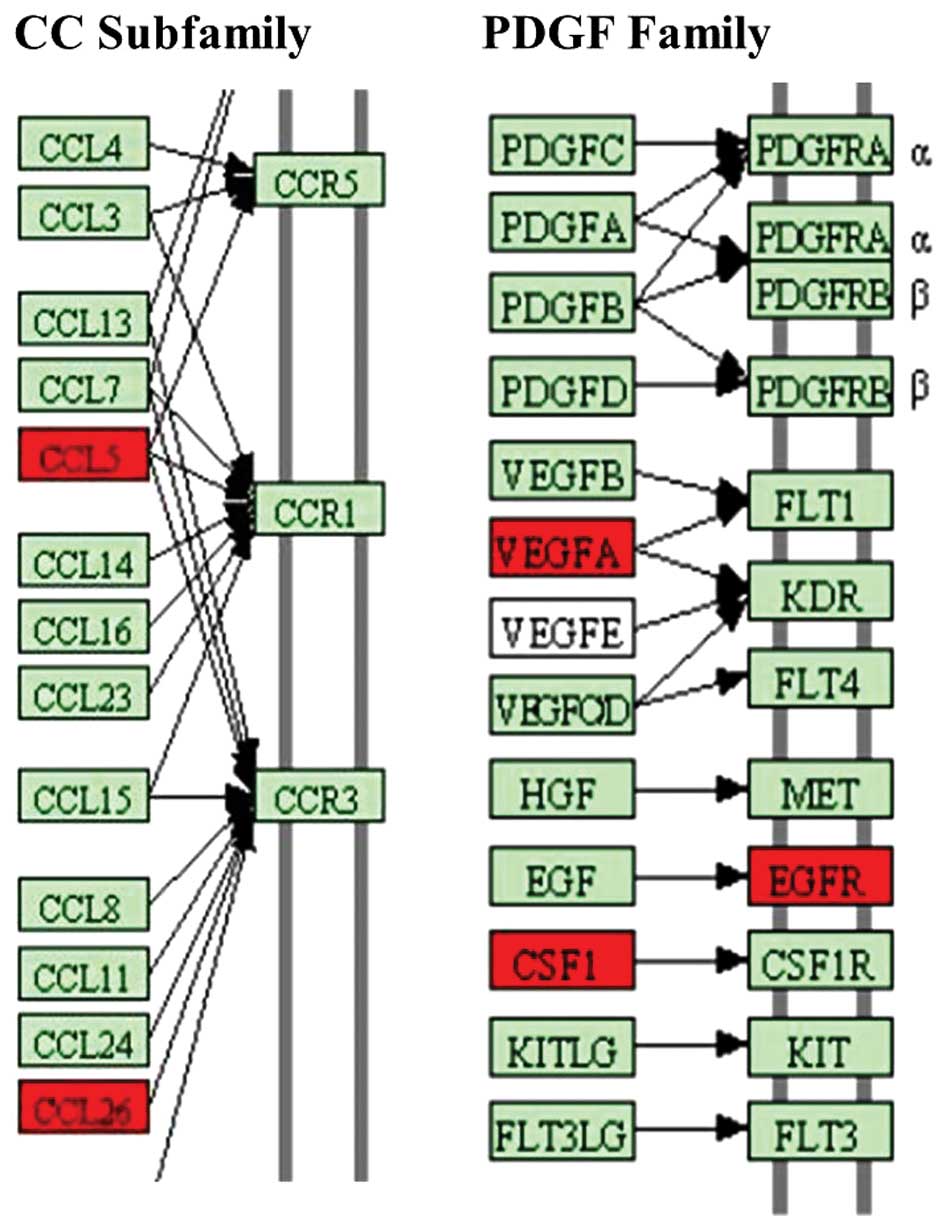
was influenced by HMGB1. Here, the secretion of CCL5, CCL26, VEGFA
and CSF1 was induced (Fig. 3). The
results demonstrate that the differentially secreted cytokines
serve an important role in the biological function of MSCs.
HMGB1-treated MSCs activate Ras/MAPK
pathway to promote MSC osteogenic differentiation
Antibody array assay analysis demonstrated that the
expression of EGFR was markedly increased in the MSCs following
stimulation with HMGB1. It has previously been reported that the
interaction between EGF and EGFR activates Ras/MAPK signaling
pathway (21). To investigate
whether Ras/MAPK signaling pathway was activated in MSCs, the
protein levels of GTP-Ras, p-p38 and p-ERK were determined in the
control and HMGB1-treated MSCs by western blotting. HMGB1 treatment
induced a marked increase in the protein expression levels of
GTP-Ras and this effect was sensitive to incubation with the Ras
inhibitor. Notably, HMGB1 treatment increased the expression levels
of p-p38 and p-ERK and this effect was sensitive to the incubation
with the Ras inhibitor. These results suggests that HMGB1 activates
the Ras signaling pathway and subsequently stimulates the p38 and
ERK pathways of MSCs (Fig. 4A).
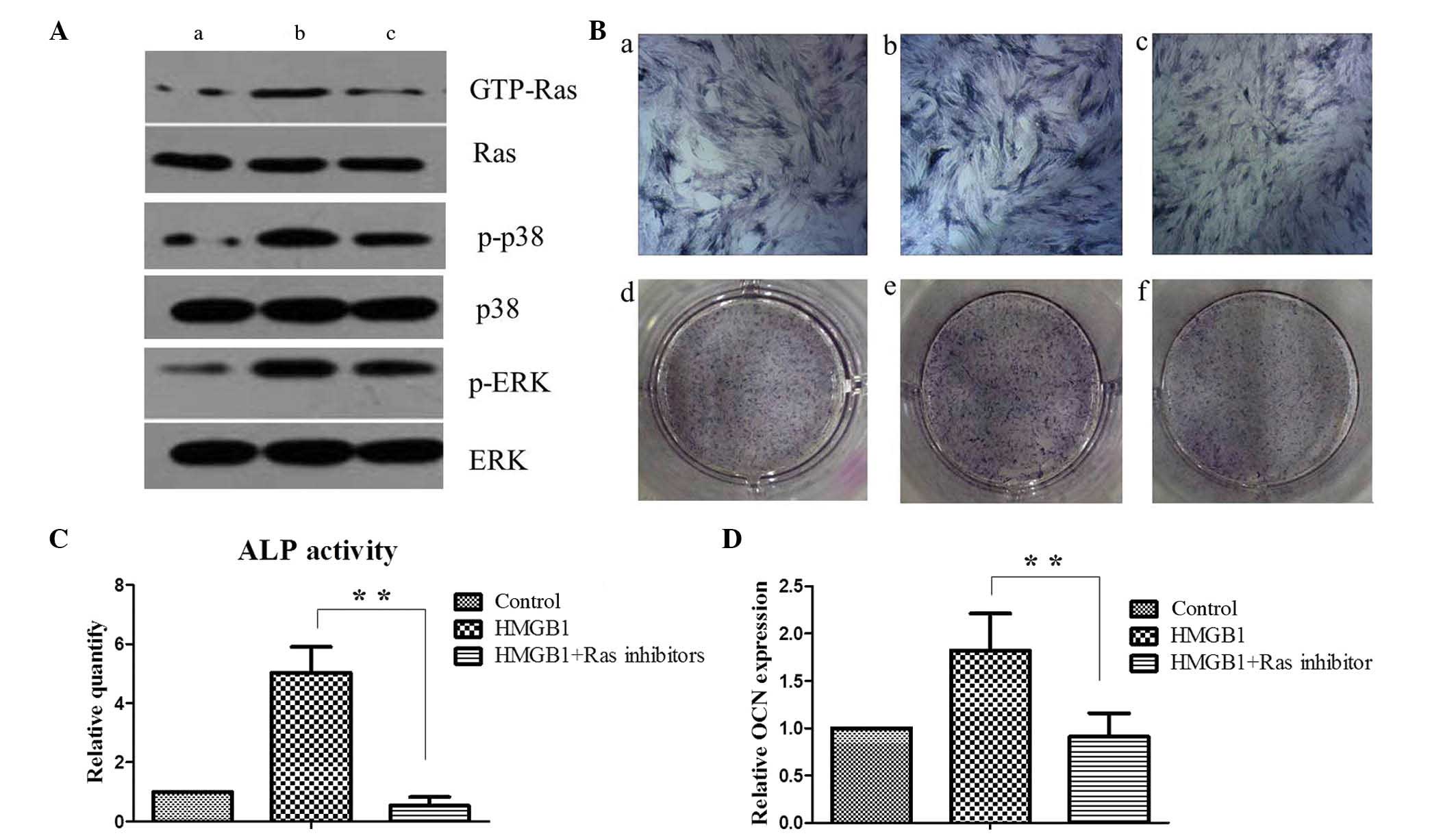 | Figure 4.Ras activation is required for p38 and
ERK activation and MSC osteogenesis. (A) Western blot analysis of
Ras, p38, and ERK signaling activity was performed on (a) MSCs
maintained in the basal medium alone, (b) with 25 ng/ml HMGB1 or
(c) with 25 ng/ml HMGB1 and 5 µM Ras inhibitor. (B) On day 5, ALP
staining was performed on (a and d) MSCs maintained in the basal
medium alone, (b and e) with 25 ng/ml HMGB1 or (c and f) with 25
ng/ml HMGB1 and 5 µM Ras inhibitor. (C) Quantification of ALP
activity among the groups. (D) Expression levels of the late
osteogenesis marker OCN were determined by reverse
transcription-quantitative polymerase chain reaction analysis in
MSCs upon HMGB1 and/or Ras inhibitor treatment as
indicated.**P<0.01. HMGB1, high mobility group box 1; GTP,
guanosine triphosphate; p-, phosphorylated; ERK, extracellular
signal-regulated kinase; MSC, mesenchymal stem cell; ALP, alkaline
phosphatase; OCN, osteocalcin. |
ALP staining and quantification of the expression of
the late OCN was performed in MSCs. ALP activity and OCN gene
expression levels were significantly higher in the HMGB1-stimulated
MSCs, as compared with the control cells (P<0.01). Furthermore,
as compared with the results in the HMGB1-induced group, the
upregulation of ALP activity and OCN expression in MSCs was
significantly reduced following Ras inhibitor treatment (P<0.01;
Figs. 4B-D). These results suggest
that HMGB1 potentiates the osteogenic differentiation of MSCs
through the Ras/MAPK signaling pathway.
Discussion
Antibody array assays were performed in the present
study to investigate cytokine secretion from HMGB1-stimulated MSCs,
in order to elucidate the effects of HMGB1 on MSC functions. The
results of the present study indicated that HMGB1 promotes the
secretion of various cytokines by MSCs. According to the Gene
Ontology database for annotation, these secreted cytokines
participate in various biological processes, including cell
development, cell growth and migration, stress responses and immune
system functions.
Among the cytokines secreted upon HMGB1 treatment in
the present study, CCL5 and CCL26 are prominent chemocytokines that
belong to the CC subfamily. The CC subfamily, which is also known
as the β-chemokine subfamily, is the largest chemokine family, the
members of which mainly act on monocytes and lymphocytes (22). In the fracture microenvironment, the
secretion of CCL5 and CCL26 by HMGB1-stimulated MSCs may promote
the migration of immune cells to tissues affected by the fracture
sites, stimulating inflammatory responses and immune reactions
(23–25). M-CSF, which is also known as human
macrophage-specific colony-stimulating factor (CSF-1), is one of a
few cytokines which are directly involved in osteoclastogenesis,
osteoclast proliferation and differentiation (26,27).
VEGF is a specific heparin-binding growth factor in vascular
endothelial cells, which can induce angiogenesis in vivo
(28). It has previously been
reported that VEGF could induce osteoclastogenesis (29), and the results of the present study
indicate that VEGF and M-SCF are essential for the occurrence of
osteoclastogenesis. During fracture restoration, the secretion of
VEGF and M-SCF by MSCs was enhanced upon the HMGB1 treatment,
leading to an increase in the cell number and activity osteoclasts.
Although osteoclast cell activation will delay the coalescence of
fractures during the early phase, it will facilitate reparative
processes on the osteoblast surface during the late phase (30,31).
Therefore, further studies are necessary to investigate the effects
of the HMGB1-promoted cytokine secretion by MSCs on osteoclast
cells in detail. Such studies may provide a novel theoretical basis
for improving fracture therapy.
Notably, EGFR expression levels were was markedly
enhanced by HMGB1 stimulation in the present study. EGF is a
polypeptide that belongs to the EGF superfamily and functions as a
mutifunctional cytokine that can promote cell division and
proliferation in vivo (32).
The interaction between EGF and EGFR activates the Ras protein,
which mediates the downstream signal transduction of various
cytokines (33). Ras signaling
regulates multiple downstream signaling pathways, which are
predominantly implicated in cell proliferation and differentiation
(34). It is widely accepted that
osteogenesis depends on the activation of various key signaling
pathways and that Ras/MAPK pathway regulates osteogenic
differentiation of stem cells (35).
Although the function of HMGB1 in osteogenic differentiation of
MSCs has been established previously, the underlying mechanisms of
HMGB1 effects remain unknown. Therefore, we hypothesize that HMGB1
may promote osteogenic differentiation of MSCs via the activation
of Ras/MAPK signaling pathway in MSCs.
In the present study, it was demonstrated that the
levels of GTP-bound Ras were increased upon HMGB1. It is known that
only GTP-bound Ras interacts with Raf-1 kinase and stimulates the
latter to initiate the MAPK signaling cascade (36). Because the level of GTP-bound Ras
represents the activation of Ras (37), it is possible to conclude that Ras
signaling activity may be activated by HMGB1 treatment. Similarly,
the phosphorylation levels of p38 and ERK were upregulated in
HMGB1-treated MSCs. These observations suggest that p38 and ERK
signaling pathways were also activated as a result of Ras
activation. An inhibitor of Ras was applied to block its activity
in order to verify our hypothesis that p38 and ERK may be
stimulated via the activated Ras signaling pathway. As
hypothesized, the activation of ALP and the expression of OCN
promoted by HMGB1 treatment were remarkably downregulated following
Ras inhibitor treatment. These results demonstrated that the
osteogenic differentiation of MSCs stimulated by HMGB1 is sensitive
to Ras inhibition. Moreover, the upregulation of the expression
levels of p-p38 and p-ERK upon treatment with HMGB1 were also
reduced by the inhibition of Ras, suggesting that p38 and ERK
pathways are suppressed when Ras activity is blocked.
On the basis of these observations, we conclude that
HMGB1 activates the Ras signaling pathway and subsequently
stimulates the p38 and ERK pathways to promote the osteogenic
differentiation of MSCs. It is important to note that the
activation of the Ras/MAPK signaling pathway is only one of various
mechanisms affecting the HMGB1-induced osteogenic differentiation
of MSCs, as numerous downstream signaling pathways are regulated by
Ras signaling, including the Rap1 and PI3K-AKT cascades (38,39).
Therefore, the identity of the pathway(s) affected by the
activation of Ras upon HMGB1 treatment and their roles in the
osteogenic differentiation of MSCs should be investigated in future
studies. In addition to exocrine cytokines, MSCs also synthesize
endocrine proteins which regulate numerous biological processes
(40), and it remains to be
elucidated whether HMGB1 affects the endocrine protein synthesis.
Therefore, future studies should focus on investigating
differentially expressed endocrine proteins in HMGB1-stimulated
MSCs to gain further insight into the mechanisms of regulation of
MSC functions by HMGB1.
Acknowledgements
The present study was supported by a grant from the
National Natural Science Foundation of China (grant nos. 81271973
and 81201397), the Zhejiang Provincial Natural Science Foundation
of China (grant no. Y13H060003), the Department of Health Bureau of
Zhejiang Province (grant no. 2014KYA092) and the Zhejiang Medical
and Health Science and Technology Plan Project (grant no.
201462458).
References
|
1
|
Gardella S, Andrei C, Ferrera D, Lotti LV,
Torrisi MR, Bianchi ME and Rubartelli A: The nuclear protein HMGB1
is secreted by monocytes via a non-classical, vesicle-mediated
secretory pathway. EMBO Rep. 3:995–1001. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Fink MP: Bench-to-bedside review:
High-mobility group box 1 and critical illness. Crit Care.
11:2292007. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Degryse B, Bonaldi T, Scaffidi P, Müller
S, Resnati M, Sanvito F, Arrigoni G and Bianchi ME: The high
mobility group (HMG) boxes of the nuclear protein HMG1 induce
chemotaxis and cytoskeleton reorganization in rat smooth muscle
cells. J Cell Biol. 152:1197–1206. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Palumbo R, Sampaolesi M, De Marchis F,
Tonlorenzi R, Colombetti S, Mondino A, Cossu G and Bianchi ME:
Extracellular HMGB1, a signal of tissue damage, induces
mesoangioblast migration and proliferation. J Cell Biol.
164:441–449. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Andersson U, Erlandsson-Harris H, Yang H
and Tracey KJ: HMGB1 as a DNA-binding cytokine. J Leukoc Biol.
72:1084–1091. 2002.PubMed/NCBI
|
|
6
|
Yang H, Wang H, Czura CJ and Tracey KJ:
HMGB1 as a cytokine and therapeutic target. J Endotoxin Res.
8:469–472. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Naglova H and Bucova M: HMGB1 and its
physiological and pathological roles. Bratisl Lek Listy.
113:163–171. 2012.PubMed/NCBI
|
|
8
|
Granero-Moltó F, Weis JA, Miga MI, Landis
B, Myers TJ, O'Rear L, Longobardi L, Jansen ED and Mortlock DP:
Regenerative effects of transplanted mesenchymal stem cells in
fracture healing. Stem Cells. 27:1887–1898. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Glass GE, Chan JK, Freidin A, Feldmann M,
Horwood NJ and Nanchahal J: TNF-alpha promotes fracture repair by
augmenting the recruitment and differentiation of muscle-derived
stromal cells. Proc Natl Acad Sci USA. 108:1585–1590. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Marsell R and Einhorn TA: The biology of
fracture healing. Injury. 42:551–555. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Mingari MC, Moretta A and Moretta L:
Regulation of KIR expression in human T cells: A safety mechanism
that may impair protective T-cell responses. Immunol Today.
19:153–157. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Guo Z, Yang J, Liu X, Li X, Hou C, Tang PH
and Mao N: Biological features of mesenchymal stem cells from human
bone marrow. Chin Med J (Engl). 114:950–953. 2001.PubMed/NCBI
|
|
13
|
Guo J, Jie W, Shen Z, Li M, Lan Y, Kong Y,
Guo S, Li T and Zheng S: SCF increases cardiac stem cell migration
through PI3K/AKT and MMP2/9 signaling. Int J Mol Med. 34:112–118.
2014.PubMed/NCBI
|
|
14
|
Sidney LE, Kirkham GR and Buttery LD:
Comparison of osteogenic differentiation of embryonic stem cells
and primary osteoblasts revealed by responses to IL-1β, TNF-α and
IFN-γ. Stem Cells Dev. 23:605–617. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tso GH, Law HK, Tu W, Chan GC and Lau YL:
Phagocytosis of apoptotic cells modulates mesenchymal stem cells
osteogenic differentiation to enhance IL-17 and RANKL expression on
CD4+ T cells. Stem Cells. 28:939–954. 2010.PubMed/NCBI
|
|
16
|
Lda S Meirelles, Fontes AM, Covas DT and
Caplan AI: Mechanisms involved in the therapeutic properties of
mesenchymal stem cells. Cytokine Growth Factor Rev. 20:419–427.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yuk JM, Yang CS, Shin DM, Kim KK, Lee SK,
Song YJ, Lee HM, Cho CH, Jeon BH and Jo EK: A dual regulatory role
of apurinic/apyrimidinic endonuclease 1/redox factor-1 in
HMGB1-induced inflammatory responses. Antioxid Redox Signal.
11:575–588. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lee W, Ku SK, Bae JW and Bae JS:
Inhibitory effects of lycopene on HMGB1-mediated pro-inflammatory
responses in both cellular and animal models. Food Chem Toxicol.
50:1826–1833. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Meng E, Guo Z, Wang H, Jin J, Wang J, Wang
H, Wu C and Wang L: High mobility group box 1 protein inhibits the
proliferation of human mesenchymal stem cells and promotes their
migration and differentiation along osteoblastic pathway. Stem
Cells Dev. 17:805–813. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2−ΔΔCt method. Methods. 25:402–408. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Nonomura A, Ohta G, Nakanuma Y, Izumi R,
Mizukami Y, Matsubara F, Hayashi M, Watanabe K and Takayanagi N:
Simultaneous detection of epidermal growth factor receptor (EGF-R),
epidermal growth factor (EGF) and ras p21 in cholangiocarcinoma by
an immunocytochemical method. Liver. 8:157–166. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zlotnik A and Yoshie O: Chemokines: A new
classification system and their role in immunity. Immunity.
12:121–127. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Pham K, Luo D, Liu C and Harrison JK:
CCL5, CCR1 and CCR5 in murine glioblastoma: Immune cell
infiltration and survival rates are not dependent on individual
expression of either CCR1 or CCR5. J Neuroimmunol. 246:10–17. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lee JK, Schuchman EH, Jin HK and Bae JS:
Soluble CCL5 derived from bone marrow-derived mesenchymal stem
cells and activated by amyloid β ameliorates Alzheimer's disease in
mice by recruiting bone marrow-induced microglia immune responses.
Stem Cells. 30:1544–1555. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Errahali YJ, Taka E, Abonyo BO and Heiman
AS: CCL26-targeted siRNA treatment of alveolar type II cells
decreases expression of CCR3-binding chemokines and reduces
eosinophil migration: Implications in asthma therapy. J Interferon
Cytokine Res. 29:227–239. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
De Vries TJ, Schoenmaker T, Aerts D,
Grevers LC, Souza PP, Nazmi K, van de Wiel M, Ylstra B, Lent PL,
Leenen PJ and Everts V: M-CSF priming of osteoclast precursors can
cause osteoclastogenesis-insensitivity, which can be prevented and
overcome on bone. J Cell Physiol. 230:210–225. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Karst M, Gorny G, Galvin RJ and Oursler
MJ: Roles of stromal cell RANKL, OPG and M-CSF expression in
biphasic TGF-beta regulation of osteoclast differentiation. J Cell
Physiol. 200:99–106. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kazemi-Lomedasht F, Behdani M, Bagheri KP,
Habibi-Anbouhi M, Abolhassani M, Arezumand R, Shahbazzadeh D and
Mirzahoseini H: Inhibition of angiogenesis in human endothelial
cell using VEGF specific nanobody. Mol Immunol. 65:58–67. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Henriksen K, Karsdal M, Delaisse JM and
Engsig MT: RANKL and vascular endothelial growth factor (VEGF)
induce osteoclast chemotaxis through an ERK1/2-dependent mechanism.
J Biol Chem. 278:48745–48753. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kayal RA, Tsatsas D, Bauer MA, Allen B,
Al-Sebaei MO, Kakar S, Leone CW, Morgan EF, Gerstenfeld LC, Einhorn
TA and Graves DT: Diminished bone formation during diabetic
fracture healing is related to the premature resorption of
cartilage associated with increased osteoclast activity. J Bone
Miner Res. 22:560–568. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lee K, Kim H, Kim JM, Kim JR, Kim KJ, Kim
YJ, Park SI, Jeong JH, Moon YM, Lim HS, et al: Systemic
transplantation of human adipose-derived stem cells stimulates bone
repair by promoting osteoblast and osteoclast function. J Cell Mol
Med. 15:2082–2094. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wang CL, Jiang H and Sun JB: EGF and SCF
promote the proliferation and differentiation of mouse
spermatogenic cells in vitro. Zhonghua Nan Ke Xue. 20:679–683.
2014.(In Chinese). PubMed/NCBI
|
|
33
|
Kikuchi A, Amagai M, Hayakawa K, Ueda M,
Hirohashi S, Shimizu N and Nishikawa T: Association of EGF receptor
expression with proliferating cells and of ras p21 expression with
differentiating cells in various skin tumours. Br J Dermatol.
123:49–58. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Scholz ME, Meissner JD, Scheibe RJ, Umeda
PK, Chang KC, Gros G and Kubis HP: Different roles of H-ras for
regulation of myosin heavy chain promoters in satellite
cell-derived muscle cell culture during proliferation and
differentiation. Am J Physiol Cell Physiol. 297:C1012–C1018. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Peng S, Zhou G, Luk KD, Cheung KM, Li Z,
Lam WM, Zhou Z and Lu WW: Strontium promotes osteogenic
differentiation of mesenchymal stem cells through the Ras/MAPK
signaling pathway. Cell Physiol Biochem. 23:165–174. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Yao B, Zhang Y, Delikat S, Mathias S, Basu
S and Kolesnick R: Phosphorylation of Raf by ceramide-activated
protein kinase. Nature. 378:307–310. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Mor A and Philips MR: Compartmentalized
Ras/MAPK signaling. Annu Rev Immunol. 24:771–800. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zeiller C, Blanchard MP, Pertuit M,
Thirion S, Enjalbert A, Barlier A and Gerard C: Ras and Rap1 govern
spatiotemporal dynamic of activated ERK in pituitary living cells.
Cell Signal. 24:2237–2248. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Hubbard PA, Moody CL and Murali R:
Allosteric modulation of Ras and the PI3K/AKT/mTOR pathway:
Emerging therapeutic opportunities. Front Physiol. 5:4782014.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Binger T, Stich S, Andreas K, Kaps C,
Sezer O, Notter M, Sittinger M and Ringe J: Migration potential and
gene expression profile of human mesenchymal stem cells induced by
CCL25. Exp Cell Res. 315:1468–1479. 2009. View Article : Google Scholar : PubMed/NCBI
|