Introduction
Coronary heart disease (CHD), synonymously known as
coronary artery disease (CAD) is the most predominant type of
cardiovascular disease in developing countries (1). Acute myocardial infarction (AMI) is one
of the most prevalent types of CHD (2), and may lead to an irreversible loss of
cardiomyocytes and scar formation in the infarct area, which are
major factors in the progression of heart failure (3,4).
Therefore, investigation of novel treatments to improve the
prognosis of AMI is an area of intense activity.
Prior studies have reported the effects of various
herb-derived compounds on clinical symptoms, biomarkers and
mortality in AMI patients and animal models (5–7).
However, the mechanisms underlying these effects require further
investigation and systematic review.
The use of a combination of multiple herbs is
designed to exploit the additive or synergistic activities of
individual herbs, as well as to balance or neutralize the toxic
effects of certain herbal components by others in the mixture
(8). Guanmaitong (GMT) consists
primarily of the herbs Salvia miltiorrhiza, Safflower
(Carthamus tinctorius) and Polygonum multiflorum, has
been applied to the treatment of CHDs such as angina pectoris and
myocardial ischemia in China (9).
The three medicinal herbs are commonly used in traditional Chinese
medicine and have previously been shown to be physiologically
active in human vascular endothelial cells (10). Tetrahydroxystilbene-glucoside, one of
the active ingredients of F. multiflorum, has been reported
to exert a protective effect on the cardiovascular system by
influencing cellular antioxidant capacity and inhibiting
doxorubicin-induced apoptosis in cardiomyocytes (11,12). The
pharmacological effects of S. miltiorrhiza extracts include
antioxidative, anti-apoptotic and vasodilatory activities, which
may be affected by the inhibition of intercellular adhesion
molecule 1 (ICAM-1) expression to protect endothelial function and
inhibit atherogenesis, and promoting the role of S.
miltiorrhiza in cardiovascular and cerebrovascular systems
(13–15). In previous studies, Safflower has
been demonstrated to reduce cardiovascular disease risk in rats
(16,17).
The aim of the present study was investigate the
cardioprotective effects of GMT and to elucidate possible
mechanisms underlying its effect on myocardial apoptosis and
inflammatory response in rats with AMI.
Materials and methods
Animals
A total of 60 healthy adult male Sprague-Dawley (SD)
rats, aged 6–8 weeks and weighing 220–250 g, were provided by the
Experimental Animal Center of PLA Academy of Military Medical
Sciences (Beijing, China) and acclimated for at least three days
[license no. SCXK (Army) 2007–004]. All animals were housed in
separated cages with laboratory chow and tap water ad
libitum. All animal experiments were performed in accordance
with the National Institute of Health Guide for the Care and Use of
Laboratory Animals, and experiments were approved by the University
Laboratory Animal Research Committee of Tianjin Medical University
(Tianjin, China).
Experimental design and protocol
SD rats were randomly allocated into five equal
groups (n=12/group): Sham-operated control group (S), model group
(M), and small (0.55 g/kg/day; GL), medium (1.1 g/kg/day; GM), and
large (2.2 g/kg/day; GH) dosage GMT groups. GMT was provided by
Tianjin Tongrentang Group Co., Ltd. (Tianjin, China). Control and
model groups received an equal quantity of vehicle. After 14 days
of treatment, animals underwent AMI surgery.
Animal model establishment
An AMI model was established in rats by ligation of
the left anterior descending coronary artery for 24 h. The surgical
procedure was performed according to a previous study (18), with minor modifications. Briefly, SD
rats were anesthetized with 10% chloral hydrate (0.3 ml/100 g,
intraperitoneally; Sigma-Aldrich, St. Louis, MO, USA), then a left
thoracotomy was performed. The incised area was extended using
forceps and the pericardium was opened. Following tracheal
intubation, the rats were ventilated using a respirator (ALC-V8;
Alcott Biotech Co., Ltd., Shanghai, China) with room air at a tidal
volume of 25 ml/min and a respiratory rate of 70 breaths/min. The
heart was exteriorized and ligated at the proximal left anterior
descending coronary artery 2–3 mm from its origin between the
pulmonary artery conus and the left atrium using a 5-0 Prolene
suture (WEGO Inc., Shandong, China). The heart was returned to its
normal position and the thorax was closed. Sham-operated rats
underwent an identical surgical procedure as described above except
that the suture was not tightened around the coronary artery.
Measurement of myocardial infarct size
and histological analysis
A 2,3,5-triphenyltetrazolium chloride (TTC) assay
was used to determine myocardial infarct size. TTC was provided by
Amresco (Amresco LLC, Solon, OH, USA). In brief, the heart was
transversely cut across the left ventricle, and sections 2–3 mm
thick were incubated in 0.5% TTC solution prepared in phosphate
buffer (pH 7.4; Sangon Biotech Co., Ltd., Shanghai, China) for 30
min at 37°C, following which they were fixed with 10% formalin
(Sangon Biotech Co., Ltd.). Non-ischemic and viable ischemic
myocardium were stained red, while the infarcted myocardium
appeared pale grey or white. For histological analysis, 5-µm
sections from the left ventricle were stained with hematoxylin and
eosin (HE; Beijing Zhongshan Golden Bridge Biotechnology Co., Ltd.,
Beijing, China). The pathological features were observed using a
microscope (BX53; Olympus Corporation, Tokyo, Japan) at a
magnification of ×400.
Measurement of cardiac marker enzyme
activity
Abdominal aortic blood samples (4 ml) was separated
by centrifugation at 840 × g for 10 min at 4°C. The
activities of serum creatine kinase (CK; 812060103), creatine
kinase-MB (CK-MB; 812060202), and lactate dehydrogenase (LDH;
812060403) were measured spectrophotometrically according to the
specifications of commercial diagnostic kits (Shanghai Kehua
Medical Instruments Co., Ltd., Shanghai, China).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) analysis
Rats were sacrificed with 10% chloral hydrate (2
ml/100 g) and total RNA was extracted from the rat heart tissues
using TRIzol (Invitrogen; Thermo Fisher Scientific, Inc., Waltham,
MA, USA) according to the manufacturer's instructions. RNA yields
and purity were assessed by spectrophotometric analysis
(BioPhotometer Plus; Eppendorf, Shanghai, China) at 260 and 280 nm.
Total RNA (1 µg) from each well was subjected to reverse
transcription with oligo dT (19)
primers, dNTPs (both Takara Bio, Inc., Otsu, Japan) and M-MLV
reverse transcriptase (Promega Corporation, Madison, WI, USA) in a
total reaction volume of 20 µl. Following DNase treatment
(Sigma-Aldrich), the 20-µl RT-qPCR reaction system consisted of 10
µl SYBR Green Mix (Takara Bio, Inc.,), 0.5 µl of each primer
(Table I) (Invitrogen), 1 µl cDNA
and 8 µl double-distilled water. RT-qPCR was performed using an ABI
7300 Real-Time PCR System (Applied Biosystems; Thermo Fisher
Scientific, Inc.) and data were analyzed using the accompanying ABI
7300 software. Thermal cycling conditions were as follows: Initial
denaturation at 95°C for 5 min; 40 cycles at 95°C for 30 sec and
58°C for 30 sec; elongation at 72°C for 30 sec; a final cycle at
95°C for 15 sec and 60°C for 15 sec; followed by dissociation at
95°C for 15 sec. All values obtained with the tumor necrosis
factor-α (TNF-α), interleukin (IL-1) or ICAM-1 primers were
normalized against the values obtained with the GAPDH primers,
according to the 2−∆∆Cq method (20). The results are expressed as the
relative integrated intensity. Negative controls (no cDNA) and RT
controls (no reverse transcriptase) were performed. All reactions
were performed in triplicate.
 | Table I.Polymerase chain reaction primer
sequences. |
Table I.
Polymerase chain reaction primer
sequences.
| Gene | Sequence (5′-3′) |
|---|
| IL-1 | F,
AAGACAAGCCTGTGTTGCTGAAGG |
|
| R,
TCCCAGAAGAAAATGAGGTCGGTC |
| TNF-α | F,
AAATGGGCTCCCTCTCATCAGTTC |
|
| R,
TCTGCTTGGTGGTTTGGCTACGAC |
| ICAM-1 | F,
GGGTTGGAGACTAACTGGA |
|
| R,
GCACCGCAGGATGAGGTTCTT |
| GAPDH | F,
AACGACCCCTTCATTGACCT |
|
| R,
CCCCATTTGATGTTAGCGGG |
Enzyme-linked immunosorbent assay
(ELISA) detection of IL-1
ELISA measurements of IL-1 expression were performed
in duplicate using a specific, commercially available IL-1 ELISA
kit (Cusabio Biotech Co., Ltd., Wuhan, China) in accordance with
the manufacturer's instructions, and analyzed using an ELISA reader
(Tecan Trading AG, Männedorf, Switzerland) at 450 nm.
Western blot analysis
Myocardial tissue (100 mg) was grinded in liquid
nitrogen and incubated with radioimmunoprecipitation assay lysis
buffer, containing 20 mM HEPES, 0.5% NP-40 (both Sigma-Aldrich), 1%
protease inhibitor cocktail (Promega Corporation), 200 mM KCl, 20%
glycerol and 0.5 mM EDTA (all Sangon Biotech Co., Ltd.), to extract
total protein. Subsequently, 30 µg protein/lane was subjected to
10% SDS-PAGE and transferred onto nitrocellulose membranes (EMD
Millipore, Billerica, MA, USA). Membranes were blocked with 5%
bovine serum albumin and subsequently incubated with antibodies
against B-cell lymphoma 2 (Bcl-2; 1:1,000; 2876), Bcl-2-associated
X protein (Bax; 1:1,000; 2772), TNF-α (1:2,000; MAB510) ICAM-1
(1:1,000; ab124760) and GAPDH (1:2,000; AB-M-M001), as the internal
control, at 4°C overnight. Following washing six times for 30 min
with Tris-buffered saline with Tween 20 (TBST), the membranes were
incubated with horseradish peroxidase (HRP)-conjugated goat
anti-rabbit IgG (7074) or horse anti-mouse IgG (7076) secondary
antibodies (both 1:3,000; Cell Signaling Technology, Inc., Danvers,
MA, USA) for 1 h at room temperature to detect the primary
antibody. Following further washing with TBST for 30 min, the
intensity of immunoreactive bands was estimated using an imaging
densitometer (Gene Tools 3.06; Gene Company Ltd., Hong Kong).
Rabbit polyclonal anti-Bax and anti-Bcl-2 antibodies were purchased
from Cell Signaling Technology, Inc., monoclonal anti-TNF-α
antibody from R&D Systems (Minneapolis, MN, USA), rabbit
polyclonal anti-ICAM-1 antibody from Abcam (Cambridge, MA, USA) and
anti-GAPDH antibody (1:2,000; AB-M-M001) from Hangzhou Xianzhi
Biotechnology (Hangzhou, China).
Immunohistochemical detection of Bcl-2
and Bax expression
Tissues were conventionally fixed with 10% formalin,
then dehydrated with alcohol, embedded with paraffin wax and
continuously sectioned at 5 µm. Sections were incubated overnight
at 4°C with primary anti-Bcl-2 (BA0412) and anti-Bax (BA0315)
antibodies (both 1:500; Wuhan Boster Biological Technology Ltd.,
Wuhan, China). Negative control were performed which involved the
omission of primary antibody and use of phosphate-buffered saline
(PBS). Sections were then rinsed with PBS and incubated for 1 h
with HRP-conjugated secondary antibody (1:2,000; ZB2301; Beijing
Zhongshan Golden Bridge Biotechnology Co., Ltd.). The reaction was
visualized using a solution of 3,3′-diaminobenzidine (Beijing
Zhongshan Golden Bridge Biotechnology Co., Ltd.). For
quantification, the integral optical density of Bax and Bcl-2
staining were calculated using Image-Pro Plus 6.0 software (Media
Cybernetics, Inc., Rockville, MD, USA).
Statistical analysis
Data are reported as the mean ± standard deviation.
Statistical significance was determined using one-way analysis of
variance tests followed by Dunnett's test. Statistical analyses
were performed using the software package StatView 5.0J (SAS
Institute, Cary, NC, USA). P<0.05 was considered to indicate a
statistically significant difference.
Results
GMT reduces infarct area and
pathological changes in cellular morphology
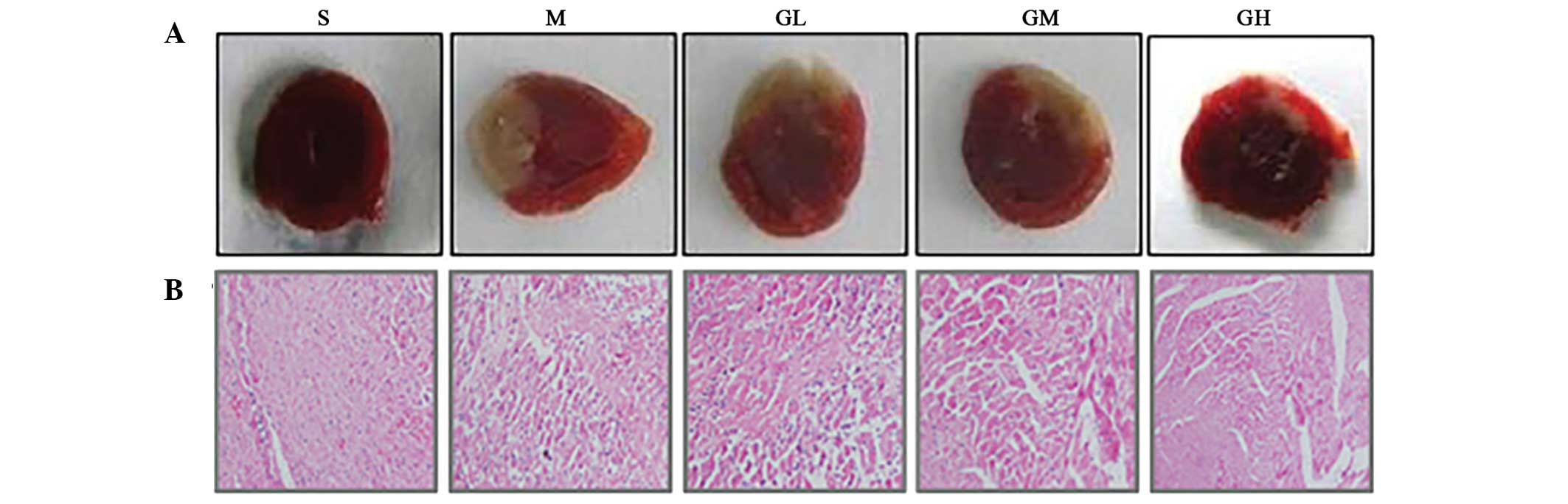
Representative illustrations of infarction tissue
size (pale grey areas) as stained by TTC are shown in Fig. 1A. Sham-operated rats exhibited no
evidence of infarcted tissue in the left ventricle. The model group
presented with a large unstained area (30.02±4.32% vs. sham group;
Table II). The infarction size
significantly reduced in GMT groups compared with the model group,
and the size was smallest in the high dosage group (15.71±3.32%)
compared with the medium (17.83±2.37%) and small (23.16±4.01%)
dosage groups. Regarding HE staining, the model group showed
augmentation and loose arrangement of the myocardial fibers,
staining asymmetry, deformity of the nucleus and marked edema and
infiltration. By contrast, tissues from the SD rats treated with
GMT exhibited markedly improved pathological changes compared with
model group (Fig. 1B).
 | Table II.Effect of Guanmaitong on myocardial
infarct tissue size. |
Table II.
Effect of Guanmaitong on myocardial
infarct tissue size.
| Group | Ventricle weight
(g) | Infarction weight
(g) | Infarction rate
(%) |
|---|
| Model | 0.61±0.08 | 0.19±0.02 |
30.02±4.32a |
| Sham | 0.57±0.06 | – | – |
| Low dosage | 0.62±0.05 | 0.15±0.05 |
23.16±4.01b |
| Medium dosage | 0.65±0.09 | 0.11±0.03 |
17.83±2.37b |
| High dosage | 0.63±0.04 | 0.09±0.04 |
15.71±3.32b |
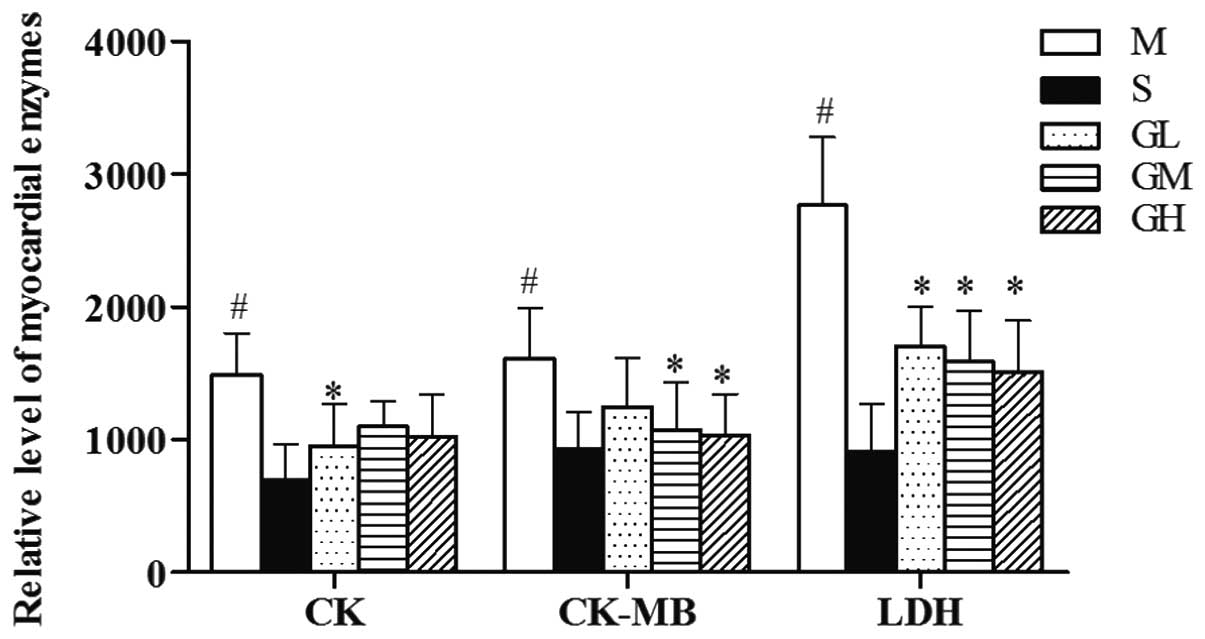
Effects of GMT on levels of cardiac
marker enzyme activity
Fig. 2 shows that
compared with the sham-operated group, the serum CK, CK-MB and LDH
activities in the model group increased significantly (P<0.05).
Treatment with GMT attenuated these elevations, and the various GMT
dosages produced varying degrees of reduction (Fig. 2).
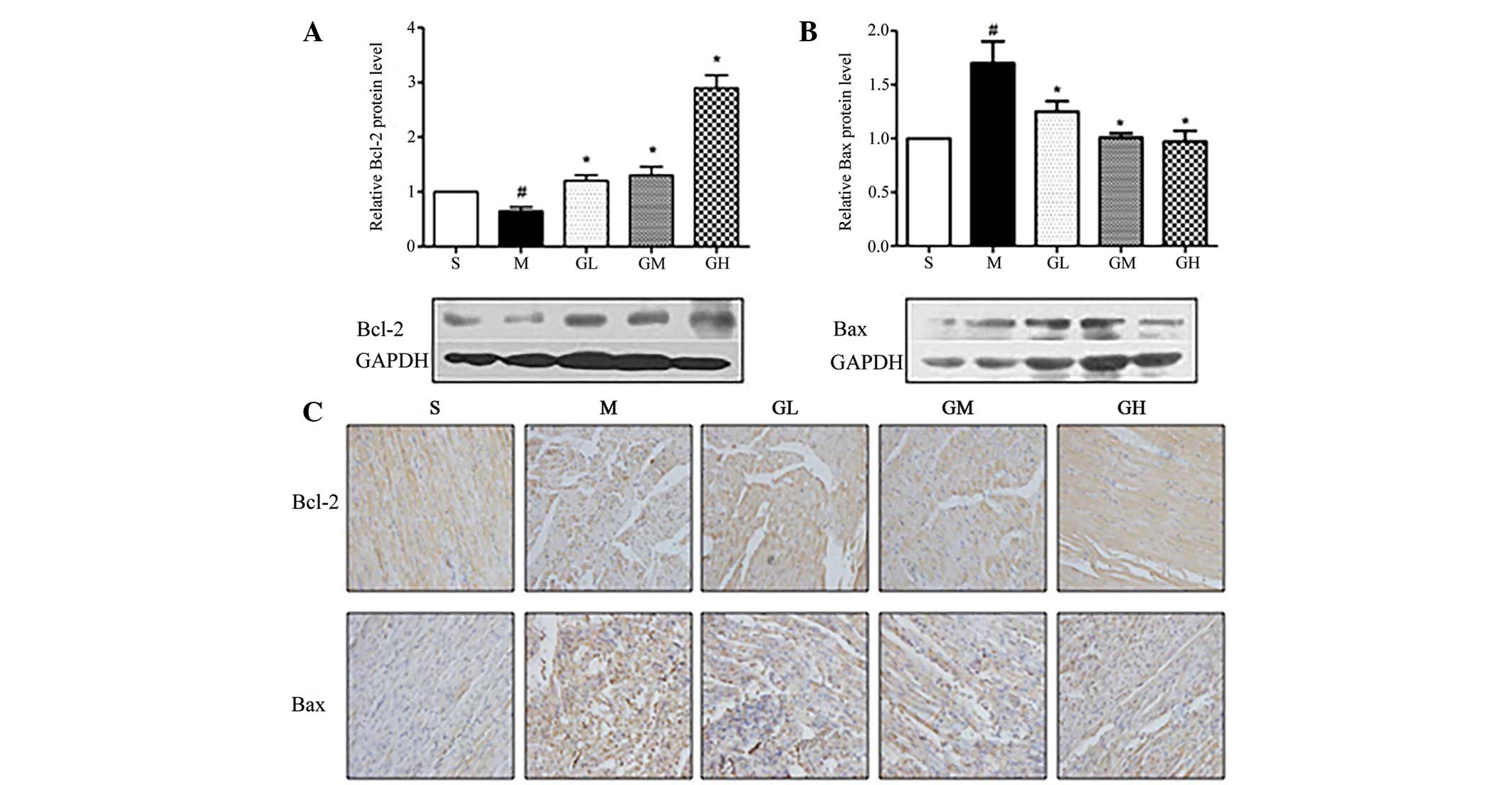
Effect of GMT on cardiomyocyte
apoptosis
Adult cardiomyocytes are post-mitotic cells, and
thus have a limited response capability to damage (21). Prior studies have suggested that
apoptosis is increased in acute and chronic heart pathologies, were
the process appears to be crucially involved (21). To clarify the possible mechanism
underlying anti-apoptotic effect exerted by GMT, two key factors of
intrinsic pathway, Bcl-2 and Bax, in the infarct heart tissues.
Using western blot and immunohistochemical assays (Fig. 3), a significant reduction in Bcl-2
expression and an increase in Bax expression was observed in the
cardiocytes of the model group, as compared with the sham-operated
group. Treatment with GMT resulted in enhanced Bcl-2 expression,
reduced Bax expression and attenuated the ratio of Bcl-2/Bax
(P<0.05) (Table III).
Furthermore, the higher dosage groups (1.1 and 2.2 g/kg/day)
exhibited more marked differences compared with the low dosage
group (0.55 g/kg/day).
 | Table III.Effect of Guanmaitong on cell
apoptosis. |
Table III.
Effect of Guanmaitong on cell
apoptosis.
| Group | Apoptosis index
(%) | η (Bcl-2)% | η (Bax)% |
|---|
| Model |
31.8±1.9a |
13.1±1.3a |
52.7±2.0a |
| Sham |
2.3±0.9 | 11.4±0.9 | 13.3±1.0 |
| Low dosage |
25.1±2.1b | 15.3±1.0 | 49.5±5.1 |
| Medium dosage |
23.2±1.6b |
18.6±1.8a |
45.1±2.7b |
| High dosage |
18.5±5.1b |
21.7±2.2a |
34.4±3.3b |
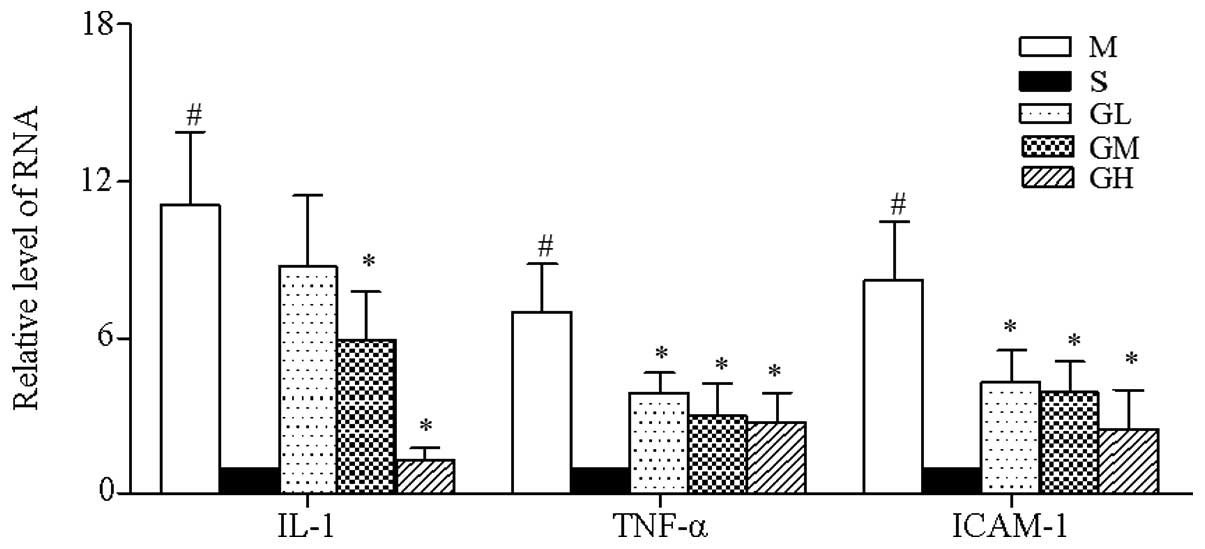
Expression of inflammation-related
cytokine mRNAs in AMI via RT-qPCR assay
At 24 h after AMI induction the mRNA expression
levels of IL-1, TNF-α and ICAM-1 in the model group significantly
increased (P<0.05 vs. sham group; Fig. 4), and a reversed trend was observed
in GMT groups. In particular, the mRNA expression levels of TNF-α
and ICAM-1 were significantly reduced in all three GMT-treated
groups (P<0.05 vs. model group), and were dosage-correlated. The
expression levels of IL-1 mRNA were significantly reduced in the
medium and high dosage groups (P<0.05 vs. model group), whereas
no significant difference was detected in the low dosage group.
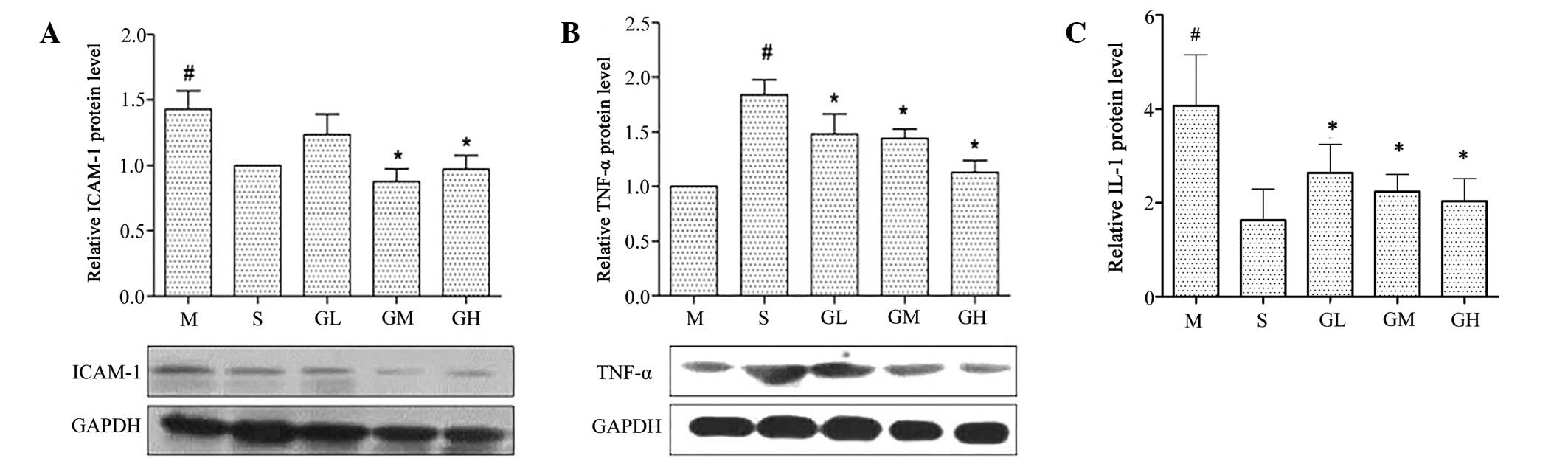
Effect of GMT on protein expression
levels of TNF-α, ICAM-1 and IL-1
The western blot analysis results indicated that
ICAM-1 expression increased in the model group (vs. sham group) and
decreased significantly in the medium and high dosage GMT groups
(P<0.05 vs. model group; Fig.
5A). By contrast, a statistically significant elevation in
TNF-α protein expression was detected in all three GMT groups
(P<0.05 vs. model group; Fig.
5B). The results of the ELISA assay indicate that the protein
expression levels of IL-1 increased significantly in the model
group (P<0.05 vs. sham group), and that this elevation was
significantly inhibited in all three GMT groups (P<0.05 vs.
model group).
Discussion
Apoptosis, the physiological process of programmed
cell death, may contribute to various cardiac disorders (22). Apoptosis has been reported to
contribute to the loss of cardiomyocytes and is recognized as a
predictor of adverse outcomes in patients with cardiac diseases or
heart failure (23). Consequently,
the interruption of apoptotic pathways may facilitate the
development of novel strategies to reverse or attenuate heart
failure (24,25). Certain active components of GMT have
been reported to have anti-apoptotic effects, and are used as a
classic prescription in traditional Chinese medicine for the
treatment of cardiovascular diseases (12–15).
Therefore, it was speculated for the purposes of the present study
that GMT could salvage these cardiocytes and prevent ischemic cell
loss induced by apoptosis. In the present study, GMT treatment
upregulated the expression of the anti-apoptotic protein, Bcl-2,
and downregulated the expression of the proapoptotic protein, Bax.
Upregulation of Bcl-2 enhanced the formation of heterodimers with
Bax, resulting in fewer available Bax proteins for the formation of
homodimers. It is well known that if Bax homodimers predominate
cell death will occur (26,27).
AMI is currently speculated to involve the process
of inflammation, which is a hallmark throughout the distinct stages
of atherosclerosis and plaque rupture (28). TNF-α is a key inflammatory cytokine
which exerts pleiotropic biological effects and is crucially
involved in cardiovascular diseases such as AMI (29). The inflammatory reaction caused by
TNF-α is able to induce upregulation of IL-1 and ICAM-1; however,
the increased expression of TNF-α may also regulate apoptosis by
inhibiting the expression levels of the anti-apoptosis factor Bcl-2
(30). Another inflammatory
cytokine, IL-1, has been proposed as a crucial mediator in the
inflammatory response and AMI. In patients with ST-segment
elevation AMI, IL-1 blockade with the IL-1 receptor antagonist
anakinra is safe and ameliorates left ventricular remodeling, and
can significantly increase and induce the expression of ICAM-1 in
ischemic heart disease (31,32). ICAM-1 belongs to the super-family of
immunoglobulin-like adhesion molecules and is critical for various
physiological and pathological processes (33). It has previously been shown that
ICAM-1 is able to induce and aggravate AMI, and its expression
levels are closely associated with the extent of myocardial damage
(34).
In the present study, the expression of a number of
inflammatory cytokines increased rapidly in model group, while GMT
treatment effectively reversed this change by influencing the
expression of these factors and apoptosis regulators. Therefore,
the present data support the cardioprotective capacity of GMT and
suggest possible mechanisms underlying the observed
anti-inflammatory and anti-apoptosis effects. Further mechanistic
studies aimed at identifying the detailed signaling pathways
upstream of TNF-α and Bcl-2 are required, in order to elucidate the
molecular mechanisms underlying GMT and provide a theoretical basis
for its clinical application.
Acknowledgements
This study was supported by grants from the Second
Hospital of Tianjin Medical University (grant no. y1106) and
Tianjin Tongrentang Group Co., Ltd. (Tianjin, China).
References
|
1
|
Pranavchand R and Reddy B: Current status
of understanding of the genetic etiology of coronary heart disease.
J Postgrad Med. 59:30–41. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Li C, Pei F, Zhu X, Duan DD and Zeng C:
Circulating microRNAs as novel and sensitive biomarkers of acute
myocardial infarction. Clin Biochem. 45:727–732. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Frangogiannis NG: The immune system and
the remodeling infarcted heart: Cell biological insights and
therapeutic opportunities. J Cardiovasc Pharmacol. 63:185–195.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Dauwe DF and Janssens SP: Stem cell
therapy for the treatment of myocardial infarction. Curr Pharm Des.
17:3328–3340. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Liang X, Chen X, Liang Q, Zhang H, Hu P,
Wang Y and Luo G: Metabonomic study of Chinese medicine Shuanglong
formula as an effective treatment for myocardial infarction in
rats. J Proteome Res. 10:790–799. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wang Y, Qian P, Liu P, Wei L, Cao M, Zhou
L, Zhou D and Lin ZX: Effects of Panax notoginseng flower extract
on the TGF-β/Smad signal transduction pathway in heart remodeling
of human chymase transgenic mice. Mol Med Rep. 5:1443–1448.
2012.PubMed/NCBI
|
|
7
|
Chen KJ and Xu H: The integration of
traditional Chinese medicine and Western medicine. European Review.
11:225–235. 2003.
|
|
8
|
Zhu YP and Woerdenbag HJ: Traditional
Chinese herbal medicine. Pharmacy World and Science. 17:103–112.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Pan SY, Chen SB, Dong HG, Yu ZL, Dong JC,
Long ZX, Fong WF, Han YF and Ko KM: New perspectives on Chinese
herbal medicine (Zhong-Yao) research and development. Evid Based
Complement Alternat Med. 2011:4037092011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ling S, Nheu L, Dai A, Guo Z and
Komesaroff P: Effects of four medicinal herbs on human vascular
endothelial cells in culture. Int J Cardiol. 128:350–358. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hong CY, Lo YC, Tan FC, Wei YH and Chen
CF: Astrafalus membranaceus and Polygonum multiflourm protect rat
heart mitochondria against lipid peroxidation. Am J Chin Med.
22:63–70. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhang SH, Wang WQ and Wang JL: Protective
effect of tetrahydroxystilbene glucoside on cardiotoxicity induced
by doxorubicin in vitro and in vivo. Acta Pharmacol Sin.
30:1479–1487. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zheng CS, Xu XJ, Ye HZ, Wu GW, Xu HF, Li
XH, Huang SP and Liu XX: Computational pharmacological comparison
of Salvia miltiorrhiza and Panax notoginseng used in the therapy of
cardiovascular diseases. Exp Ther Med. 6:1163–1168. 2013.PubMed/NCBI
|
|
14
|
Fong CC, Wei F, Chen Y, Yu WK, Koon CM,
Leung PC, Fung KP, Lau CB and Yang M: Danshen-Gegen decoction
exerts proliferative effect on rat cardiac myoblasts H9c2 via MAPK
and insulin pathways. J Ethnopharmacol. 138:60–66. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wing-Shing Cheung D, Koon CM, Ng CF, Leung
PC, Fung KP, Kar-Sing Poon S and Bik-San Lau C: The roots of Salvia
miltiorrhiza (Danshen) and Pueraria lobata (Gegen) inhibit
atherogenic events: A study of the combination effects of the
2-herb formula. J Ethnopharmacol. 143:859–866. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wan LH, Chen J, Li L, Xiong WB and Zhou
LM: Protective effects of Carthamus tinctorius injection on
isoprenaline-induced myocardial injury in rats. Pharm Biol.
49:1204–1209. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Han SY, Li HX, Ma X, Zhang K, Ma ZZ, Jiang
Y and Tu PF: Evaluation of the anti-myocardial ischemia effect of
individual and combined extracts of Panax notoginseng and Carthamus
tinctorius in rats. Ethnopharmacol. 145:722–727. 2013. View Article : Google Scholar
|
|
18
|
Maclean D, Fishbein MC, Braunwald E and
Maroko PR: Long-term preservation of ischemic myocardium after
experimentalcoronary artery occlusion. J Clin Invest. 61:541–551.
1978. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Whelan RS, Kaplinskiy V and Kitsis RN:
Cell death in the pathogenesis of heart disease: mechanisms and
significance. Annu Rev Physio. 72:19–44. 2010. View Article : Google Scholar
|
|
20
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2−ΔΔCt method. Methods. 25:402–408. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Favaloro B, Allocati N, Graziano V, Di
Ilio C and De Laurenzi V: Role of apoptosis in disease. Aging
(Albany NY). 4:330–349. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lee SD, Chu CH, Huang EJ, Lu MC, Liu JY,
Liu CJ, Hsu HH, Lin JA, Kuo WW and Huang CY: Roles of insulin-like
growth factor II in cardiomyoblast apoptosis and in hypertensive
rat heart with abdominal aorta ligation. Am J Physiol Endocrinol
Metab. 291:E306–E314. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Liou CM, Tsai SC, Kuo CH, Ting H and Lee
SD: Cardiac Fas-dependent and mitochondria-dependent apoptosis
after chronic cocaine abuse. Int J Mol Sci. 15:5988–6001. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sinha-Hikim I, Shen R, Nzenwa I, Gelfand
R, Mahata SK and Sinha-Hikim AP: Minocycline suppresses oxidative
stress and attenuates fetal cardiac myocyte apoptosis triggered by
in utero cocaine exposure. Apoptosis. 16:563–573. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Gill C, Mestril R and Samali A: Losing
heart: The role of apoptosis in heart disease-a novel therapeutic
target? FASEB J. 16:135–146. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Bansal N, Marchion DC, Bicaku E, Xiong Y,
Chen N, Stickles XB, Sawah EA, Wenham RM, Apte SM, Gonzalez-Bosquet
J, et al: BCL2 antagonist of cell death kinases, phosphatases and
ovarian cancer sensitivity to cisplatin. J Gynecol Oncol. 23:35–42.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Xu Z, Dong Y, Wu X, Zhang J, McAuliffe S,
Pan C, Zhang Y, Ichinose F, Yue Y and Xie Z: The potential dual
effects of anesthetic isoflurane on Aβ-induced apoptosis. Curr
Alzheimer Res. 8:741–752. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lin XM, Zhang ZY, Wang LF and Zhang L, Liu
Y, Liu XL, Yang XC, Cui L and Zhang L: Attenuation of tumor
necrosis factor-alpha elevation and improved heart function by
postconditioning for 60 seconds in patients with acute myocardial
infarction. Chin Med J (Engl). 123:1833–1839. 2010.PubMed/NCBI
|
|
29
|
Tuttolomondo A, Di Raimondo D, Pecoraro R,
Arnao V, Pinto A and Licata G: Inflammation in ischemic stroke
subtypes. Curr Pharm Des. 18:4289–4310. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Chen M, Quintans J, Fuks Z, Thompson C,
Kufe DW and Weichselbaum RR: Suppression of Bcl-2 messenger RNA
production may mediate apoptosis after ionizing radiation, tumor
necrosis factor and ceramide. Cancer Res. 55:991–994.
1995.PubMed/NCBI
|
|
31
|
Arnson Y: Circulating levels of adhesion
molecules ICAM-1, VCAM-1, E-Selectin, VEGF and their associations
with age and troponin in patients with acute coronary syndrome. J
Am Coll Cardiol. 63:A1772014. View Article : Google Scholar
|
|
32
|
Abbate A, Kontos MC, Grizzard JD,
Biondi-Zoccai GG, Van Tassell BW, Robati R, Roach LM, Arena RA,
Roberts CS, Varma A, et al: VCU-ART Investigators: Interleukin-1
blockade with anakinra to prevent adverse cardiac remodeling after
acute myocardial infarction(Virginia Commonwealth University
Anakinra Remodeling Trial [VCU-ART] Pilot study). Am J Cardiol.
105:1371–1377. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Projahn D and Koenen RR: Platelets: key
players in vascular inflammation. J Leukoc Biol. 92:1167–1175.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
van den Akker F, Deddens JC, Doevendans PA
and Sluijter JP: Cardiac stem cell therapy to modulate inflammation
upon myocardial infarction. Biochim Biophys Acta. 1830:2449–2458.
2013. View Article : Google Scholar : PubMed/NCBI
|