Introduction
Pseudoexfoliation syndrome (PEX) was first described
by Lindberg in 1917, but its exact etiology remains unknown
(1). PEX is a common, age-associated
systemic disorder of elastic fibers, affecting the lungs, liver,
kidney, gall bladder and cerebral meninges (2,3). Cardio-
and cerebrovascular diseases, such as angina, aortic aneurysm and
dementia have been linked to PEX (4–6);
however, this association remains controversial (7–9).
The prevalence of pseudoexfoliation syndrome is
known to vary widely between geographic regions; for instance, the
Reykjavik Eye Study in Iceland reported an overall prevalence of
10.6% in patients over 50 years of age increasing to 40.6% in
individuals over 80 years of age (10). Another population-based study, which
was conducted in the Blue Mountains, west of Sydney, Australia,
revealed an overall prevalence of 2.3% in individuals over 49 years
of age (11). Between these, the
reported prevalence in Greece is 27% among age-associated cataract
patients (12).
Pseudoexfoliation material has been identified in
many human organs (2–6). Epidemiological data reveal that this
disease occurs worldwide, and that prevalence increases with age;
however, PEX is not an integral part of normal aging (13). PEX manifests in eyes through the
accumulation of an abnormal fibrillar material on the extracellular
matrix of tissues of the anterior segment. The pathological
material appears as concentric rings of greyish fringes and flakes
on the pupillary border of the iris and the peripheral lens capsule
(14,15). These deposits are diagnosed
clinically via slit lamp examination, but it is unclear how this
material is produced and accumulates.
Previous studies have reported an increased
incidence of intraoperative complications during extracapsular
cataract extraction in the eyes of patients with PEX compared with
the eyes of patients without this syndrome (16–20).
Serious complications in these patients appear to be predominantly
caused by zonular weakness (21).
Weakened zonules may manifest clinically as iridodonesis,
phacodonesis, anterior chamber depth asymmetry or spontaneous lens
subluxation or dislocation (16,22,23).
Intraoperatively, patients with PEX are at greater risk of zonular
dialysis, posterior capsule tear, vitreous loss and displaced
nucleus or fragment in the vitreous body; postoperatively, these
patients have a higher incidence of inflammation in the form of
increased aqueous flare and cellular response, fibrin reaction,
posterior synechiae, posterior capsule opacification, anterior
capsule phimosis and late intraocular lens decentration and
dislocation (22).
Phacoemulsification has become a typical procedure within routine
cataract surgery, and previous studies have demonstrated an
increased incidence of intraoperative, immediate postoperative and
late complications caused by cataract surgery in the eyes of
patients with PEX syndrome (21,24,25). The
pathology of PEX is therefore hypothesized to alter capsular
rigidity, elasticity and other biomechanical properties of the eyes
(26,27).
One of the main secondary effects of PEX is blockage
of the aqueous outflow mechanism of the eye (28). This may lead to elevation of
intraocular pressure, causing loss of vision due to glaucomatous
damage to the optic nerve, which is the second leading cause of
irreversible blindness worldwide (29).
Although the causes of PEX are not well understood
at the molecular level and the specific pathogenic mechanism(s)
behind PEX are unknown, it is hypothesized that inflammation and
oxidative stress are partially responsible (30,31). The
cellular antioxidant defense system comprises enzymatic
antioxidants, including repair enzymes such as microsomal
glutathione S-transferase 1 (MGST1). A role of clusterin in
preventing the deposition of pseudoexfoliative material has also
been proposed (32).
Therefore, the aims of the present study were to
determine the morphology and ultrastructure of epithelial lens
cells in PEX (using light and transmission electron microscopy,
respectively) and to evaluate the expression of MGST1 and clusterin
by immunohistochemistry (IHC).
Materials and methods
Tissue extraction
In the present study, anterior lens capsules and
adherent lens epithelial cells (LECs) were obtained from 24
patients (comprising 13 PEX and 11 control patients) who underwent
routine cataract surgery over the course of 3 months between
January and March 2015 in the Department of Ophthalmology, Nicolaus
Copernicus University Collegium Medicum in Bydgoszcz (Poland).
Written informed consent was obtained from each patient prior to
tissue sample acquisition, and approval of the study was granted by
the Bioethics Committee of the Nicolaus Copernicus University
Collegium Medicum in Bydgoszcz. Two groups of patients were
categorized, as follows: i) Patients with typical PEX [with
deposits of pathological material observed, during slit lamp
examination, as concentric rings of greyish fringes and flakes on
the pupillary border of the iris and the peripheral lens capsule
(14,15)]; ii) control patients with no clinical
signs of this disease.
Following phacoemulsification, surgical specimens
were immediately fixed in 2% (w/v) paraformaldehyde (Serva
Electrophoresis GmbH, Heidelberg, Germany), dehydrated in graded
ethanol concentrations (Avantor Performance Materials, Gliwice,
Poland) and embedded in paraffin. Tissue was then cut into thin
sections (5-nm) on a Reichert microtome (Reichert Technologies,
Depew, NY, USA), placed on SuperFrost Plus microscopic slides and
used in IHC staining (Thermo Fisher Scientific, Inc., Waltham, MA,
USA).
IHC staining
Surgical specimens of human anterior lens capsules
with adherent LECs on the above slides were immersed in xylene,
xylene/ethanol and series of ethanol in descending concentrations
(100–50%; Avantor Performance Materials, Gliwice, Poland). For
antigen retrieval, tissue on slides was placed in Target Retrieval
Solution (Dako; Agilent Technologies, Inc., Santa Clara, CA, USA)
and irradiated in a microwave at 800 W for 10 min and 400 W for 10
min. The EnVision System-HRP (AEC+), used in accordance with the
manufacturer's protocol, was applied for IHC staining (cat. no.
K4008; Dako; Agilent Technologies, Inc.). Non-specific binding
between the primary antibodies and the tissue was prevented using
Peroxidase Blocking Reagent (Dako; Agilent Technologies, Inc.) at
room temperature for 5 min. The samples were exposed to antibodies
and incubated in a humid chamber at room temperature for 30 min.
IHC was performed using rabbit antibodies against MGST1 (dilution,
1:1,000; cat. no. HPA044840; Sigma-Aldrich, Merck Millipore,
Darmstadt, Germany) and clusterin (dilution, 1;1,000; cat. no.
SAB3500199; Sigma-Aldrich; Merck Millipore). Following a series of
washes, the slides were incubated with horseradish
peroxidase-labelled polymer anti-rabbit for 1 h at room temperature
(cat. no. K4008; Dako, Agilent Technologies, Inc.). The
visualization of bound antibodies was performed using AEC+
substrate chromogen at room temperature for 15 min (as above).
Counterstaining was performed using Mayer's hematoxyline (10 min,
room temperature; Dako; Agilent Technologies, Inc.) and the slides
were mounted using Aqua-Poly/Mount (Polysciences, Inc., Warminster,
PA, USA). The surgical specimens were examined using an Eclipse
E800 microscope (Nikon Corporation, Tokyo, Japan) with NIS-Elements
Viewer v. 3.30 image analysis system and a CCD camera (DS-5Mc-U1;
Nikon Corporation).
Transmission electron microscopy
To observe the morphological changes in the anterior
lens capsule and LECs on the ultrastructural level, the surgical
specimens were fixed in 2.5% glutaraldehyde (30 min, room
temperature; Polysciences, Inc., Warminster, PA, USA), washed three
times with 0.1 M sodium cacodylate buffer (Carl Roth GmbH and Co.
KG, Karlsruhe, Germany) and post-fixed with OsO4 (1%, 1
h; Serva Electrophoresis GmbH, Heidelberg, Germany) in the same
buffer at room temperature. Next, the tissues were dehydrated in a
graded series of ethanol (30–90%; Avantor Performance Materials)
and acetone (90–100%; Avantor Performance Materials). Following
this, the material was embedded in epoxy resin (glycidether 100;
Carl Roth GmbH and Co. KG) with the addition of accelerator
[2,4,6-tris (dimethylaminomethyl) phenol-30; Carl Roth GmbH and Co.
KG] and hardeners (methyl nadic anhydride and DDSA; Carl Roth GmbH
and Co. KG), polymerized for 24 h at 37°C, then for 120 h at 65°C.
Finally, the material was cut into ultra-thin sections using a
Reichert Om U3 ultramicrotome (Leica Microsystems, Inc., Buffalo
Grove, IL, USA) and placed on copper grids (Sigma-Aldrich; Merck
Millipore). These ultra-thin slices were stained in uranyl acetate
(Ted Pella, Inc., Redding, CA, USA) and analyzed using a
transmission electron microscope (JEM-100 CXII; JEOL, Ltd., Tokyo,
Japan).
Thickness of cells and quantitation of
IHC staining
Changes in the thickness of the LECs were analyzed
on slides from human anterior lens capsules with adherent LECs,
both from patients with PEX and from patients without clinical
signs of these disease. To determine the mean thickness of the
LECs, 100 micrographs were evaluated in each group; a measurement
was recorded of the straight-line distance between the basolateral
and the apical domains of cells, through the center of the cell
nucleus. This analysis was performed using Fiji software (ImageJ
1.47i; imagej.nih.gov/ij/).
The IHC expression intensity of MGST1 and clusterin
was also measured using Fiji software with a Colour Deconvolution
plug-in, on 100 micrographs obtained with the same CCD camera
parameters. The IHC images were distributed to individual color
channels by vectors specific to AEC staining, and the value of the
integrated density was defined as the intensity of the IHC reaction
multiplied by the area of positive signal for the appropriate
protein. The results were averaged for control and PEX cases.
Statistical analysis
The data are presented as the mean ± standard error
of the mean. Statistical evaluation of significant differences
between the groups of patients was performed using a two-tailed
Mann-Whitney U test (for the length of cells and the intensity of
AEC IHC staining). P<0.05 was considered to indicate a
statistically significant difference, and GraphPad Prism 5.0
(GraphPad Software, Inc., La Jolla, CA, USA) was used for these
statistical analyses.
Results
Staining and quantitation of IHC
The IHC staining of MGST1 and clusterin in
paraffin-embedded surgical specimens were first determined by light
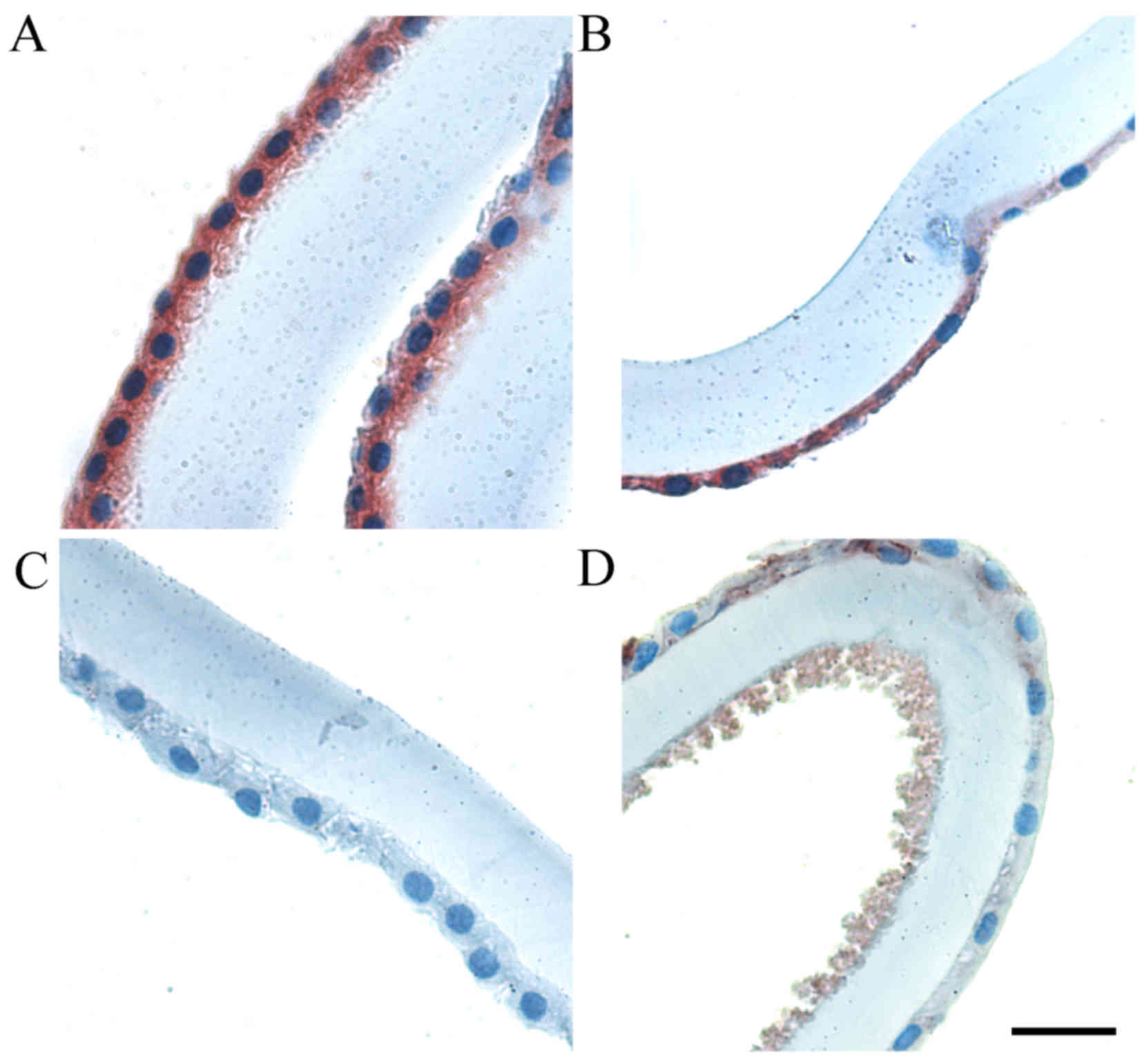
microscopy (Figs. 1 and 2, respectively). Positive MGST1 expression
was visible in adherent LECs in control patients (Fig. 1A and B). However, examination of IHC
labeling of this protein in PEX patients revealed an absence or
only traces of IHC labeling (Fig. 1C and
D, respectively). Notably, in the same paraffin-embedded
anterior lens capsules and adherent LECs, the accumulation of
pseudoexfoliation material was detected (Fig. 1D). MGST1 expression was significantly
lower in PEX patients compared with control group patients
(P<0.001). The PEX LECs were characterized by a lower level of
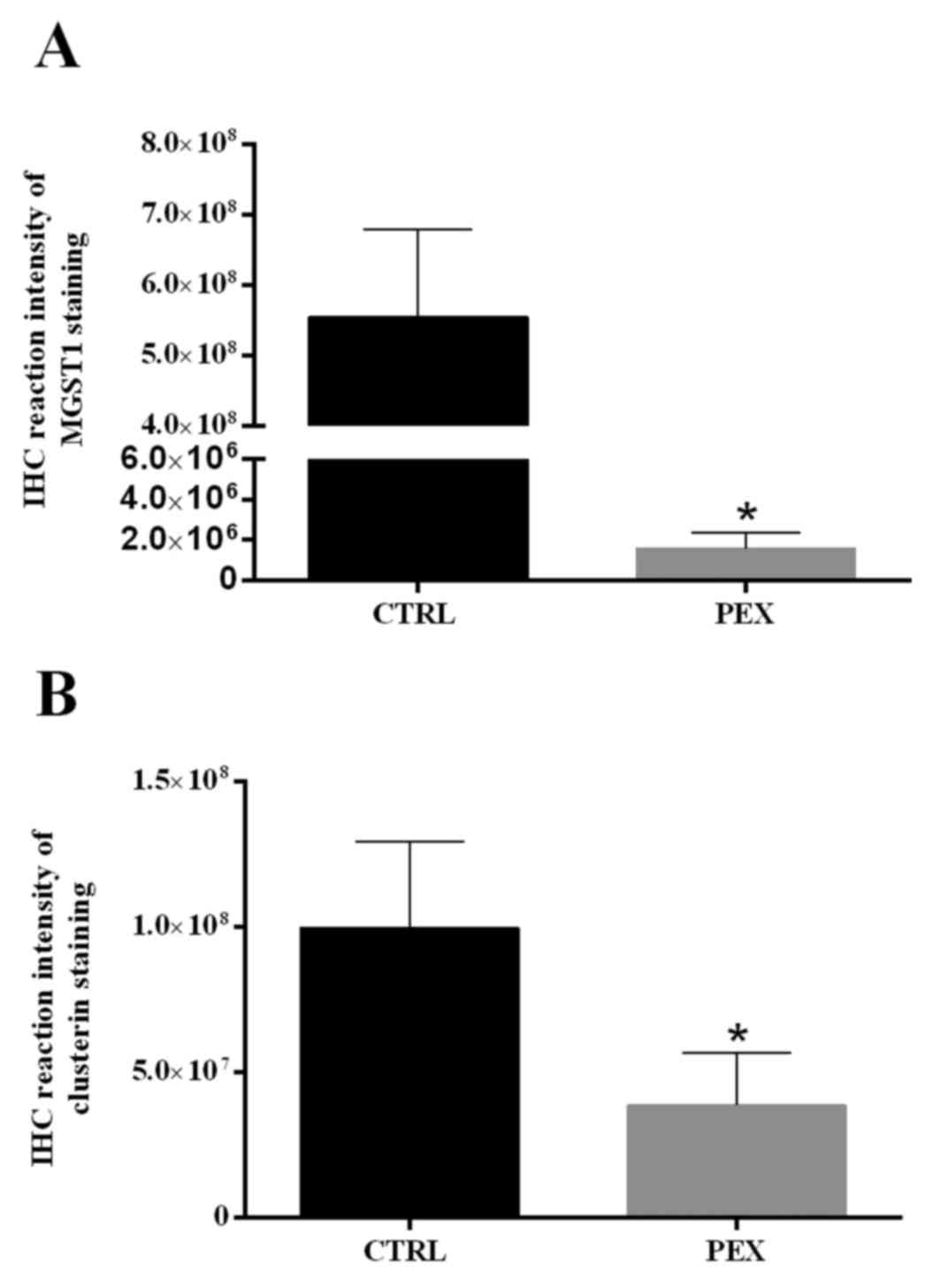
MGST1 expression compared with the control (Fig. 3A).
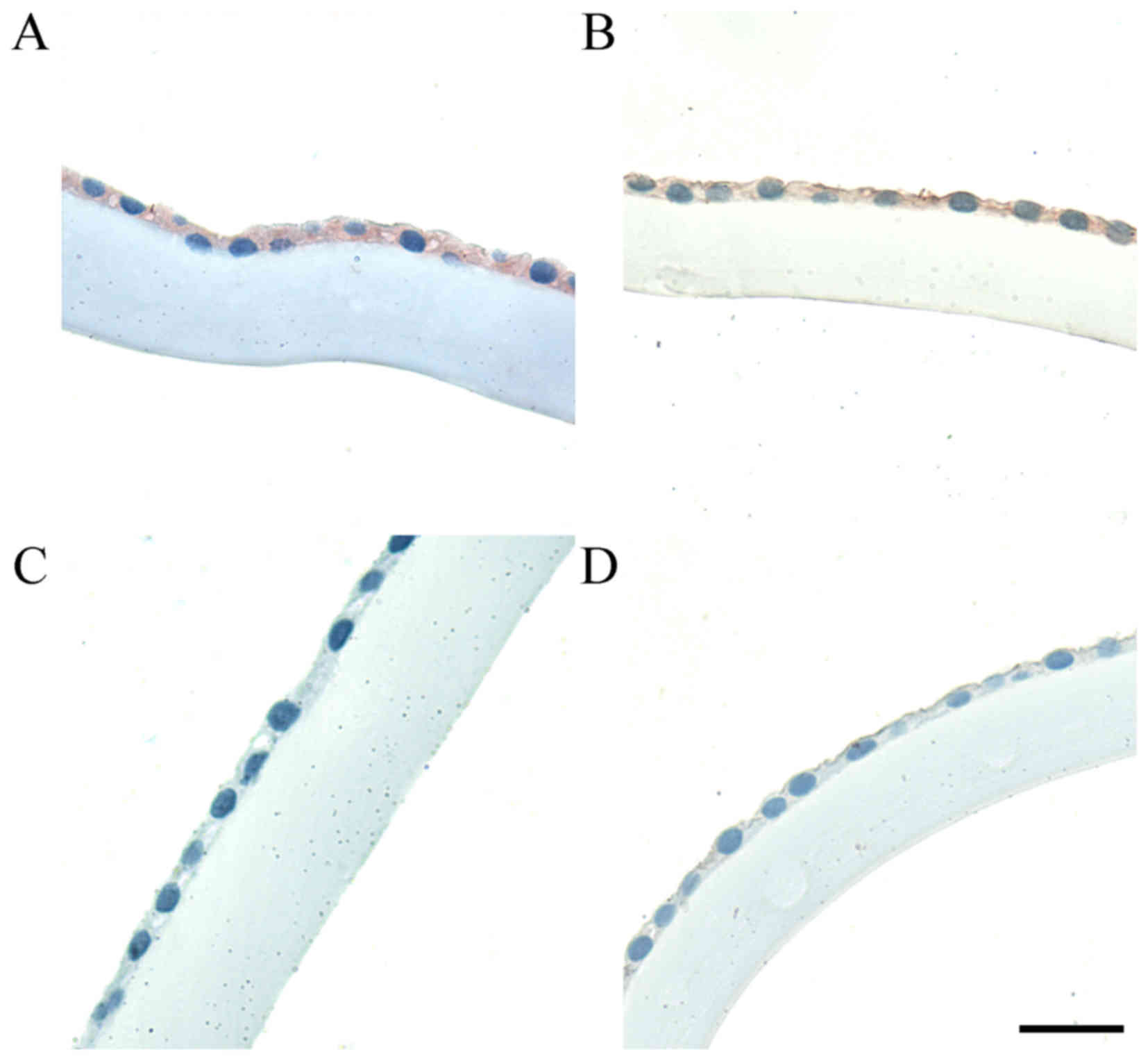
The LECs in the control group also revealed higher
expression of clusterin, whereas the PEX patients demonstrated
absent or weakly positive clusterin-staining (Fig. 2C and D, respectively). Two types of
clusterin immunoreactivity are presented, in both disease and
control cases, in order to demonstrate the variation in staining
intensity; the light microscopic observations were supported by a
statistical analysis of the IHC reaction intensity of clusterin. As
reported in Fig. 3B, the intensity
of clusterin staining was statistically higher in the LECs of
patients compared with the control group (P<0.001; Fig. 3B).
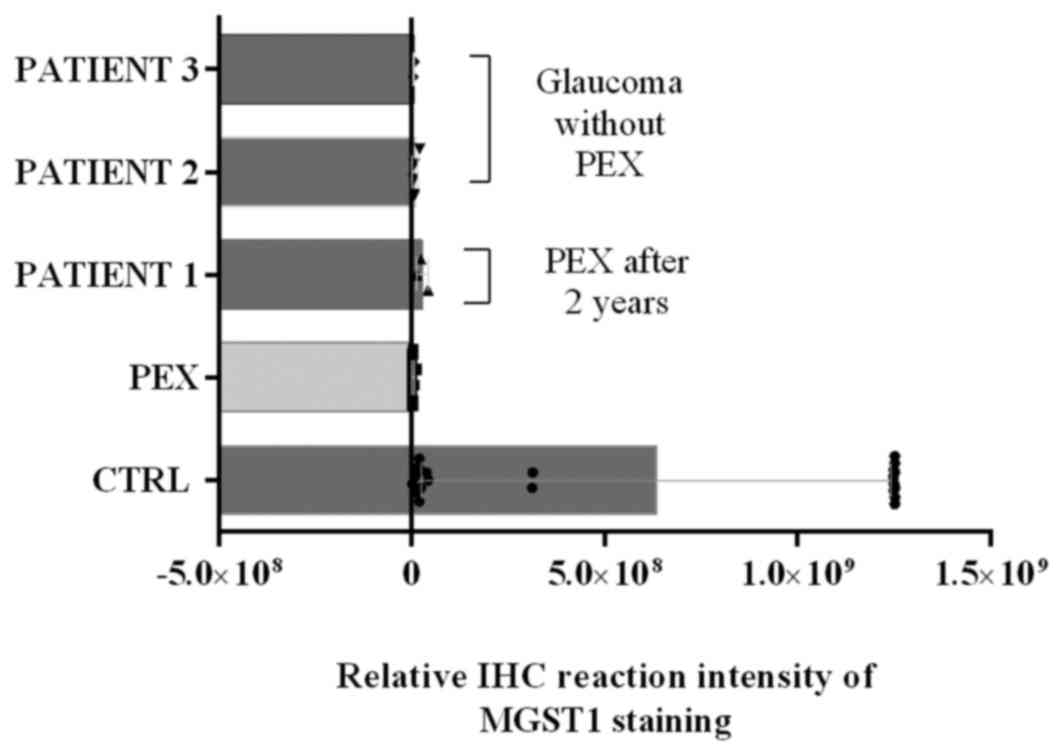
IHC quantitation illustrated that the
immunoreactivity of MGST1, as determined by the protein expression
level, was similar in a few control group cases as in patients with
PEX; for this reason, the disease history of selected patients was
investigated (Fig. 4). The collected
data revealed that a number of patients from control groups were
classified as patients diagnosed as PEX after 2 years. Furthermore,
two patients classified as control patients have since been
diagnosed with glaucoma. These data are reported in Fig. 4.
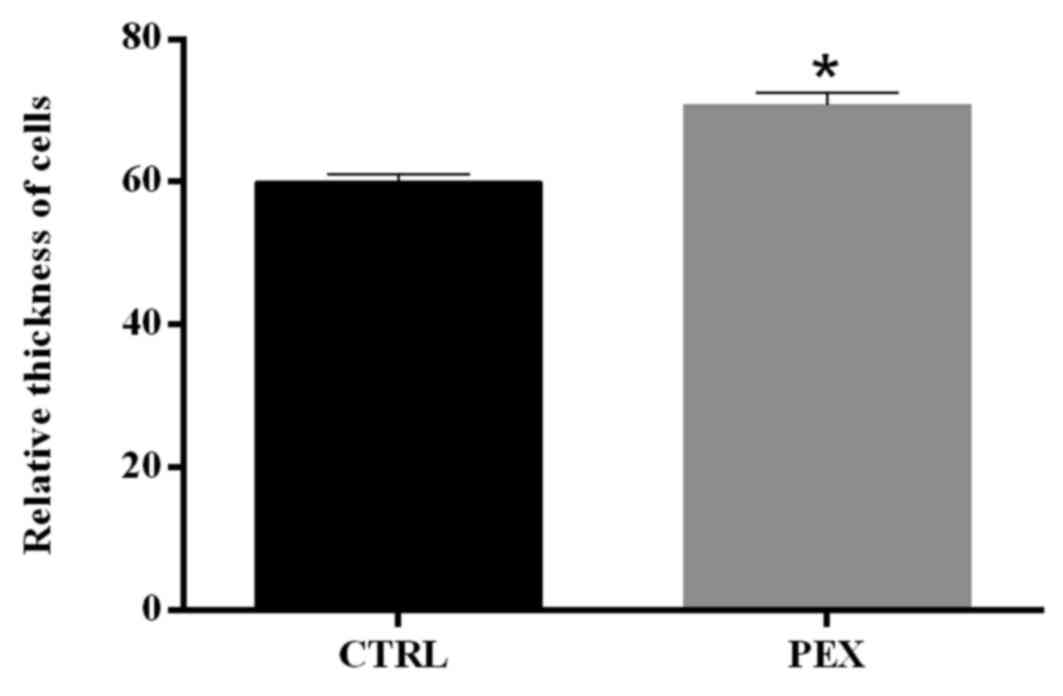
Thickness of cells
As the mechanism of accumulation of
pseudoexfoliation material, and in light of the present evaluation
of IHC images and differences in the LECs, the differences in the
thickness of cells were investigated. PEX LECs were characterized
by a statistically significant increase in the vertical thickness
compared with control cells (P<0.001; Fig. 5), which may suggest upregulated
secretion and/or changes in organization of junctions between
LECs.
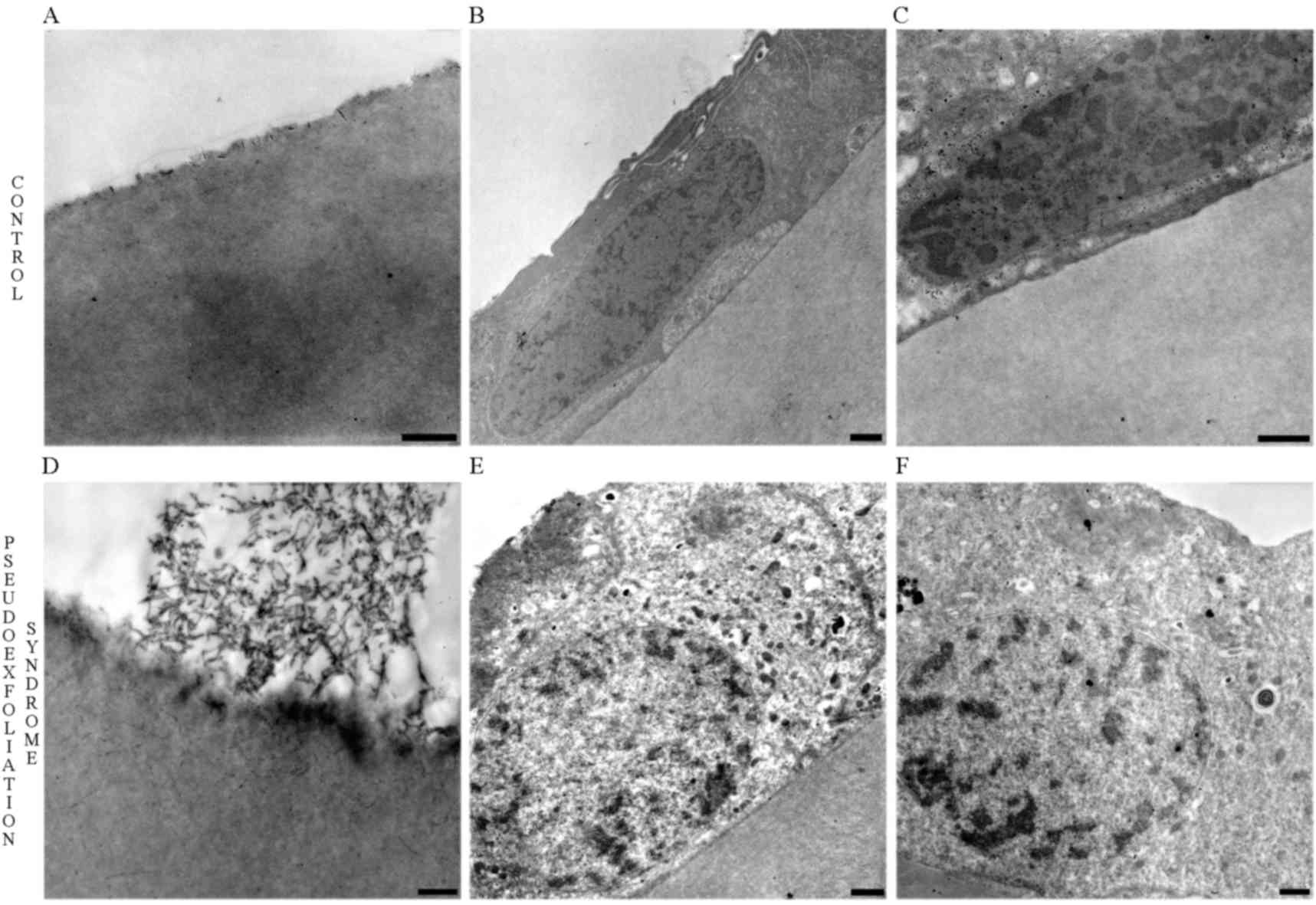
Transmission electron microscopy
In order to confirm the differences in the thickness
of the cells, increased secretion and changes in the organization
of cell-cell junctions, transmission electron microscopy was used.
The above results were confirmed, and are illustrated in Fig. 6. The normal lenses of control group
patients are presented in Fig. 6A.
The evaluation of morphological alterations in LECs revealed that
control LECs and their nuclei were characterized by a flattened and
stretched appearance (Fig. 6B and
C). Accumulation of PEX material was observed in the front side
of the lens (Fig. 6D) and PEX LECs
appeared to be thicker and contained oval nuclei (Fig. 6E and F). Furthermore, ultrastructural
analysis of PEX LECs revealed a markedly increased number of
electron-dense secretory granules and enlargement of the cell-cell
junctional area (Fig. 6E and F).
Discussion
Several mechanisms of pseudoexfoliation material
formation are possible, but a molecular background is more
frequently suggested (30,33–35).
Oxidative stress is believed to contribute to the pathogenesis of
many diseases. Reactive oxygen species (ROS) are formed as normal
metabolic products and are important in normal cellular
functioning, but their production may be increased under
pathological conditions and cause cellular damage (3,36).
Exogenous factors (e.g., exposure to environmental
chemicals, radiation, and atmospheric oxygen) may increase their
production. The imbalance between ROS production and the
antioxidant defense mechanisms results in oxidative stress leading
to cellular damage (37,38). Lobo et al (39) and Sies (40) distinguished three levels of the
antioxidant defense system, which is comprised of various enzymes
such as superoxide dismutase, catalase and glutathione peroxidase,
the low molecular weight antioxidants such as vitamins, and enzymes
involved in this pathway such as aldehyde dehydrogenase 1 and
repair enzymes such as glutathione peroxidase and microsomal
glutathione S-transferase 1.
The present results demonstrated a significantly
lower MGST1 IHC reaction intensity in the PEX group compared with
the control group (P=0.0001). Strzalka-Mrozik et al
(41) revealed significantly higher
mRNA levels of SOD2, ALDH1A1, and MGST1 in the anterior lens
capsules of patients with PEX and cataracts compared with control
cataracts subjects. Zenkel et al (42) reported that the expression of
MAPKp38, heat shock proteins (HSP40, HSP60) and superoxide
dismutase (SOD2) were increased up to threefold in PEX specimens.
In contrast, a large set of cytoprotective gene products, including
antioxidant defense enzymes (the glutathione S-transferases MGST1
and GSTT1), ubiquitin-conjugating enzymes (UBE2A, UBE2B), the DNA
repair protein MLH1 and the stress-inducible transcription factor
GADD153, were found to be consistently downregulated up to
threefold in PEX specimens on both the mRNA and protein levels
(42). These findings correspond
with the present results.
The available data suggest that chronic oxidative
stress, in combination with weakened cytoprotective and repair
strategies, affects the abnormal matrix metabolism by induction of
a persistent proinflammatory state and activation of the
profibrotic growth factor TGF-beta1. Oxidative stress, therefore,
appears to represent a modifiable risk factor in the management of
patients with PEX syndrome/glaucoma (43).
In the current research, divergence in the
distribution of IHC measurements and the immunoreactivity of MGST1
were observed in three cases from the control group. These were
similar to the protein expression level in PEX patients and,
therefore, their disease history was followed. Notably, one control
patient developed PEX two years following study commencement and,
in two others, glaucoma has been diagnosed in a longer term follow
up. This suggests that it may be possible to detect
pseudoexfoliation syndrome/glaucoma prior to its clinical
manifestation.
Clusterin is a highly efficient extracellular
chaperone, and its deficiency in the LECs of eyes with PEX may
therefore promote the stress-induced aggregation and stable
deposition of the characteristic pathologic extracellular matrix
product (32). Zenkel et al
(44) observed that clusterin levels
in the aqueous humor were significantly reduced in the eyes of PEX
patients compared with patients with normal and glaucomatous
control eyes. The present findings confirm this observation. A
marked immunopositive reaction for clusterin was observed in the
control group, whereas the PEX patients were characterized by
unstained or weakly positive LECs. The obtained relative results
were statistically significant (P=0.0005).
In fact, subtle chronic inflammatory processes, also
termed ‘molecular inflammation’, have been suggested to underlie
the causes of numerous age-associated chronic degenerative
diseases, such as Alzheimer's disease, atherosclerosis and
cardiovascular disorders (45). In
accordance with this hypothesis, one major causative factor in
chronic tissue injury is believed to be oxidative stress; in older
individuals, oxidative stress combined with weakened cytoprotective
strategies and stress-response mechanisms may lead to a persistent
proinflammatory state (30). One of
the multiple functions of clusterin is to act as an inhibitor of
the complement system, an important mediator of inflammation
(46). Significant clusterin
deficiency in the LECs of patients with PEX compared with the LECs
of patients without PEX may suggest an impaired cytoprotective
mechanism or its depletion in pseudoexfoliation syndrome.
The mechanisms controlling capsular synthesis and
the specification of the basal surface of lens cells are unclear.
During lens reconstitution from epithelium/capsule fragments in the
developing chick, cells that lose contact with the basement
membrane undergo anoikis (apoptosis due to loss of matrix
attachment) while cells attached to the capsule fragment migrate to
reestablish their normal polarity within the eye (47). It is known that the elasticity within
the capsule is directly correlated with its thickness (48). All cells involved in the exfoliation
syndrome process demonstrated common ultrastructural signs of
active fibrillogenesis and metabolic activation, such as increased
vesicular transport to the cell surface, extracellular material
formation within invaginations of cellular surfaces and a prominent
rough endoplasmic reticulum (49).
To our knowledge, this is the first report comparing LEC thickness
and nuclei shape in patients with PEX to controls. Significant
differences in cell morphology alterations may be due to the high
metabolic activation of LECs in PEX, but this requires additional
investigation.
The present findings suggest that low MGST1 and
clusterin expression level may be an early clinical sign of
pseudoexfoliation syndrome and that oxidative stress may serve an
important role in the etiology of PEX, but additional investigation
may serve to provide further clarity.
References
|
1
|
Lindberg JG: Clinical investigations on
depigmentation of the pupillary border and translucency of the
iris: In cases of senile cataract and in normal eyes in elderly
persons. Acta Ophthalmol Suppl. 190:1–96. 1989.PubMed/NCBI
|
|
2
|
Schlötzer-Schrehardt UM, Koca MR, Naumann
GO and Volkholz H: Pseudoexfoliation syndrome. Ocular manifestation
of a systemic disorder? Arch Ophthalmol. 110:1752–1756. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Streeten BW, Li ZY, Wallace RN, Eagle RC
Jr and Keshgegian AA: Pseudoexfoliative fibrillopathy in visceral
organs of a patient with pseudoexfoliation syndrome. Arch
Ophthalmol. 110:1757–1762. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Mitchell P, Wang JJ and Smith W:
Association of pseudoexfoliation syndrome with increased vascular
risk. Am J Ophthalmol. 124:685–687. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Schumacher S, Schlötzer-Schrehardt U,
Martus P, Lang W and Naumann GO: Pseudoexfoliation syndrome and
aneurysms of the abdominal aorta. Lancet. 357:359–360. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ritland JS, Egge K, Lydersen S, Juul R and
Semb SO: Exfoliative glaucoma and primary open-angle glaucoma:
Associations with death causes and comorbidity. Acta Ophthalmol
Scand. 82:401–404. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Allingham RR, Loftsdottir M,
Gottfredsdottir MS, Thorgeirsson E, Jonasson F, Sverisson T, Hodge
WG, Damji KF and Stefánsson E: Pseudoexfoliation syndrome in
Icelandic families. Br J Ophthalmol. 85:702–707. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Stafiej J, Malukiewicz G, Lesiewska-Junk
H, Rość D and Kaźmierczak K: Endothelial cell markers in patients
with pseudoexfoliation syndrome. ScientificWorldJournal.
2012:8639492012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Tarkkanen A: Is exfoliation syndrome a
sign of systemic vascular disease? Acta Ophthalmol. 86:832–836.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Arnarsson A, Damji KF, Sverrisson T,
Sasaki H and Jonasson F: Pseudoexfoliation in the Reykjavik Eye
Study: Prevalence and related ophthalmological variables. Acta
Ophthalmol Scand. 85:822–827. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Mitchell P, Wang JJ and Hourihan F: The
relationship between glaucoma and pseudoexfoliation: The Blue
Mountains Eye Study. Arch Ophthalmol. 117:1319–1324. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Andrikopoulos GK, Mela EK, Georgakopoulos
CD, Papadopoulos GE, Damelou AN, Alexopoulos DK and Gartaganis SP:
Pseudoexfoliation syndrome prevalence in Greek patients with
cataract and its association to glaucoma and coronary artery
disease. Eye(Lond). 23:442–447. 2009.PubMed/NCBI
|
|
13
|
Forsius H: Exfoliation syndrome in various
ethnic populations. Acta Ophthalmol Suppl. 184:71–85.
1988.PubMed/NCBI
|
|
14
|
Ritch R: Exfoliation syndrome and
occludable angles: Trans. Am Ophthalmol Soc. 92:848–855. 1994.
|
|
15
|
Damji KF, Bains HS, Stafansson E,
Loftsdottir M, Sverrisson T, Thorgeirsson E, Jonasson F,
Gottfredsdottir M and Allingham RR: Is pseudoexfoliation syndrome
inherited? A review of genetic and nongenetic factors and a new
obserwation. Ophthalmic Genet. 19:175–185. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Alfaiate M, Leite E, Mira J and Cunha-Vaz
JG: Prevalance and surgical complications of pseudoexfoliation
syndrome in Portuguese patients with senile cataract. J Cataract
Refract Surg. 22:972–976. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Guzek JP, Holm M, Cotter JB, Cameron JA,
Rademaker WJ, Wissinger DH, Tonjum AM and Sleeper LA: Risk factors
for intraoperative complicatons in 1000 extracapsular cataract
cases. Ophthalmology. 94:461–466. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Abbasoğlu OE, Hoşal B, Tekeli O and Gürsel
E: Risk factors for vitreous loss in cataract surgery. Eur J
Ophthalmol. 10:227–232. 2000.PubMed/NCBI
|
|
19
|
Drolsum L, Haaskjold E and Davanger M:
Pseudoexfoliation syndrome and extracapsular cataract extraction.
Acta Ophthalmol (Copenh). 71:765–770. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lumme P and Laatikainen L: Exfoliation
syndrome and cataract extraction. Am J Ophthalmol. 116:51–55. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hyams M, Mathalone N, Herskovitz M, Hod Y,
Israeli D and Geyer O: Intraoperative complications of
phacoemulsification in eyes with and without pseudoexfoliation. J
Cataract Refract Surg. 31:1002–1005. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Küchle M, Viestenz A, Martus P, Händel A,
Jünemann A and Naumann GO: Anterior chamber depth and complications
during cataract surgery in eyes with pseudoexfoliation syndrome. Am
J Ophthalmol. 129:281–285. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Freissler K, Küchle M and Naumann GO:
Spontaneous dislocation of the lens in pseudoexfoliation syndrome.
Arch Ophthalmol. 113:1095–1096. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sufi AR, Singh T, Mufti AA and Rather MH:
Outcome of Phacoemulsification in patients with and without
Pseudoexfoliation syndrome in Kashmir. BMC Ophthalmol. 12:132012.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ascaso FJ, Huerva V and Grzybowski A:
Epidemiology, etiology, and prevention of late IOL-capsular bag
complex dislocation: Review of the literature. J Ophthalmol.
2015:8057062015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Guo S, Gewirtz M, Thaker R and Reed M:
Characterizing pseudoexfoliation syndrome through the use of
ultrasound biomicroscopy. J Cataract Refract Surg. 32:614–617.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ünsal E, Eltutar K, Muftuoglu I, Akcetin
TA and Acar Y: Ultrasound biomicroscopy in patients with unilateral
pseudoexfoliation. Int J Ophthalmol. 8:754–758. 2015.PubMed/NCBI
|
|
28
|
Johnson M: What controls aqueous humour
outflow resistance. Exp Eye Res. 82:545–557. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Barbosa AM, Frare AB, Costa NB, Silva RE
and Moura KK: GSTM1 polymorphism in patients with primary
open-angle glaucoma. Genet Mol Res. 11:3256–3262. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zenkel M, Lewczuk P, Jünemann A, Kruse FE,
Naumann GO and Schlötzer-Schrehardt U: Proinflammatory cytokines
are involved in the initiation of the abnormal matrix process in
pseudoexfoliation syndrome/glaucoma. Am J Pathol. 176:2868–2879.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Tanito M, Kaidzu S, Takai Y and Ohira A:
Status of Systemic oxidative stresses in patients with primary
open-angle glaucoma and pseudoexfoliation syndrome. PLoS One.
7:e496802012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Burdon KP, Sharma S, Hewitt AW, McMellon
AE, Wang JJ, Mackey DA, Mitchell P and Craig JE: Genetic analysis
of the clusterin gene in pseudoexfoliation syndrome. Mol Vis.
14:1727–1736. 2008.PubMed/NCBI
|
|
33
|
Schlötzer-Schrehardt U: Molecular
pathology of pseudoexfoliation syndrome/glaucoma-new insights from
LOXL1 gene associations. Exp Eye Res. 88:776–785. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Elhawy E, Kamthan G, Dong CQ and Danias J:
Pseudoexfoliation syndrome, a systemic disorder with ocular
manifestations. Hum Genomics. 6:222012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Schlötzer-Schrehardt U: Genetics and
Genomics of Pseudoexfoliation Syndrome/Glaucoma. Middle East Afr J
Ophthalmol. 18:30–36. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Schlötzer-Schrehardt UM, Koca MR, Naumann
GO and Volkholz H: Pseudoexfoliation syndrome. Ocular manifestation
of a systemic disorder? Arch Ophthalmol. 110:1752–1756. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Comhair SA and Erzurum SC: Redox control
of asthma: Molecular mechanisms and therapeutic opportunities.
Antioxid Redox Signal. 12:93–124. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Yildirim Z, Ucgun NI and Yildirim F: The
role of oxidative stress and antioxidants in the pathogenesis of
age-related macular degeneration. Clinics (Sao Paulo). 66:743–746.
2011.PubMed/NCBI
|
|
39
|
Lobo V, Patil A, Phatak A and Chandra N:
Free radicals, antioxidants and functional foods: Impact on human
health. Pharmacogn Rev. 4:118–126. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Sies H: Total antioxidant capacity:
Appraisal of a concept. J Nutr. 137:1493–1495. 2007.PubMed/NCBI
|
|
41
|
Strzalka-Mrozik B, Prudlo L, Kimsa MW,
Kimsa MC, Kapral M, Nita M and Mazurek U: Quantitative analysis of
SOD2, ALDH1A1 and MGST1 messenger ribonucleic acid in anterior lens
epithelium of patients with pseudoexfoliation syndrome. Mol Vis.
19:1341–1349. 2013.PubMed/NCBI
|
|
42
|
Zenkel M, Kruse FE, Naumann GO and
Schlötzer-Schrehardt U: Impaired cytoprotective mechanisms in eyes
with pseudoexfoliation syndrome/glaucoma. Invest Ophthalmol Vis
Sci. 48:5558–5566. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Schlötzer-Schrehardt U: Oxidative stress
and pseudoexfoliation glaucoma. Klin Monbl Augenheilkd.
227:108–113. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Zenkel M, Kruse FE, Jünemann AG, Naumann
GO and Schlötzer-Schrehardt U: Clusterin deficiency in eyes with
pseudoexfoliation syndrome may be implicated in the aggregation and
deposition of pseudoexfoliative material. Invest Ophthalmol Vis
Sci. 47:1982–1990. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Maggio M, Guralnik JM, Longo DL and
Ferrucci L: Interleukin-6 in aging and chronic disease: A
magnificent pathway. J Gerontol Biol Sci Med Sci. 61:575–584. 2006.
View Article : Google Scholar
|
|
46
|
Calero M, Rostagno A, Frangione B and
Ghiso J: Clusterin and Alzheimer's disease. Subcell Biochem.
38:273–298. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Danysh BP and Duncan MK: The Lens Capsule.
Exp Eye Res. 88:151–164. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Krag S and Andreassen TT: Mechanical
properties of the human lens capsule. Prog Retin Eye Res.
22:749–767. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Zenkel M and Schlötzer-Schrehardt U: The
composition of exfoliation material and the cells involved in its
production. J Glaucoma. 23(8): Suppl 1. S12–S14. 2014. View Article : Google Scholar : PubMed/NCBI
|