Introduction
Colon cancer is the third most common cancer among
women (after breast and lung cancers) and men (following prostate
and lung cancers) and the third most common cancer-related cause of
mortality globally, particularly in western and developed nations
(1,2). In addition, the incidence of colon
cancer is higher in men than women and strongly increases with age
(3). At present, colon cancer is one
of the most frequently encountered malignant tumors, and the
incidence of colon cancer ranks third among global gastrointestinal
tumors (4). Economic development and
improvements in living standards have caused marked changes in
people's daily diets, and the morbidity and mortality of colon
cancer have exhibited concurrent rapid increases in the last two
decades. Morbidity and mortality are significantly higher in
developed coastal areas than in the mainland. The incidence of
colon cancer has strongly increased globally and is closely
associated with elements of the so-called western lifestyle
(5).
Colon cancer has long been associated with apoptosis
and polygenic mutations (4,6). The genetics of colon cancer have been
actively researched in the final decades of the past century. The
major genes identified as being associated with colon cancer are
adenomatous polyposis coli (APC), B-cell lymphoma 2 (Bcl-2),
Bcl-2-associated X protein (Bax) and cytochrome c (7). Studies have shown that APC is a tumor
suppressor gene. APC gene mutations can be detected in nearly all
familial large adenomas and in 60–80% of sporadic colorectal
cancers (CRCs) (8,9). Apoptosis is regulated in part by the
Bcl-2 gene, which promotes cell survival and lengthens cell life by
hindering programmed cell death (1,10). The
expression of the Bcl-2 gene in colon cancer is higher than that in
normal colonic mucosa and gradually increases as adenomas progress
to early adenocarcinomas (11).
Additionally, Bcl-2 can prevent the release of cytochrome c
from the mitochondria to the cytoplasm and thus inhibit apoptosis
(12). Bax is a member of the Bcl-2
gene family whose products are associated with the Bcl-2-homologous
proteins (13). The biological
activities of Bcl-2 are antagonized by Bax. The main function of
Bax is to accelerate cell apoptosis (14). Cytochrome c is a water-soluble
protein that is encoded by nuclear genes with a molecular weight of
12–13 kDa. The main function of cytochrome c is to adjust
mitochondrial energy metabolism (15). Studies have revealed that cytochrome
c also plays an important role in cell apoptosis via the
transmission of the apoptosis signal and amplification of the
regulation of apoptosis (15,16).
The occurrence of colon cancer is attributable to a
number of causes, but epidemiological studies have shown that
dietary factors are important in the prevention of human colon
cancer (17). Understanding the
cause of colon cancer would undoubtedly contribute to better
surveillance and early prevention and thus reduce cancer morbidity
(18). Increasing the amount of
fiber in the diet should reduce the incidence of cancers,
particularly those of the colon and rectum (19,20).
Furthermore, resistant starch (RS) can reduce the incidence of
colon cancer (21). Therefore,
improved eating habits and greater dietary adjustments are the most
economical and effective means of prevention and control.
RS is a starch, the chemical structure of which is
different from that of fiber, and the properties of RS are similar
to those of soluble fiber. RS is a new food ingredient and has a
low glycemic index. RS is termed an anti-digested starch on the
basis of the fraction of the starch that cannot be digested in the
small intestine and is instead partially fermented in the large
intestine to produce short-chain fatty acids and other products
(22). In our previous study, RS was
prepared from indica starch using a new method that combines
α-amylase, pullulanase and heat-moisture treatment. Indica rice
resistant starch (IR-RS) products produce a mixture of B- and
V-type X-ray diffraction patterns and a crystallinity of 51.0%
(23). In vivo experiments
revealed that IR-RS is able to improve the symptoms associated with
high blood sugar and the complications of diabetes in mice.
Building upon our previous study, this study primarily focuses on
the effect of IR-RS on azoxymethane (AOM)-induced colon cancer in
mice and illuminates the mechanism of action of this effect. The
results of this study should provide a scientific basis for colon
cancer prevention and control measures, and be highly significant
for the prevention of chronic diseases.
Materials and methods
Materials
Dual modification-treated (DMT) IR-RS was prepared
according to the procedure outlined by Zhou et al (23). The RS content of the DMT IR-RS was
51.3%. AOM was purchased from Sigma-Aldrich (St. Louis, MO, USA).
Methylene blue, 10% buffered formalin and other chemicals and
reagents were analytical grade.
Animals
One hundred male SCXK (su) 2011-001 mice (SPF mice
that are free of specific microbes and parasites and so reduce
experimental interference) were purchased from Changzhou Card Vince
Laboratory Animal Co., Ltd. (‘Changzhou, China). The experiments
were approved by the ethical committee of the Animal Experiment
Committee of China and conform to national guidelines on the care
and use of laboratory animals (24).
AOM-induced colon cancer mice
During the experiments, the mice were housed in an
approved laboratory animal facility and maintained under controlled
temperature (25°C) and lighting (12 h light/dark cycle) conditions
for a 7-day adaptation period. AOM (10 mg/kg) diluted in normal
saline was administered to the mice by intraperitoneal injection
once per week for 2 consecutive weeks.
Experimental diets and general
observation
Seven days after the first AOM injection, the model
mice were placed in cages with 12 animals each and divided into the
following five groups: Low-dose group (LG), middle-dose group (MG),
high-dose group (HG), model control (MC) and normal control (NC).
The NC did not receive any treatment and other groups were treated
with AOM. After the modeling and immediately after grouping, the
mice in the LG, MG and HG groups were administered 2, 4 and 8 g/kg
DMT IR-RS aqueous solutions, respectively, once daily for 6 weeks
by gavage. The mice in the MC and NC groups were given the same
dose of distilled water by gavage. Each group had free access to
food and water. The water bottles were filled twice daily. The
drinking, diets and activities of the mice were observed daily, and
the mice were weighed once per week.
Gastrointestinal emptying of mice
Gastrointestinal emptying was measured as previously
described, with slight modifications (25,26).
Normal mice (n=5) were fasted for >12 h and had free access to
water. The fasted mice were gavaged with different amounts of DMT
IR-RS (2, 4 and 8 g/kg) or distilled water (0.4 ml). Two hours
later, each mouse was gavaged with 0.4 ml blue ink. The mice were
then sacrificed by cervical dislocation. The gut of each mouse was
immediately exposed by laparotomy. The guts were expanded and a
standard measuring tape was used to measure the distance that the
blue ink had advanced in the gut (from the start of the gastric
pylorus to the point at which the blue ink ended). Gastrointestinal
emptying (%) was calculated according to the following formula:
Gastrointestinal emptying (%) = distance advanced/total length of
the gut × 100.
Observation of aberrant crypt foci
(ACFs)
After 8 weeks of gavage, all mice were sacrificed by
cervical dislocation, and the colons were removed immediately,
flushed with ice-cold normal saline to remove the intestinal
contents, cut with scissors longitudinally, placed mucosal side up
on strips of filter paper, and another filter paper was used to
cover the mucosal surface. Finally, the colons were fixed in 10%
buffered formalin for 24 h. Each colon was then cut into 2–3 cm
long strips. All segments were put into Petri dishes that contained
0.5% methylene blue solution for 2.5 min and then placed in another
Petri dish with a buffer to wash away the excess dye. ACFs were
immediately observed under a Gel Imaging System (BIS910; Beijing
Dongsheng Innovative Biotechnology Co., Ltd., Beijing, China).
Reverse transcription-polymerase chain
reaction (RT-PCR) for the detection of APC, Bax, Bcl-2 and
cytochrome c mRNA
The primer sequences for APC, Bax, Bcl-2, cytochrome
c and GADPH were designed using Primer Premier 5 (Premier
Biosoft International, Palo Alto, CA, USA) and synthesized by
Shanghai Sangon Biological Engineering Technology & Service Co.
Ltd (Shanghai, China); the primer sequences are listed in Table I.
 | Table I.Primer design for RT-PCR. |
Table I.
Primer design for RT-PCR.
| Gene | Primer sequence
(5′-3′) | Product size
(bp) |
|---|
| Cytochrome
c | Forward:
ACCAAATCTCCACGGTCTGTTC | 192 |
|
| Reverse:
GTCTGCCCTTTCTCCCTTCTTC |
|
| Bax | Forward:
ACAGATCATGAAGACAGGGG | 298 |
|
| Reverse:
AAAGTAGAAGAGGGCAACCA |
|
| Bcl-2 | Forward:
GTCACAGAGGGGCTACGAGT | 212 |
|
| Reverse:
GGGTCAGATGGACCACAGG |
|
| APC | Forward:
GGAAGATTGGTTGTAAGTGAAAGGA | 525 |
|
| Reverse:
CAAAAAGCAGAGTTAGAACAGGAGG |
|
| GAPDH | Forward:
CCCTTCATTGACCTCAACTAC | 244 |
|
| Reverse:
CCACGACTCATACAGCACC |
|
The total RNA of each mouse was extracted from the
colon according to the method developed by Yuan et al
(27) with slight modifications. The
RNA extracts were placed in ultra-low temperature freezers (−80°C)
until use. cDNA was synthesized from total RNA with the Revert Aid
First Strand cDNA Synthesis kit (Invitrogen; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) according to the manufacturer's
protocol, and amplified by PCR. PCR was conducted for 30 cycles.
Each amplification cycle consisted of 30 sec at 93°C for
denaturation, 30 sec of cytochrome c primer exposure at
59°C, Bax primer exposure at 55°C, Bcl-2 primer exposure at 55°C,
APC primer exposure at 62°C or GADPH primer exposure at 55°C for
primer annealing, and 1 min at 72°C for extension. All PCR products
were mixed with 2 µl goldview loading dye (Invitrogen; Thermo
Fisher Scientific, Inc.) and subjected to electrophoresis using
1.5% agarose gels containing 0.5 µg/ml ethidium bromide
(Invitrogen; Thermo Fisher Scientific, Inc.).
Statistical analysis
All experiments were repeated ≥10 times. All
experimental data are expressed as the mean ± standard deviation.
Differences between test subjects and model controls were evaluated
using Student's t-tests. P<0.05 was considered to indicate a
statistically significant difference.
Results and Discussion
Observations of the daily activities
and body weights of the mice
During the experiment, each group of mice exhibited
normal eating, drinking and activity. The changes in body weight in
each group of mice during the experiment were analyzed, and the
results are summarized in Table II.
The results revealed no significant differences (P>0.05) in
initial body weight (25.65±0.33 g) prior to the AOM injections
between the five experimental groups. After the AOM injections
(week 2), the body weights of the mice in the NC group increased by
22.4%, while the other four groups of mice gained less weight than
did the normal group, and there were no significant difference
between the four experimental groups. After 6 weeks of feeding with
RS solution (LG, 2 g/kg; MG, 4 g/kg; HG, 8 g/kg), the gains in body
weight of the mice in the LG, MG and HG groups were lower than
those of the NC group, and the weight gain of the HG group was
significantly different from that of the NC group (P<0.05). The
body weights of the MC, LG and MG groups increased by 57.5, 52.7
and 26.4%, respectively, and there were no significant differences
relative to the NC group (P>0.05).
 | Table II.Body weight of each mouse. |
Table II.
Body weight of each mouse.
|
| Body weights
(g) |
|---|
|
|
|
|---|
| Group | Week 1 | Week 2 | Week 4 | Week 6 | Week 8 |
|---|
| NC | 25.79±0.45 | 31.56±0.15 | 36.41±0.07 | 38.98±0.35 | 41.34±0.65 |
| MC | 25.88±0.15 |
27.91±0.28a | 35.61±0.20 | 39.37±0.27 | 40.76±0.51 |
| LG | 25.14±0.33 |
27.24±0.45a |
30.47±0.32a | 33.65±0.45 | 38.36±0.08 |
| MG | 25.52±0.22 |
27.53±0.32a |
26.60±0.26b |
28.46±0.13a | 32.25±0.11 |
| HG | 25.94±0.25 |
26.95±0.35a |
26.75±0.06b |
27.52±0.09a |
28.22±0.65a |
The results revealed that the body weights of the MC
group increased rapidly and were not significantly different
(P>0.05) from those of the NC group at 8 weeks. This finding
indicates that the inchoate colon cancer induced by AOM had no
clear effect on the body weights of the mice. However, the body
weight gains in the RS-treated groups exhibited a dose-dependent
reduction, which indicates that RS effectively controlled the
increases in the body weights of the mice. In addition, some
differences were observed in the food intake of the mice in
different treatment groups. The amount of food intake decreased in
mice after feeding with the RS solution, and the NC and MC groups
exhibited normal eating. RS does not readily degrade in the small
intestine; thus, RS can reduce the postprandial glycemic response
and food intake and increase satiety (28). RS can also be fermented to
short-chain fatty acids by microorganisms in the colon (29). Following the rapid absorption of
short-chain fatty acids by colorectal tissue, energy is stored and
the osmotic pressure is reduced; short-chain fatty acids are
important in maintaining the morphology and function of the normal
colon and colonic epithelial cell function (30). Therefore, RS can reduce the
intestinal pH and the quantity of carcinogens, and promote the
absorption of trace elements.
Effects of RS on gastrointestinal
emptying
Gastrointestinal emptying plays different and
important roles in accommodating gastrointestinal function
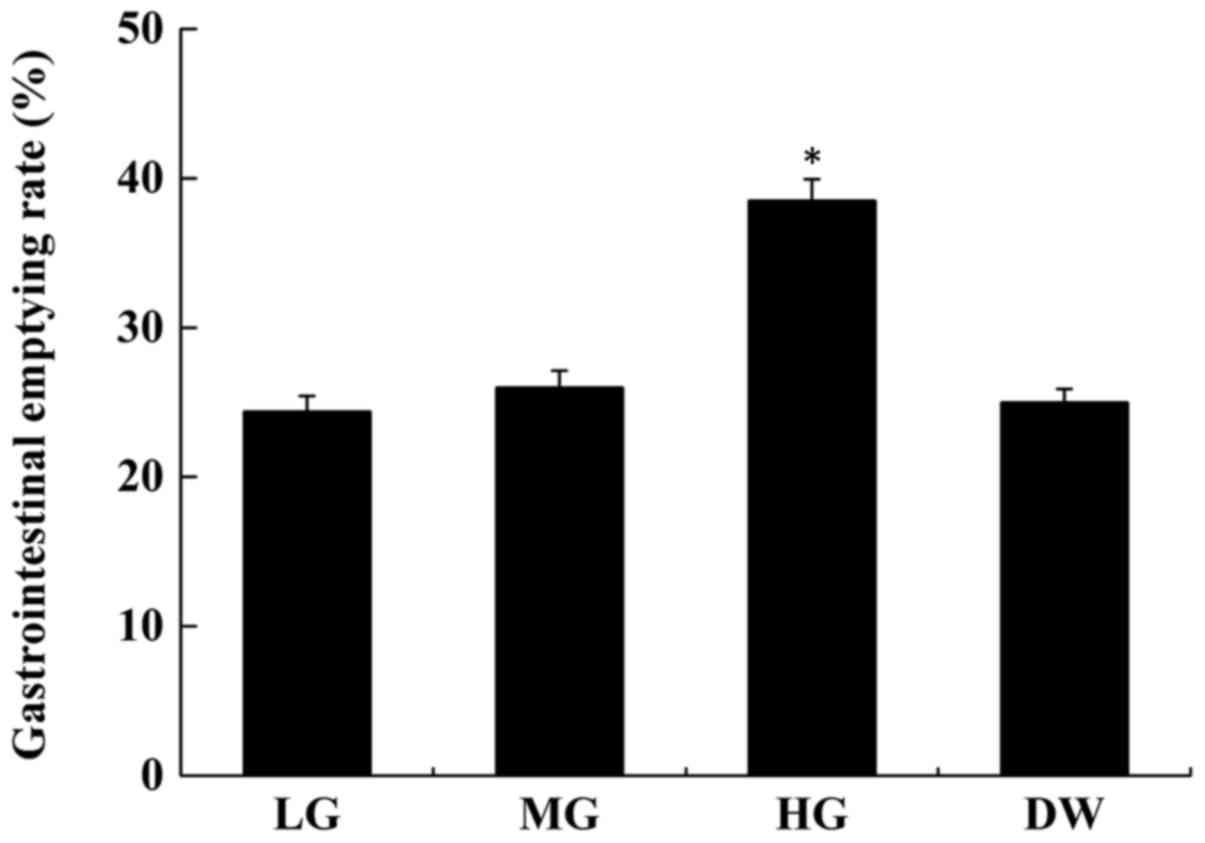
(31). As shown in Fig. 1, the gastrointestinal emptying rate
of the normal mice increased following DMT IR-RS gavage. The
gastrointestinal emptying rate of the high-dose group (38.5±0.9%)
was significantly different from that of the mice treated with
distilled water only (P<0.01). Treatment with 2 or 4 g/kg IR-RS
did not markedly affect gastrointestinal emptying rates, which
indicates that a certain amount of RS helps to improve
gastrointestinal emptying and promote gastrointestinal motility.
These results should aid the analysis of the role of RS in
gastrointestinal motility and may provide a new strategy for future
studies of colon cancer.
Effects of RS on ACFs in the colons of
mice
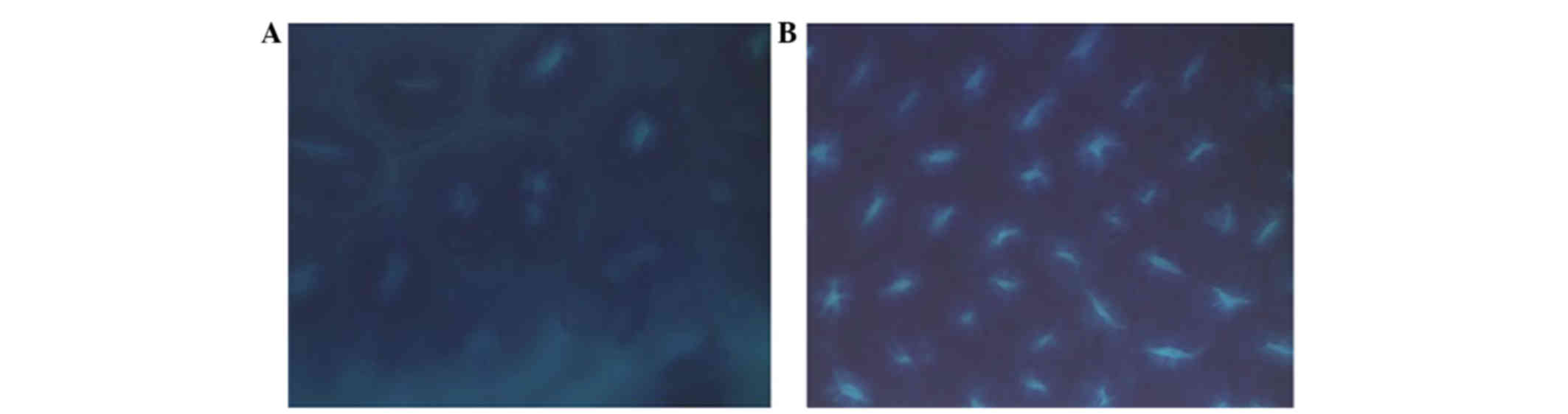
Following the intraperitoneal injection of AOM, the
incidence of ACFs in the mice was 100%. The ACF configurations are
presented in Fig. 2A. The ACF
incidence in the NC group was 0%, and the configuration is shown in
Fig. 2B. The ACF incidence revealed
that the colon cancer model was successful. In comparison with the
NC group, the ACF characteristics of the other groups included
comparatively large crypt foci, deep staining, oval cavity recesses
and additional layers of epithelial cells.
Table III
summarizes the effect of RS on the ACFs of the mice. The numbers of
ACFs in the RS-treated groups exhibited a dose-dependent reduction
compared with those in the MC group, while the MG and HG groups had
significantly different numbers of ACFs from the MC group
(P<0.05). In the RS-treated groups, the numbers of aberrant
crypts in the LG group did not differ from those of the MG group
(P>0.05), and they were also not significantly different from
those in the MC group. The number of aberrant crypts in the HG
group was significantly different from that of the MC group
(P<0.01). The average numbers of aberrant crypts in each ACF of
the RS-treated groups were not dose-dependent, and the differences
were not statistically significant. These results indicate that a
specific diet of RS can effectively reduce the numbers of ACFs and
aberrant crypts.
 | Table III.Influence of RS on colonic ACFs in
mice. |
Table III.
Influence of RS on colonic ACFs in
mice.
| Group | ACF incidence | Number of ACFs | Number of aberrant
crypts | Mean number of
aberrant crypts |
|---|
| NC | 0/16 | 0 | 0 | 0 |
| MC | 16/16 | 11±2 | 32±5 | 3.0±0.13 |
| LG | 16/16 |
9±1 | 25±5 | 2.9±0.45 |
| MG | 16/16 |
8±1a | 26±4 | 3.2±0.25 |
| HG | 16/16 |
7±1b | 20±3b | 3.0±0.16 |
ACFs were first proposed by Bird et al
(32) in 1987 and can be observed in
CRC when viewing early colorectal mucosal lesions under an optical
microscope. ACFs typically consist of one, several or even hundreds
of abnormal aberrant crypts (33).
The mechanism of the effect of RS on ACFs is mediated by RS
inducing increases in diet and intestinal emptying rate, reducing
fecal pH, and increasing the levels of intestinal short chain fatty
acids, which dilutes carcinogens, accelerates their excretion, and
reduces their likelihood of coming into contact with the intestinal
epithelium, and thereby suppresses the occurrence of ACFs (34).
Effect of RS on the expression of APC,
Bax, cytochrome c and Bcl-2 genes in the colon
RT-PCR assays were used to detect the specific mRNA
expression levels of APC, Bax, cytochrome c and Bcl-2, and
the results are shown in Fig. 3.
GAPDH served as an internal reference for the calculation of the
relative expression levels of the mRNAs. At 6 weeks of RS gavage,
the expression levels of the amplification products of APC, Bax and
cytochrome c in the NC group were higher than those of the
MC group. It was also observed that RS treatment promoted the
expression of APC, Bax and cytochrome c and that these
increases exhibited a trend toward being dose-dependent. By
contrast, the expression of the anti-apoptotic gene Bcl-2 in the NC
group was lower than that of the MC group. The results showed that
RS treatment inhibited the expression of Bcl-2, and these
reductions exhibited a trend towards being dose-dependent.
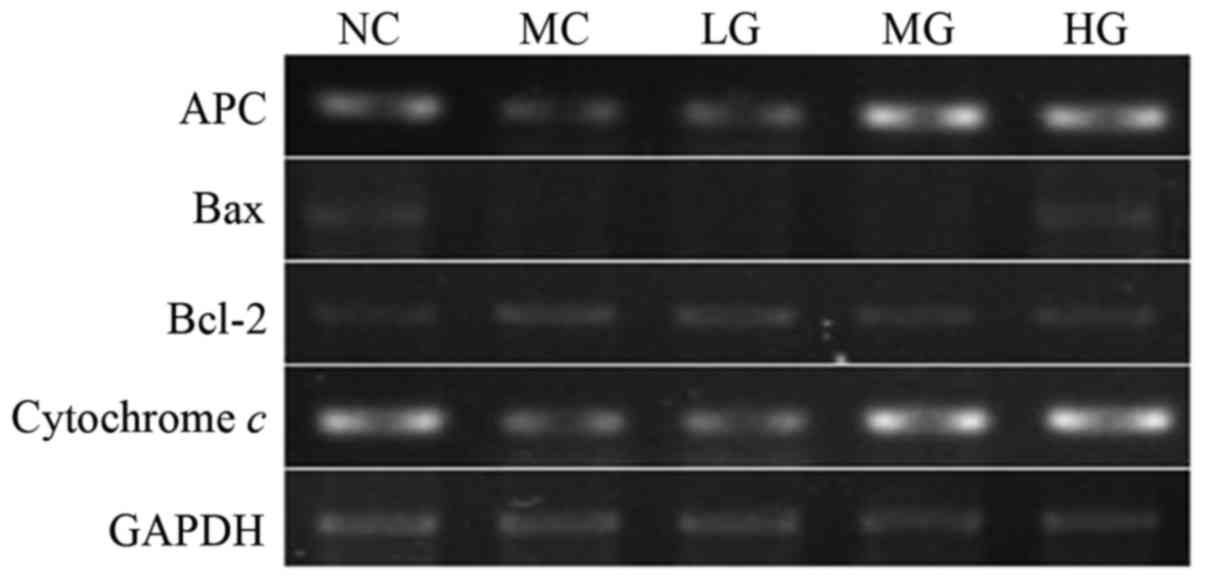 | Figure 3.Expression of APC, Bax, cytochrome
c and Bcl-2 in the colon. Reverse transcription-polymerase
chain reaction assays were used to detect the specific expression
levels of APC, Bax, cytochrome c and Bcl-2 genes. APC,
adenomatous polyposis coli; Bcl-2, B-cell lymphoma 2; Bax,
Bcl-2-associated X protein; NC, normal control; MC, model control;
LG, low-dose group; MG, middle-dose group; HG, high-dose group. |
The occurrence of CRC followed the ‘adenoma to
adenocarcinoma’ law of development that was first proposed by Muto
et al (35) in 1975. Fearon
and Vogelstein (36) subsequently
noted that the progression of ‘adenoma to adenocarcinoma’ involves
changes in suppressor genes and oncogenes. Additionally, the
mechanism of CRC also involves changes in signaling pathways. The
occurrence of CRC is caused by changes in these genes and their
long-term accumulation, and its mechanism is essentially a
polygenic multi-step process. The APC gene is an important tumor
suppressor gene in colon cancer and is located on human chromosome
5q21 (9). Deletions or mutations of
the APC gene are closely associated with the occurrence of early
CRC; 85% of colon cancers are accompanied by deletions and
inactivation of the APC gene. The protein product of the APC gene
typically combines with β-connexin protein (β-catenin) to control
excessive cell proliferation. When APC is mutated, its ability to
regulate cell proliferation is reduced. Therefore, the
APC-β-catenin pathway is considered to be an important therapeutic
target for early colon cancer (9,37). In
the present study, it was found that the expression of APC in the
MC group was lower than that in the NC group; however, after 6
weeks of gavage treatment, APC expression was higher than that of
the MC group and increased in a dose-dependent manner. These
results may be due to the ability of dietary RS to reduce the
methylation of the APC gene, enabling APC and β-catenin to bind and
thereby inhibit excessive cell proliferation.
The Bcl-2 family, which includes Bcl-2 and Bax
genes, plays an important role in the regulation of apoptosis
(38). In normal colonic mucosa, the
Bcl-2 gene was found to be expressed in the mucosal crypts, and no
cell apoptosis occurred, whereas the Bax gene was expressed in
epithelial cells and promoted cell apoptosis. These findings
indicate that these genes coordinate in normal mucosa to control
cell proliferation and apoptosis so that the epithelial cells,
which are produced by the mucosal crypts can migrate upward until
death due to cellular aging (39).
It has been found that Bcl-2 can antagonize the pro-apoptotic
effect of Bax on cells and that the Bcl-2/Bax ratio is closely
associated with apoptosis; indeed, this ratio is considered to be a
molecular switch for the initiation of apoptosis (13). The present study found that the
expression of the Bax gene in the MC group was weaker than that in
the NC group, and that the expression of the Bcl-2 gene in the MC
group was stronger than that in the NC group, in which the
Bcl-2/Bax ratio was relatively high. However, after 6 weeks of
gavage treatment, Bax gene expression increased, and Bcl-2 gene
expression decreased to produce a relatively low Bcl-2/Bax ratio.
These results demonstrate that RS improved the abnormal expression
of the Bcl-2 family of pro- and anti-apoptotic genes in the colon
mucosa to bring the Bcl-2/Bax ratio close to that of the MC group
and induce apoptosis.
Cytochrome c is a water-soluble protein that
regulates the energy metabolism of the mitochondria and also
regulates apoptosis (15).
Cytochrome c plays a negative regulatory role and inhibits
the growth of tumor cells in the process of tumor development. The
mechanism of action of cytochrome c is mediated by apoptotic
signal conduction and amplification that results in the regulation
of apoptosis (12). Cytochrome
c is closely associated with the Bcl-2 family. The Bcl-2
family can participate in the process of cytochrome c
release and the formation of an integral membrane protein. Bcl-2
stabilizes the mitochondrial membrane and suppresses the release
and activation of cytochrome c and caspase, which changes
the oxidation-reduction reaction within the nucleus and ultimately
inhibits apoptosis. It has been reported that the content of
cytochrome c in colorectal tissues adjacent to cancerous
tissue is significantly higher than that in the cancerous tissues,
which suggests that tumors may be associated with inhibition of the
release of cytochrome c. In the present study, it was found
that the expression of cytochrome c mRNA in the MC group was
weaker than that in the NC group. However, after 6 weeks of gavage
treatment, cytochrome c mRNA expression increased, and this
result demonstrates that RS effectively increased the release of
cytochrome c from the colon mucosa cells. The results for
APC, Bax, cytochrome c and Bcl-2 expression levels in the
colon indicate that DMT IR-RS promoted the expression of APC, Bax
and cytochrome c and inhibited the expression of Bcl-2.
These findings indicate that DMT IR-RS may induce colonic
epithelial cell apoptosis and decrease the effects of colon cancer
in mice.
In conclusion, the present study demonstrated that
the DMT IR-RS product can induce apoptosis and has beneficial
health effects in mice with AOM-induced early colon cancer. The
body weight gains, gastrointestinal emptying rates and numbers of
ACFs in the RS-treated groups decreased dose-dependently. In
addition, DMT IR-RS diets may induce the occurrence of apoptosis
and reduce the effects of colon cancer in mice. RS is difficult to
digest in the small intestine and is fermented by microorganisms to
increase the amounts of short-chain fatty acids, particularly
butyric acid. Butyric acid can reduce carcinogen levels in the gut
and inhibit tumor cells (40). In
our future studies, we will examine how RS changes the intestinal
microbial system to affect the generation and discharge of
intestinal toxins and how toxins affect glucose metabolism in
mice.
Acknowledgements
This study was funded by the National College
Students Innovation Training Program (Grant No. 201210359058), the
Department of Science and Technology, Anhui Province Natural
Science Fund (Grant No. 1208085MC56) and The State Natural Science
Fund (Grant No. 31370371).
Glossary
Abbreviations
Abbreviations:
|
IR-RS
|
indica rice resistant starch
|
|
DMT
|
dual modification-treated
|
|
LG
|
low-dose group
|
|
MG
|
middle-dose group
|
|
HG
|
high-dose group
|
|
PC
|
positive control
|
|
MC
|
model control
|
|
NC
|
normal control
|
|
DW
|
distilled water
|
|
AOM
|
azoxymethane
|
|
CRC
|
colorectal cancer
|
|
ACF
|
aberrant crypt foci
|
|
RT-PCR
|
reverse transcription-polymerase chain
reaction
|
References
|
1
|
John CR: Mechanisms of apoptosis. Am J
Pathol. 157:1415–1430. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Homan SG, Steward BR and Armer JM: Public
health and cooperative group partnership: A colorectal cancer
intervention. Semin Oncol Nurs. 30:61–73. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Brenner H, Kloor M and Pox CP: Colorectal
cancer. Lancet. 383:1490–1502. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lv Q, Xing SY and Zhao ZH: Research status
and prospect of colon cancer. Zhong Guo Shi Yan Zhen Duan Xue.
13:1134–1137. 2009.
|
|
5
|
Zheng S and Cai S: Colorectal cancer
epidemiology and prevention study in China. Chinese-German J Clin
Oncol. 2:72–75. 2003. View Article : Google Scholar
|
|
6
|
Tsukuda K, Tanino M, Soga H, Shimizu N and
Shimizu K: A novel activating mutation of the K-ras gene in human
primary colon adenocarcinoma. Biochem Biophys Res Commun.
278:653–658. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tang N and Wang YY: Research progress of
colon cancer related genes. Yi Xue Zong Shu. 18:207–209. 2012.(In
Chinese).
|
|
8
|
Tang WZ, Gao F and Li W: APC mutation in
sporadic colorectal cancer in China. Zhonghua Shi Yan Wai Ke Za
Zhi. 22:1357–1359. 2005.(In Chinese).
|
|
9
|
Wu L, Qian YB and Zhu LX: Promoter
methylation and mRNA expression of APC gene in hepatocellular
carcinoma. Zhonghua Gan Dan Wai Ke Za Zhi. 15:378–381. 2007.(In
Chinese).
|
|
10
|
Cory S and Adams JM: The Bcl 2 family:
Regulators of the cellular life-or-death switch. Nat Rev Cancer.
2:647–656. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhu YS, Lu TY, Wang R, Huang M, Ma Q, Zhao
L, Gao P, Lei X, Ni B, Lin J, et al: Functional conversion of Bcl-2
into a pro-apoptotic molecule to regulate mitochondrial cytochrome
c. Sheng Ming Ke Xue. 23:1076–1080. 2011.(In Chinese).
|
|
12
|
Hockenbery D, Nuñez G, Milliman C,
Schreiber RD and Korsmeyer SJ: Bcl-2 is a inner mitochorndrial
membrane protein that blocks programmed cell death. Nature.
348:334–336. 1990. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Jia XD and Han C: Biomarkers in the
studies on chemoprevention of colorectal cancer. Wei Sheng Yan Jiu.
29:109–111. 2000.(In Chinese). PubMed/NCBI
|
|
14
|
Gong XZ and Liu W: The role of bcl-2 and
bax gene in colon cancer. Qi Lu Yi Xue Za Zhi. 17:367–370. 2002.(In
Chinese).
|
|
15
|
Chen NL, Chen H, Bai L, Zhang C and Zhao
WH: Expression of cytochrome c in colorectal cancer tissue
and the ultramicro-structural change of mitochondria. Shiyong Yixue
Zazhi. 24:4037–4039. 2008.(In Chinese).
|
|
16
|
Zhang J, Xu B, Cai WS and Li SH: The
effect of Coixenolide extract on the histopathological changes
induce by azoxymethane in rat colon cancer model. Zhongguo Yi Liao
Qian Yan. 6:20–21. 2011.(In Chinese).
|
|
17
|
Rogers AE, Zeisel S and Groopman J: Diet
and carcinogenesis. Carcinogenesis. 14:2205–2217. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Peh YC: Recent advances in colorectal
cancer genetics and diagnostics. Crit Rev Oncol Hematol. 69:45–55.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Liu RP and Xu GF: Effects of resistant
starch on colonic preneoplastic aberrant crypt foci in rats. Food
Chem Toxicol. 46:2672–2679. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Coleman LJ, Landström EK, Royle PJ, Bird
AR and McIntosh GH: A diet containing alpha-cellulose and fish oil
reduces aberrant crypt foci formation and modulates other possible
markers for colon cancer risk in azoxymethane-treated rats. J Nutr.
132:2312–2318. 2002.PubMed/NCBI
|
|
21
|
Brouns F, Kettlitz B and Arrigoni E:
Resistant starch and ‘the butyrate revolution’. Trends Food Sci
Tech. 13:251–261. 2002. View Article : Google Scholar
|
|
22
|
Englyst HM, Kingman SM and Cummings JH:
Classification and measurement of nutritionally important starch
fractions. Eur J Clin Nutr. 46:(Suppl 2). S33–S50. 1992.PubMed/NCBI
|
|
23
|
Zhou Y, Meng S, Chen D, Zhu X and Yuan H:
Structure characterization and hypoglycemic effects of dual
modified resistant starch from indica rice starch. Carbohyd Polym.
103:81–86. 2014. View Article : Google Scholar
|
|
24
|
Institute of Laboratory Animal Resources
(US). Committee on Care, Use of Laboratory Animals, and National
Institutes of Health (US)Division of Research Resources: Guide for
the care and use of laboratory animals. 8th. National Academies
Press; Washington, DC: 2011
|
|
25
|
Kim WK, Chung MK, Kang NE, K0im MH and
Park OJ: Effect of resistant starch from corn or rice on glucose
control, colonic events and blood lipid concentrations in
streptozotocin-induced diabetic rats. J Nutr Biochem. 14:166–172.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Toden S, Bird AR, Topping DL and Conlon
MA: Resistant starch prevents colonic DNA damage induced by high
dietary cooked red meat or casein in rats. Cancer Biol Ther.
5:267–272. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yuan HB, Gong ZB, Meng SH and He G:
Hypoglycemic and hypolipidemic effects of a triterpenoid-rich
extract from Euryale shell on streptozotocin-induced
diabetic mice. Pharmazie. 68:227–231. 2013.PubMed/NCBI
|
|
28
|
Zhao GH, Kan JQ, Li HJ and Chen Z: The
research progress of resistant starch in food. Liang Shi Yu You
Zhi. 14:37–40. 1999.(In Chinese).
|
|
29
|
Ma HB and Jia L: Progress in the stady of
resisaant starch. Di Si Jun Yi Da Xue Ji Lin Jun Yi Xue Yuan Xue
Bao. 24:174–176. 2002.(In Chinese).
|
|
30
|
Xu YJ, Fang RJ and Dai QZ: Physiological
role of short-chain fatty acids in nutrition. Si Liao Yan Jiu.
8:26–28. 2007.(In Chinese).
|
|
31
|
Gaede P, Vedel P, Lavsen N, Jensen GV,
Parving HH and Pedersen O: Multifactorial intervention and
cardiovascular disease in patients with type 2 diabetes. New Engl J
Med. 348:383–393. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Bird AR, Brown IL and Topping DL:
Starches, resistant starches, the gut microflora and human health.
Curr Issues Intest Microbiol. 1:25–37. 2000.PubMed/NCBI
|
|
33
|
Luebeck EG and Moolgavkar SH: Multistage
carcinogenesis and the incidence of colorectal cancer. Proc Natl
Acad Sci USA. 99:15095–15100. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Archer MC, Bruce WR, Chan CC, Corpet DE,
Medline A, Roncicci L, Stamp D and Zhang XM: Aberrant crypt foci
and microadenoma as markers for colon cancer. Environ Health
Perspect. 98:195–197. 1992. View
Article : Google Scholar : PubMed/NCBI
|
|
35
|
Muto T, Bussey HJ and Morson BC: The
evolution of cancer of the colon and rectum. Cancer. 36:2251–2270.
1975. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Fearon ER and Vogelstein B: Genetic model
for colorectal tumorigenesis. Cell. 61:759–767. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Lee S, Lee HJ, Kim JH, Lee HS, Jang JJ and
Kang GH: Aberrant CpG island hypermethylation along multistep
hepatocarcinogenesis. Am J Pathol. 163:1371–1378. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Watson AJ, Merritt AJ, Jones LS, Askew JN,
Anderson E, Becciolini A, Balzi M, Potten CS and Hickman JA:
Evidence for reciprocity of bcl-2 and p53 expression in human
colorectal adenomas and carcinomas. Br J Cancer. 73:889–895. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Yin XY, Oltval ZN and Korsmeyer SJ: BH1
and BH2 domains of bcl-2 are required for inhibition of apoptosis
and heterodimerization with bax. Nature. 369:321–323. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Zhan Y: Butyric acid and tumor. Int J
Cancer. 30:350–353. 2003.
|