Introduction
In recent years, there has been an increasing
incidence of inflammatory bowel diseases (IBD), broadly classified
as ulcerative colitis (UC) or Crohn's disease (CD) (1). Affected patients frequently experience
diarrhea, abdominal pain and rectal bleeding, due to aberrant
intestinal inflammation. These chronic and relapsing
bowel-inflammatory symptoms greatly decrease patient quality of
life (2). However, IBD remains
largely incurable, since the individual's genetic susceptibility,
external environment, intestinal microbial flora and immune
responses are all involved and are functionally integrated in the
etiology of IBD (3). Pharmaceutical
treatments for IBD are classified into five major categories,
namely anti-inflammatory drugs, immunosuppressants, biological
agents, antibiotics and drugs for symptomatic relief (4). Unfortunately, the effects of these
treatments are far from satisfying.
Pogostemonis Herba, the dried aerial part of
Pogostemon cablin (Blanco) Benth. (Labiatae), commonly known
as Cablin Patchouli, has been traditionally used as a principal
component of a variety of renowned Chinese medicinal formulae, such
as Baoji Pill and Huoxiangzhengqi capsule, to treat
gastrointestinal diseases involving diarrhea, abdominal pain,
vomiting and IBD. Patchouli oil (PO) is the essential oil produced
by hydro-distilling the dry leaves of Pogostemon cablin.
Studies have proven that PO has multiple activities, including
anti-inflammatory (5),
immunomodulatory (6) and
antimicrobial actions (7). Thus, in
the present study, it was speculated that PO may alleviate IBD by
attenuating inflammation and inhibiting intestinal
microorganisms.
Currently used animal models of IBD include
transgenic, congenic, chemically induced and adoptive cell-transfer
models (8), in which rodent colitis
induced by 2,4,6-trinitrobenzenesulfonic acid (TNBS) by stimulation
of transmural oxidative stress and inflammatory factors is one of
the most widely used models that closely resembles the pathogenesis
of CD (9). In a previous metabolic
profiling study by our group, five urinary metabolites potentially
associated with the pathology of TNBS-induced colitis were
identified using ultra-fast liquid chromatography-ion trap
quadrupole time of flight mass spectrometry (UFLC-IT-QTOF-MS)
(10).
The purpose of the present study was to investigate
the potential therapeutic effect of PO on TNBS-induced rat colitis.
The effect of PO was examined by evaluating the disease activity
index (DAI), macroscopic and microscopic colonic damage scores and
the myeloperoxidase (MPO) activity in the colon. Furthermore, a
targeted metabolite analysis using the UFLC-IT-qTOF-MS approach was
performed to characterize the metabolic changes in rat urine that
accompanied the amelioration of the disease. The present study not
only confirmed previously identified potential metabolic markers,
but also suggested that the therapeutic effect of PO is closely
associated with the adjustment of tryptophan metabolism and
modulation of intestinal microorganisms.
Materials and methods
Animals
A total of 50 male Sprague-Dawley rats (age, 6–7
weeks; weight, 220±20 g) were purchased from the animal center of
Guangzhou University of Chinese Medicine (Guangzhou, China) and
experimental protocols were approved by the Committee for Animal
Care and Use at Guangzhou University of Chinese Medicine [project
identification code, SYXK (yue) 2013-0085]. All animals received
humane care in accordance with the Guide for the Care and Use of
Laboratory Animals, published by the US National Institutes of
Health (Bethesda, MD, USA). Animals were housed at a temperature of
23±2°C and humidity of 55±10% in a specific pathogen-free
environment with a 12-h light/dark cycle and given free access to a
standard laboratory diet and water. Prior to the start of the
experiment, mice were acclimatized for at least one week.
Chemical reagents and materials
PO was purchased from Nanhai Zhongnan Co., Ltd.
(Foshan, China; Lot 140801) and its quality was confirmed by gas
chromatography-flame ionization detection in a previous study by
our group (11). Chloral hydrate,
TNBS, formic acid and D,L-4-chlorophenylalanine were purchased from
Sigma-Aldrich (Merck Millipore, Darmstadt, Germany).
N-phenylacetylglycine was purchased from Toronto Research
Chemicals Inc. (Toronto, ON, Canada). All other chemicals were of
analytical grade.
Drug administration and dose
selection
Following 1 week acclimatization, rats were randomly
divided into five groups (n=10 per group). Animals in the control
and TNBS groups received intra-rectal instillation of vehicle [0.5%
(w/v) aqueous sodium carboxy methylcellulose (CMC-Na)] once daily
for 7 days, and animals in the PO group received parallel
intra-rectal instillation of PO (at the dose of 65, 135 and 270
mg/kg dissolved in 3 ml vehicle). The results showed that the
effect of PO at 65 and 135 mg/kg did not achieve statistical
significance (Fig. 1A). Therefore,
the dose of 270 mg/kg was selected for the subsequent histological
and metabolomics evaluations.
TNBS-induced colitis and sample
collection
All rats were anesthetized with chloral hydrate (140
mg/kg, by intraperitoneal injection) purchased from Damao Chemical
Reagent Factory (Tianjin, China). In the TNBS and PO groups,
colitis was induced by intra-rectal administration of TNBS
dissolved in 50% ethanol at 25 mg/3 ml/kg body weight, at 8 cm from
the anal verge after anesthetization (12). Control rats received an intra-rectal
injection of saline but not TNBS. The animals then received vehicle
with or without PO for 7 subsequent days. On the 5th day after
colitis induction, rats were placed into metabolic cages for
acclimatization and two days later, urine samples were collected
from each animal. All collected urine samples were immediately
stored at −80°C for UFLC-IT-QTOF-MS [Infusion Pump (LC-20ADXR*2),
Degasser (DGU-20A3), Autosampler (SIL-20AC), Column
Heaters (CTO-20AC), Ion trap-time-of-flight mass spectrometer
(LCMS-IT-TOF), Profiling workstation (LCMS-solution Ver. 3.6)]
analysis (Shimadzu Corporation, Kyoto, Japan). Animals were
sacrificed with chloral hydrate (0.1 g/ml, by intraperitoneal
injection, Damao Chemical Reagent Factory). After macroscopic
scoring, the colonic segments were immediately removed following
longitudinal opening and then rinsed with ice-cold physiological
saline, blotted on filter paper and excised in two sections. One
section was fixed in 10% buffered formalin at 4°C for 24 h,
embedded in paraffin, sectioned at 5-µm thickness and finally
stained with hematoxylin and eosin for routine histological
examination performed with a microscope (BH22; Olympus Corporation,
Tokyo, Japan). The other section was frozen in liquid nitrogen and
stored at −80°C for measurement of MPO activity.
Assessment of colonic damage
During the experimental period, animals were
observed daily. Each day, the DAI scores were calculated by scoring
body weight loss, trait of stool and hematochezia according to the
classic scoring system by Cooper et al (13). For occult blood testing, a Hemoccult
Sensa (REF 64151; Beckman Coulter, Brea, CA, USA) was used. In
addition, a combination of micro- and macroscopic score, applied in
order to estimate the degree of colitis, were determined by an
observer who was blinded to the grouping according to previously
established criteria (14). Each
specimen was opened longitudinally and examined for immediate
macroscopic scoring of injuries according the following criteria:
0, normal appearance; 1, focal hyperemia, no ulcers; 2, ulceration
with inflammation at 1 site; 3, two or more sites of ulceration and
inflammation; 4, major sites of damage extending>1 cm along
length of colon; 5–10, when an area of damage extended >2 cm
along the length of the colon, the score was increased by 1 for
each additional cm of involvement. Colon sections were embedded in
paraffin and cut into sections 5-µm thick, then stained with
hematoxylin and eosin for routine histological examination. Colonic
mucosal damage in the histology specimens was assessed using
previously established histological scoring criteria (14). The MPO activity was measured using a
modified previous method (15) as
follows: Pre-weighed colonic tissues (0.1 g) were cut into pieces
and homogenized with an Ultra Turrax (T18 Basic; IKA, Staufen,
Germany) in 0.5 ml ice-cold 50 mM phosphate-buffered saline (PBS;
pH 6.0) containing 0.5% hexadecyltrimethylammonium bromide
(Sigma-Aldrich; Merck Millipore) at 4°C. The homogenized samples
were freeze-thawed and sonicated three times for 20 sec each,
followed by centrifugation at 3,000 × g for 20 min at 4°C.
Subsequently, 20 µl resulting supernatant was mixed with 180 µl 50
mM PBS containing 0.167 g/l O-dianisidine hydrochloride
(Sigma-Aldrich; Merck Millipore) and 0.005 g/l
H2O2. The absorbance of the mixture was
measured at 460 nm on a spectrophotometer (RT2100C; Rayto,
Shenzhen, China) within 2 min. MPO activity was expressed as
units/mg wet tissue.
Urine sample preparation and
UFLC-IT-qTOF-MS analysis
For UFLC/MS analysis, urine samples were prepared
following a method described in a previous study by our group
(10). In brief, each fresh urine
sample was centrifuged at 1,400 × g and the supernatant was
collected and stored at 20°C until use. Urine samples (200 µl) were
mixed with 1.2 ml organic solvent mixture [acetonitrile-methanol
(3:1), spiked with 20 µg of internal standard
D,L-4-chlorophenylalanine] and centrifuged at 18,000 × g at
4°C for 10 min for de-proteinization. The supernatant was dried
under a flow of nitrogen to remove organic solvents and was then
lyophilized. The residue was stored at −4°C and reconstituted in
500 µl methanol prior to analysis. Chromatographic separations of
urine were performed on a Shim-pack XR-ODS III chromatographic
column (2.2 µm, 2.0×150 mm; Shimadzu Corporation) using a
Prominence UFLCXR system (Shimadzu Corporation). The column was
maintained at 40°C and eluted with a linear gradient of 5–90% B,
where A=water with 0.1% formic acid and B=acetonitrile. The
gradient program was as follows: 0–1 min, 5% B; 1–15 min, 5–50% B;
15–18 min, 50–90% B; 18–20 min, 90% B; 20.5–24 min, 5% B; flow
rate, 300 µl/min. The injection volume was 5 µl. The column eluent
was analyzed by QTOF mass spectrometry using positive and negative
ion electro spray ionization (ESI). The column eluent was directed
to the mass spectrometer without split. All MS and MSn
mass spectra were acquired on an LCMS-IT-TOF mass spectrometer
(Shimadzu Corporation) operating in either positive or negative ESI
mode. The desolvation gas was set to 10 l/min at a temperature of
200°C. The cone gas was set to 1.5 l/min and the source temperature
was set to 200°C. The voltage of the ESI source and detector were
+4.5, −3.5 and 1.60 kV, respectively. All data were collected in
the full scan mode (100–1,000 m/z). The dwell time for each scan
was set to 50 msec.
Data processing and multivariate data
analysis
The LC-IT-QTOF-MS raw data were pre-processed using
Profiling solution software ver. 3.6 (Shimadzu Corporation). The
resulting multi-dimensional data, comprising peak number [retention
time (RT)-m/z pair], sample name (observation) and ion intensity
(variable) were acquired as multivariate data matrices and exported
into SIMCA-P ver. 10.0 (Umetrics AB, Umea, Sweden) for multivariate
data analysis. The ion intensities for each peak detected were then
normalized, within each sample, to the sum of the peak intensities
in that sample. The relative intensities of targeted metabolites
were then normalized to that of the spiked internal standard
D,L-4-chlorophenylalanine. Pareto-scaling and supervised partial
least squares-discriminate analyses (PLS-DA) were selected for data
mining and pattern recognition after optimization.
Statistical analysis
Values are expressed as the mean ± standard
deviation and analyzed with SPSS software version 17.0 (SPSS, Inc.,
Chicago, IL, USA). The groups were compared by one-way analysis of
variance followed by the Dunnett's test and statements of
statistical significance were based on P<0.05.
Results
Pathological characteristics of
PO-treated rats
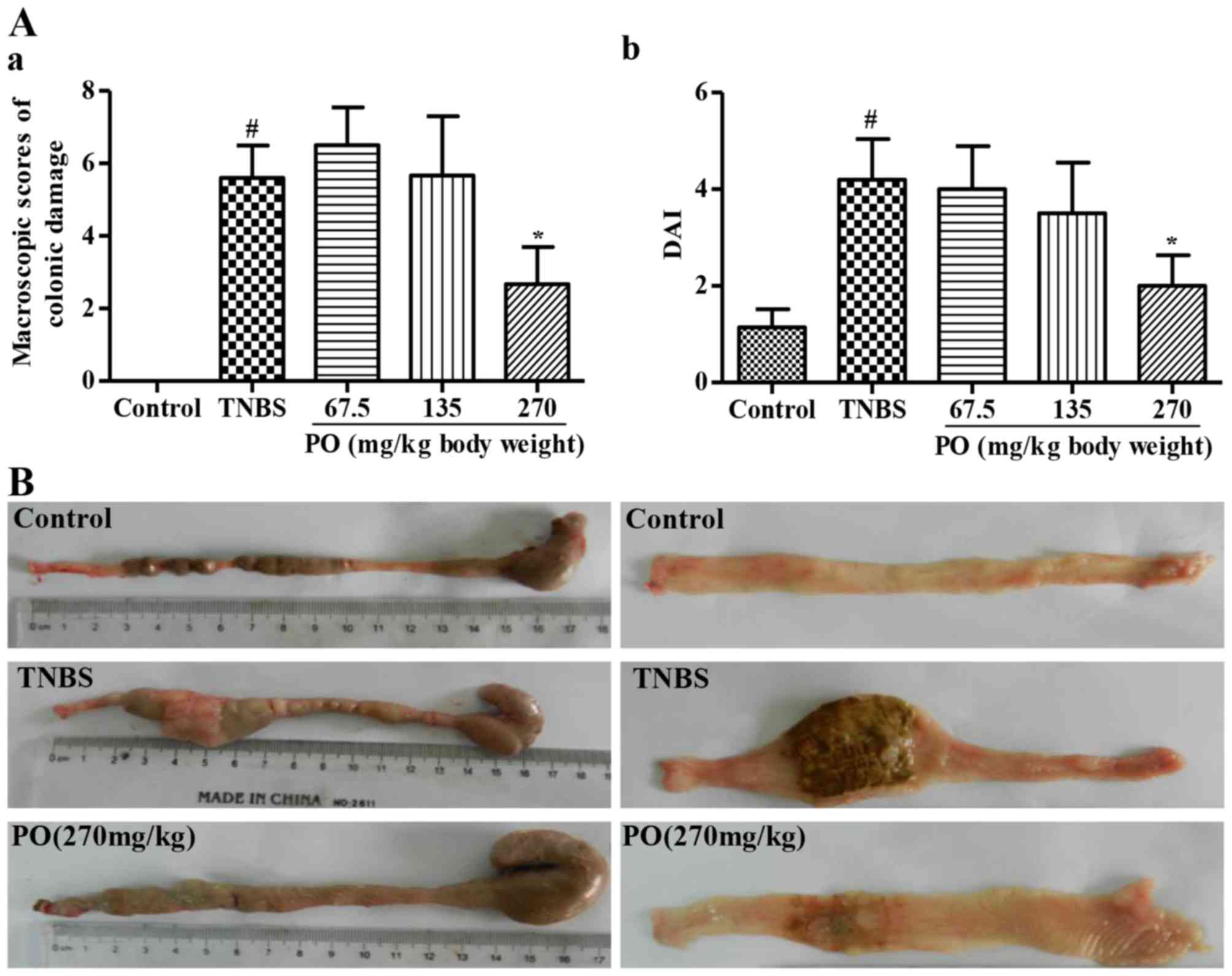
As shown in Fig. 1A,
the TNBS group had a significantly higher (P<0.05) colonic
damage score and DAI for CD (16)
than the control group. These markedly decreased following PO
treatment, particularly at the dose of 270 mg/kg. In addition, the
representative images show that rats in the control group displayed
healthy colons without ulceration, while obvious tissue necrosis
was found in the colon of TNBS-treated rats (Fig. 1B). Of note, PO attenuated the
symptoms of ulceration. Moreover, as indicated in Fig. 2A, the PO group showed significantly
lower histological scores and MPO activity, an index of neutrophil
accumulation in the tissue with correlates with severity (17), when compared with the TNBS-treated
group. Furthermore, the control group maintained an intact
architecture of the colon (Fig. 2B),
whereas TNBS-treated rats revealed significant necrosis of the
mucosa and submucosa and dense inflammatory-cell infiltration in
the submucosa and muscularis mucosae. However, PO treatment
attenuated inflammatory-cell infiltration. These results confirmed
that TNBS induced serious colitis in rats, while rectal application
of PO significantly attenuated the symptoms of colitis.
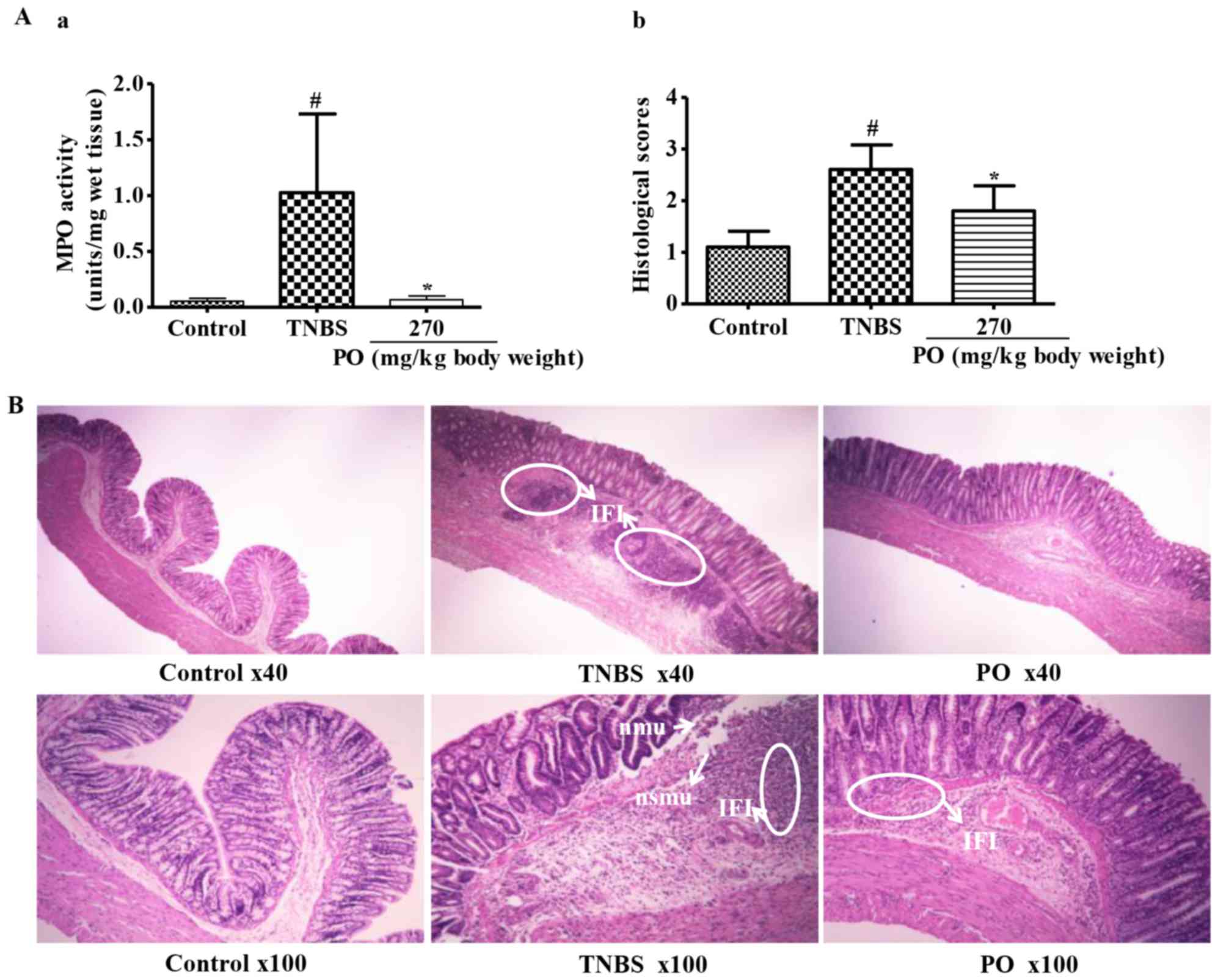 | Figure 2.Microscopic characteristics of rats in
Control, TNBS and PO groups. (A-a) MPO activity. (A-b) Histological
scores. Values are expressed as the mean ± standard deviation
(n=7–10). #P<0.05 vs. Control group; *P<0.05 vs.
TNBS group. (B) Images showing histological changes. The control
group showed normal features of mucosa with intact epithelial
surface, submucosa and muscularis layer. The TNBS group showed
mucosal erosion with loss of epithelial cells, focal nmu and nsmu
and dense IFI. The PO group had nearly intact mucosa and decreased
inflammatory cells. TNBS, 2,4,6-trinitrobenzenesulfonic acid; PO,
patchouli oil; nmu, necrosis of mucosa; nsmu, necrosis of
submucosa; IFI, inflammatory-cell infiltration. |
Targeted metabolite analysis of urine
from rats by LC-IT-QTOF-MS
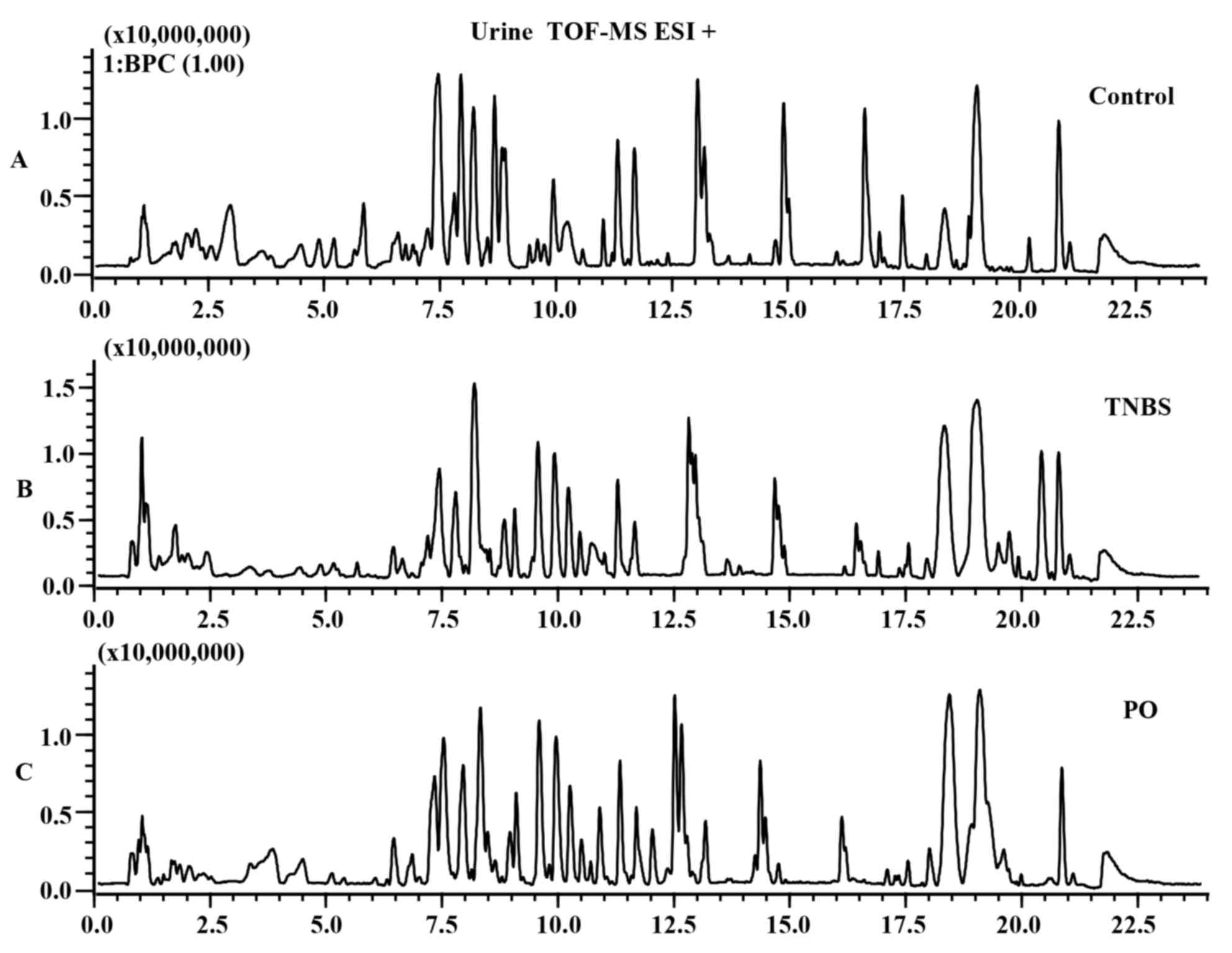
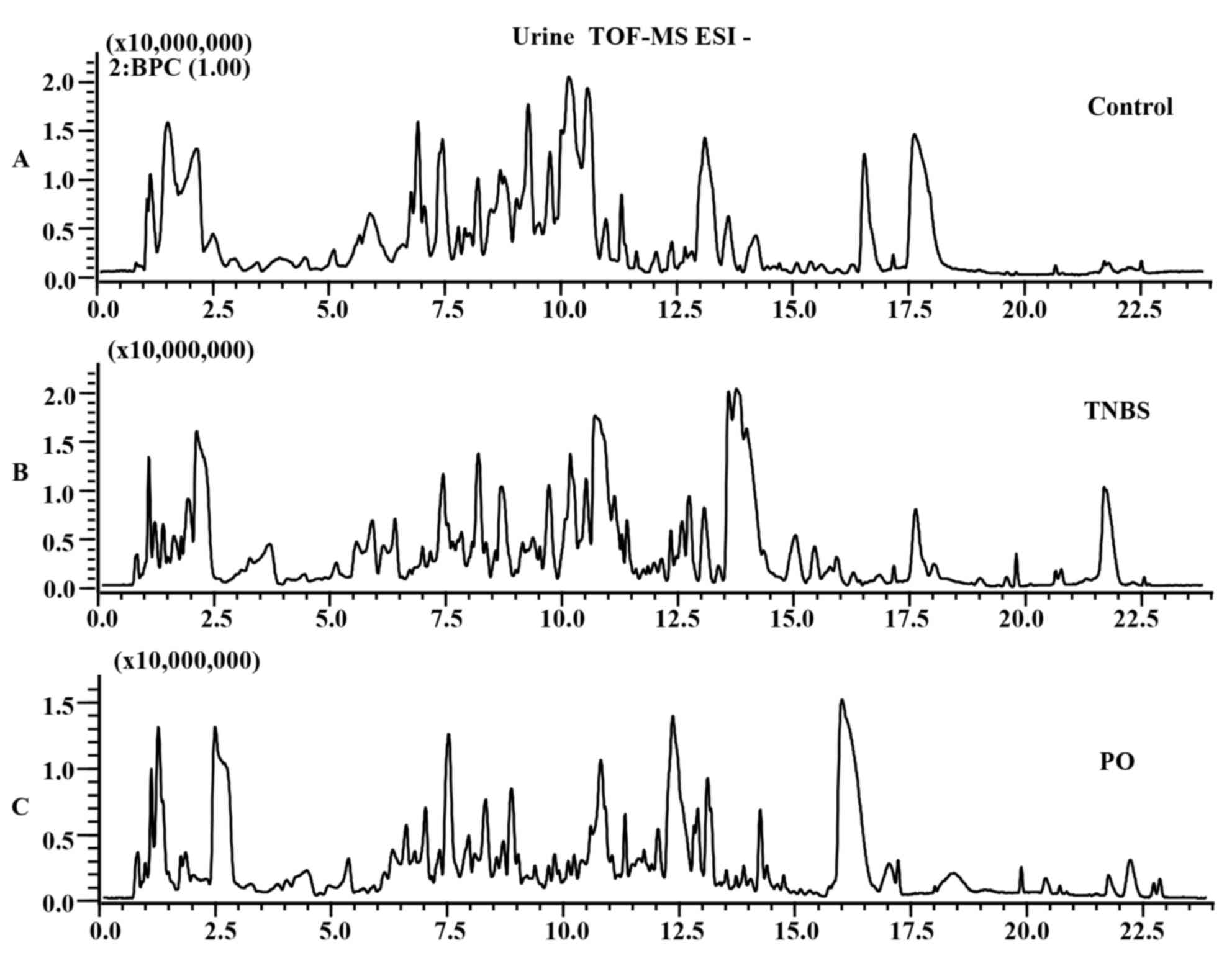
The differences in base peak intensity
chromatographs of urine samples from the control, TNBS and
PO-treated groups were displayed in ESI positive (Fig. 3A-C) and negative mode (Fig. 4A-C). In order to clearly
differentiate between groups, PLS-DA was used to investigate the
fluctuation of metabolites. As shown in Fig. 5A-D, urine samples from the TNBS group
were distinctly separated from the control and PO groups in the ESI
positive as well as negative mode.
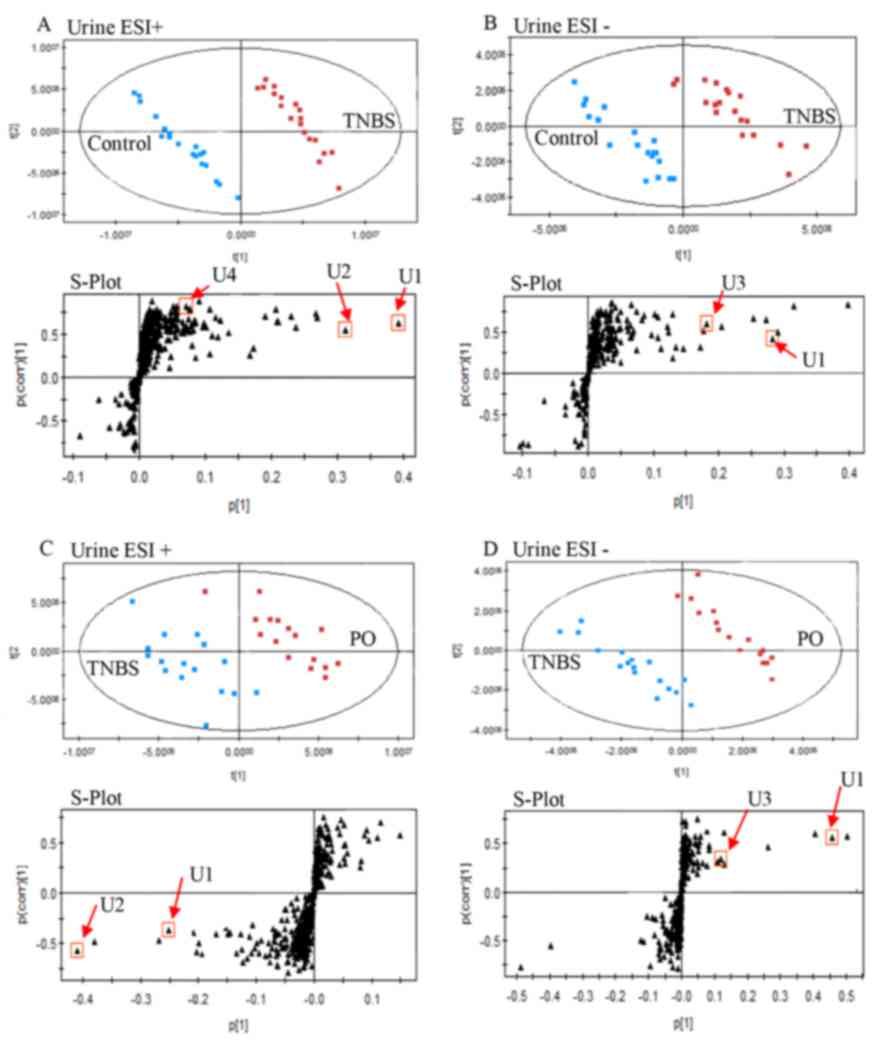 | Figure 5.Targeted metabolic analysis of urinary
samples using ultra-fast liquid chromatography-ion trap quadrupole
time of flight mass spectrometry-based metabolomics. PLS-DA score
plots and PLS S-plot based on detected urinary metabolites from
TNBS-induced colitis and control rats in (A) positive and (B)
negative ion mode. PLS-DA score plots and PLS S-plot based on
detected urinary metabolites from TNBS-induced colitis and
PO-treated rats in (C) positive and (D) negative ion mode. The
black circles represent the retention time and m/z data pairs for
each metabolite. Metabolites: U1, phenylacetylglycine; U2,
4,6-cihydroxyquinoline; U3, p-cresol glucuronide; U4,
4-(2-aminophenyl)-2,4-dioxobutanoic acid. ESI, electron spray
ionization; PO, patchouli oil; PLS-DA, partial least
squares-discriminate analysis; TNBS, 2,4,6-trinitrobenzenesulfonic
acid. |
Identification of the targeted
metabolites in urine
A previous study by our group has identified five
endogenous metabolites that are potential metabolic markers of
TNBS-induced IBD in rats (10).
Therefore, in the present study, the data of the targeted
metabolites we extracted from the urine data matrix and the
variable importance in the projection (VIP) was calculated
following PLS-DA processing. Then Student's t-test was used
to select potential metabolites worthy of preferential study.
P<0.05 was considered to indicate statistical significance which
was assessed using SPSS 17.0. Identification of the five endogenous
metabolites was performed based on the retention time, accurate
mass by MS and MS/MS, as well as elemental composition data by
comparing results from various databases such as the Human
Metabolome Database (HMDB; http://www.hmdb.ca), the METLIN Metabolite Database
(http://metlin.scripps.edu) and Mass Bank
(http://www.massbank.jp) (10). The results of the analysis are shown
in Table I. A total of four
metabolites [U1, phenylacetylglycine; U2, 4,6-cihydroxyquinoline;
U3, p-cresol glucuronide; U4, 4-(2-aminophenyl)-2,4-dioxobutanoic
acid] were identified in urine samples from the different groups in
positive ESI and/or negative ESI modes (VIP value >1).
Metabolites U1 and U3 are associated with the homeostasis of
intestinal microbiota, while U2 and U4 are tryptophan metabolites
(10). As shown in Fig. 6, the levels of U1-U4 were
significantly higher in the TNBS-treated rats than in the control
rats. By contrast, PO-treatment significantly reversed the
increases in the levels of metabolites U1-U4 that occurred in TNBS
mice. Levels of metabolite U4 in the PO-treated group was
significantly decreased to levels below the limit of detection.
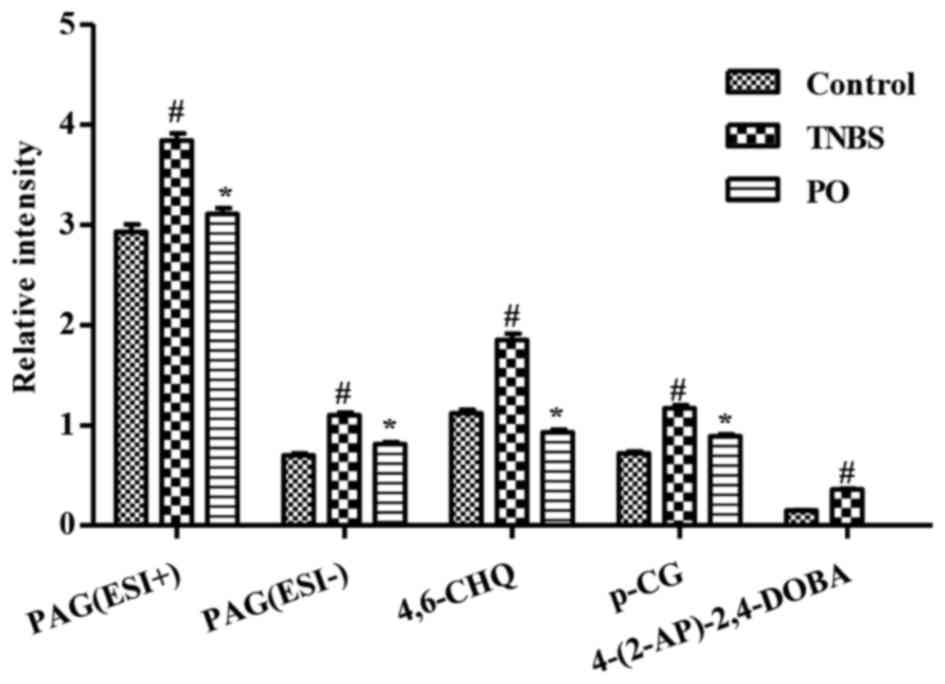 | Figure 6.Relative intensity of targeted
metabolites in urinary samples of Control, TNBS and PO groups.
Intensity of metabolites in ESI-positive and/or ESI-negative modes
used in this figure were presented as the mean ± standard error of
the mean (n=7–10). #P<0.05 vs. Control group;
*P<0.05 vs. TNBS group. PAG (U1), phenylacetylglycine; 4,6-CHQ
(U2), 4,6-cihydroxyquinoline; p-CG (U3), p-cresolglucuronide;
4-(2-AP)-2,4-DOBA (U4), 4-(2-aminophenyl)-2,4-dioxobutanoic acid;
ESI, electron spray ionization; PO, patchouli oil; TNBS,
2,4,6-trinitrobenzenesulfonic acid. |
 | Table I.Identified potential metabolites
showing the difference between urine samples from Control, TNBS and
PO group (n=7–10) in ESI+ and/or ESI- modes. |
Table I.
Identified potential metabolites
showing the difference between urine samples from Control, TNBS and
PO group (n=7–10) in ESI+ and/or ESI- modes.
| Symbol | Ion mode | RT (min) | VIP
valueb |
| Relative
intensityc | P-value | m/z | Mass error (ppm) | Formula | changed | Chemical name | Human metabolome
database ID | Compound class |
|---|
| U1a | ESI+ | 8.293 | 4.58 | C | 2.93±1.55 | 0.03139f | 194.0813 | −0.52 |
C10H11NO3 | 1.31 |
Phenylacetylglycine | HMDB00821 | Acylglycine |
|
|
|
|
| T | 3.84±1.54 |
|
|
|
| −1.34 |
|
|
|
|
|
|
|
| P | 3.11±1.12 |
0.02473g |
|
|
|
|
|
|
|
|
| ESI- | 8.275 | 2.92 | C | 0.70±0.41 |
0.01843f | 192.0686 | −10.41 |
| 1.58 |
|
|
|
|
|
|
|
| T | 1.10±0.60 |
|
|
|
| −1.50 |
|
|
|
|
|
|
|
| P | 0.81±0.43 |
0.00273g |
|
|
|
|
|
|
|
| U2 | ESI+ | 7.956 | 5.62 | C | 1.12±0.74 |
0.04704f | 162.056 | −6.17 |
C9H7NO2 | 1.64 |
4,6-dihydroxyquinoline | HMDB04077 | Aromatic |
|
|
|
|
| T | 1.85±1.37 |
|
|
|
| −2.15 |
|
| Amino |
|
|
|
|
| P | 0.93±0.55 |
0.00775g |
|
|
|
|
|
| Alcohol |
| U3 | ESI- | 8.829 | 2.86 | C | 0.72±0.42 |
0.01112f | 283.0841 | −6.36 |
C13H16O7 | 1.63 |
p-cresolglucuronide | HMDB11686 | Mammalian |
|
|
|
|
| T | 1.17±0.62 |
|
|
|
| −1.47 |
|
| Metabolite |
|
|
|
|
| P | 0.89±0.36 |
0.01748f |
|
|
|
|
|
|
|
| U4 | ESI+ | 6.912 | 1.18 | C | 0.15±0.07 |
0.00001f | 208.0599 | 2.40 |
C10H9NO4 | 2.38 |
4-(2-aminophenyl)- | HMDB00978 | Amino |
|
|
|
|
| T | 0.36±0.16 |
|
|
|
|
| 2,4-dioxobutanoic
acid |
| Ketone |
|
|
|
|
| P |
Undetectablee |
|
|
|
|
|
|
|
|
Discussion
The etiology of IBD involves a complex interaction
between the genetic, environmental or microbial factors and the
immune responses, which eventually result in recurrent diarrhea,
rectal inflammatory lesions and bleeding (18). PO, the essential oil of Pogostemon
cablin, possesses anti-inflammatory, immunomodulatory and
anti-microbial activities, indicating a potential for the
symptomatic ease of IBD. The present study demonstrated for the
first time that rectal administration of PO downregulates the
macroscopic and histological colonic damage scores, DAI and colonic
MPO activities of rats with TNBS-induced colitis. Furthermore, a
targeted metabolic analysis of urine samples based on a
high-throughput UFLC-IT-QTOF-MS approach showed that PO decreased
the levels of four differential metabolites (two gut microbial
metabolites and two tryptophan metabolites), which were markedly
increased following treatment with TNBS.
As mentioned previously, one of the major
pathological factors of IBD is the commensal bacterial flora
(19). The microbial dysbiosis may
interplay with the damage of the intestinal barrier and the
disturbance of the mucosal immune system, inducing destructive
inflammatory immune responses, considered to be the basis of IBD
pathogenesis (20). The present
study demonstrated that the levels of two gut microbial
metabolites, phenylacetylglycine and p-cresol glucuronide, were
upregulated in the urine of TNBS rats. Phenylacetylglycine is a gut
microbial co-metabolite (21),
generated by conjugation of glycine with phenylacetate, via the
phase-II detoxification mechanism in the liver or the gut mucosa
(22). P-cresol glucuronide is a
glucuronide derivative of p-cresol, a metabolic product of
Clostridium difficile in the large intestine (23). Consistent with previous studies, the
observed increases in the output of those 2 metabolites indicated
dysbiosis of the intestinal microbiota (24). However, this change was significantly
attenuated by PO. As PO has marked anti-bacterial activity
(7), it may be deduced that PO
possibly ameliorated TNBS-induced colitis via regulating intestinal
microorganisms.
In addition, IBD is initiated and perpetuated by a
dysregulated immune response to antigens (25). The present study identified two
tryptophan metabolites that are closely involved in immune
regulation and the inflammatory response. Tryptophan has a pivotal
role in immunosuppression in inflammatory diseases, immune system
regulation, protein synthesis as well as serotonin (5-HT) and
melatonin production (26,27). The present study further identified 2
metabolites associated with the homeostasis of intestinal
microbiota [4-(2-aminophenyl)-2,4-dioxobutanoic acid and
4,6-cihydroxyquinoline] in urine. The former is the intermediate
product in metabolism of kynurenine, which regulates the immune
response (28). The latter is an
intermediate metabolite of 5-hydroxytryptophan, a precursor of
5-HT, which activates immune cells to produce pro-inflammatory
mediators (29). Hence, the
increased urine levels of these two metabolites suggest accelerated
tryptophan-kynurenine and tryptophan-5-HT metabolism. The enhanced
tryptophan and 5-HT metabolism may be associated with immune
dysfunction in IBD (30).
In conclusion, the results of the present study
demonstrated that treatment with 270 mg/kg via rectal instillation
significantly ameliorated TNBS-induced rat colitis and
significantly reversed the metabolic changes of four metabolites in
the urine of the TNBS rats, which were two tryptophan metabolites
[4-(2-aminophenyl)-2,4-dioxobutanoic acid and
4,6-cihydroxyquinoline] and two gut microbial metabolites
(phenylacetylglycine and p-cresol glucuronide). These metabolic
changes may be associated with TNBS-induced colitis in rats.
Acknowledgements
The present study was supported by grants from the
National Natural Science Foundation of China (project nos.
81303200, 21377106 and 81173534), Guangdong Natural Science
Foundation (project nos. S2013010016627 and S2012010008893),
Medical Scientific Research Foundation of Guangdong Province
(project no. A2013232), Administration of Traditional Chinese
Medicine of Guangdong Province (project no. 20132142), the Special
Funds from Central Finance of China in Support of the Development
of Local Colleges and University (project no. 276, 2014), the China
state ‘12th Five year Plan’ Scientific and Technological Support
Scheme (project no. 2012BAI029B09), Hong Kong, Macao and Taiwan
Science & Technology Cooperation Program of China (no.
2014DFH30010) and the Guangdong Province Universities and Colleges
Pearl River Scholar Funded Scheme (2011). The authors gratefully
acknowledge this financial support.
References
|
1
|
Frolkis A, Dieleman LA, Barkema HW,
Panaccione R, Ghosh S, Fedorak RN, Madsen K and Kaplan GG: Alberta
IBD Consortium: Environment and the inflammatory bowel diseases.
Can J Gastroenterol. 27:e18–e24. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Maloy KJ and Powrie F: Intestinal
homeostasis and its breakdown in inflammatory bowel disease.
Nature. 474:298–306. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Danese S and Fiocchi C: Etiopathogenesis
of inflammatory bowel diseases. World J Gastroenterol.
12:4807–4812. 2006.PubMed/NCBI
|
|
4
|
Triantafillidis JK, Merikas E and
Georgopoulos F: Current and emerging drugs for the treatment of
inflammatory bowel disease. Drug Des Devel Ther. 5:185–210. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Xian YF, Suo J, Huang XD, Hou SZ, Chen J,
Ye M and Su ZR: A Pharmacological Study on Anti-inflammatory
Effects of Refined Huodan Recipe. Zhong Guo Shi Yan Fang Ji Xue Za
Zhi She. 13:54–56. 2007.(In Chinese).
|
|
6
|
Qi SS, Hu LP, Chen WN, Sun HB and Ma XD:
Immunological regulation effects of essential oil in leaves of
Cablin Patchouli herbal on mice. Chin Arch Trad Chin Med.
27:774–776. 2009.
|
|
7
|
Yang X, Zhang X, Yang SP and Liu WQ:
Evaluation of the antibacterial activity of patchouli oil. Iran J
Pharm Res. 12:307–316. 2013.PubMed/NCBI
|
|
8
|
Mizoguchi A and Mizoguchi E: Animal models
of IBD: Linkage to human disease. Curr Opin Pharmacol. 10:578–587.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lu X, Zhao X, Bai C, Zhao C, Lu G and Xu
G: LC-MS-based metabonomics analysis. J Chromatogr B Analyt Technol
Biomed Life Sci. 866:64–76. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhang XJ, Choi FF, Zhou Y, Leung FP, Tan
S, Lin S, Xu H, Jia W, Sung JJ, Cai Z and Bian Z: Metabolite
profiling of plasma and urine from rats with TNBS-induced acute
colitis using UPLC-ESI-QTOF-MS-based metabonomics-a pilot study.
FEBS J. 279:2322–2338. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lin RF, Feng XX, Li CW, Zhang XJ, Yu XT,
Zhou JY, Zhang X, Xie YL, Su ZR and Zhan JY: Prevention of UV
radiation-induced cutaneous photoaging in mice by topical
administration of patchouli oil. J Ethnopharmacol. 154:408–418.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
McCafferty DM, Sharkey KA and Wallace JL:
Beneficial effects of local or systemic lidocaine in experimental
colitis. Am J Physiol. 266:G560–G567. 1994.PubMed/NCBI
|
|
13
|
Cooper HS, Murthy SN, Shah RS and
Sedergran DJ: Clinicopathologic study of dextran sulfate sodium
experimental murine colitis. Lab Invest. 69:238–249.
1993.PubMed/NCBI
|
|
14
|
Gue M, Bonbonne C, Fioramonti J, Moré J,
Del Rio-Lachèze C, Coméra C and Buéno L: Stress-induced enhancement
of colitis in rats: CRF and arginine vasopressin are not involved.
Am J Physiol. 272:G84–G91. 1997.PubMed/NCBI
|
|
15
|
Schicho R and Storr M: Topical and
systemic cannabidiol improves trinitrobenzene sulfonic acid colitis
in mice. Pharmacology. 89:149–155. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Best WR, Becktel JM, Singleton JW and Kern
F Jr: Development of a Crohn's disease activity index. National
cooperative crohn's disease study. Gastroenterology. 70:439–444.
1976.PubMed/NCBI
|
|
17
|
Krawisz JE, Sharon P and Stenson WF:
Quantitative assay for acute intestinal inflammation based on
myeloperoxidase activity. Assessment of inflammation in rat and
hamster models. Gastroenterology. 87:1344–1350. 1984.PubMed/NCBI
|
|
18
|
Zhang YZ and Li YY: Inflammatory bowel
disease: Pathogenesis. World J Gastroenterol. 20:91–99. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Guarner F: The intestinal flora in
inflammatory bowel disease: Normal or abnormal? Curr Opin
Gastroenterol. 21:414–418. 2005.PubMed/NCBI
|
|
20
|
Stephani J, Radulovic K and Niess JH: Gut
microbiota, probiotics and inflammatory bowel disease. Arch Immunol
Ther Exp (Warsz). 59:161–177. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yap IK, Li JV, Saric J, Martin FP, Davies
H, Wang Y, Wilson ID, Nicholson JK, Utzinger J, Marchesi JR and
Holmes E: Metabonomic and microbiological analysis of the dynamic
effect of vancomycin-induced gut microbiota modification in the
mouse. J Proteome Res. 7:3718–3728. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Akira K, Hichiya H, Morita M, Shimizu A
and Mitome H: Metabonomic study on the biochemical response of
spontaneously hypertensive rats to chronic taurine supplementation
using (1)H NMR spectroscopic urinalysis. J Pharm Biomed Anal.
85:155–161. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Walsh MC, Brennan L, Pujos-Guillot E,
Sébédio JL, Scalbert A, Fagan A, Higgins DG and Gibney MJ:
Influence of acute phytochemical intake on human urinary
metabolomic profiles. Am J Clin Nutr. 86:1687–1693. 2007.PubMed/NCBI
|
|
24
|
Akira K, Masu S, Imachi M, Mitome H and
Hashimoto T: A metabonomic study of biochemical changes
characteristic of genetically hypertensive rats based on (1)H NMR
spectroscopic urinalysis. Hypertens Res. 35:404–412. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Del A, ngel-Meza AR, Dávalos-Marín AJ,
Ontiveros-Martinez LL, Ortiz GG, Beas-Zarate C, Chaparro-Huerta V,
Torres-Mendoza BM and Bitzer-Quintero OK: Protective effects of
tryptophan on neuro-inflammation in rats after administering
lipopolysaccharide. Biomed Pharmacother. 65:215–219. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kim CJ, Kovacs-Nolan JA, Yang C, Archbold
T, Fan MZ and Mine Y: l-Tryptophan exhibits therapeutic function in
a porcine model of dextran sodium sulfate (DSS)-induced colitis. J
Nutr Biochem. 21:468–475. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Frumento G, Rotondo R, Tonetti M, Damonte
G, Benatti U and Ferrara GB: Tryptophan-derived catabolites are
responsible for inhibition of T and natural killer cell
proliferation induced by indoleamine 2,3-dioxygenase. J Exp Med.
196:459–468. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Nguyen NT, Kimura A, Nakahama T, Chinen I,
Masuda K, Nohara K, Fujii-Kuriyama Y and Kishimoto T: Aryl
hydrocarbon receptor negatively regulates dendritic cell
immunogenicity via a kynurenine-dependent mechanism. Proc Natl Acad
Sci USA. 107:pp. 19961–19966. 2010; View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Linden DR, Chen JX, Gershon MD, Sharkey KA
and Mawe GM: Serotonin availability is increased in mucosa of
guinea pigs with TNBS-induced colitis. Am J Physiol Gastrointest
Liver Physiol. 285:G207–G216. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Gersemann M, Wehkamp J and Stange EF:
Innate immune dysfunction in inflammatory bowel disease. J Intern
Med. 271:421–428. 2012. View Article : Google Scholar : PubMed/NCBI
|