Introduction
The regeneration ability of articular cartilage is
limited, and tiny trauma can induce severe pain and dysfunction of
articular. A good treatment strategy for osteoarthritis (OA) is to
in vitro build cartilage tissues, which have the same
biological features as articular cartilage, thus restoring the
defects of cartilage (1). Currently,
autologous cartilage transplantation is the most common way to
restore the defects of articular cartilage. Autologous cartilage
transplantation means human articular cartilage cells are generated
from human body, cultured and amplified in vitro, and
eventually the amplified cartilage cells were transplanted to the
defected articular cartilage. However, dedifferentiation is common
with in vitro cultured articular cartilage, meaning that the
expression of collagen II and aggrecan representative of hyaline
cartilage of extracellular matrix are decreased, while collagen X
representative of cartilage hypertrophy is upregulated. Therefore,
cartilage tissues built in autologous cartilage transplantation
usually are not hyaline cartilage, and the repairing effect of
articular cartilage defects are quite limited (2).
Another replacement of autologous cartilage
transplantation is to build cartilage tissues by stem cells or
biological materials, and bone marrow mesenchymal stem cell (BMSC)
is a very attractive cell (3). To
fulfill this purpose, it is necessary to fully understand the
molecular mechanism of the differentiation of BMSCs into
chondrocytes.
miRNAs are small, single strand RNA molecules
composed of 18–25 nucleotides. Combined with related proteins to
form RNA-induced silencing complex (RISC), miRNAs could take part
in post-transcription regulation of target genes by inhibiting
translation or promoting degradation of target genes. Accumulating
evidence has shown that many miRNAs are related with the
initiation, pathologic grade, clinical stages, chemotherapy
resistance and prognosis of tumors and even directly take part in
the initiation and progression of neoplasms including leukemia,
lymphoma, lung and colon cancer. In different tumors, miRNAs
displayed particular expression patterns and have potential of
differentiating tumors types and early diagnosis (4). miR-127, a small, non-coding RNA with
overlapped gene structure, plays a role in lung development,
embryogenesis and tumor initiation (5). Previous studies revealed that miR-127
showed downregulation in various tumors including malignant
gliomas, gastric and liver cancer (6–8). It is
reported that inhibition of miR-127 expression promoted the
initiation of liver cancer (9) and
was closely linked to the oncogenesis of diffuse large B-cell
lymphoma (10). Also, miR-127 could
activate JNK signaling pathway and promote lung inflammatory injury
(11). In particular, researchers
indicated that miR-127-5p were downregulated in OA cartilage
tissues compared with normal tissues and it promoted the
development and progression of OA by regulating the expression of
MMP-13 in cartilage cells (12).
From all the above, we hypothesized that miR-127-5p may play a
critical role in the differentiation of the cartilage.
Until now, the roles of miR-127-5p in regulating rat
BMSC differentiation into cartilage remain unclear. In this study,
we investigated the effect and related mechanisms of miR-127-5p on
the cartilage differentiation of rat BMSCs to provide theoretical
basis and experimental evidence for construction of hyaline
cartilage with stable phenotypes.
Materials and methods
Materials
The following were obtained: DMEM media, F12 media,
collagen I, trypsin and fetal bovine serum (FBS) were from Gibco
Life Technologies (Carlsbad, CA, USA). miRNAs analogues were from
Guangzhou Ruibo Biological Technology Co., Ltd. (Guangzhou, China).
Lipofectamine™ RNAiMAX was from Invitrogen Life Technologies
(Carlsbad, CA, USA). Primary rabbit polyclonal Sox9 antibody
(dilution, 1:1,000; cat. no. sc-20095) and rabbit polyclonal Runx2
antibody (dilution, 1:1,000; cat. no. sc-10758) were from Santa
Cruz Biotechnology, Inc. (Santa Cruz, CA, USA) and horseradish
peroxidase coupled secondary antibodies were also from Santa Cruz
Biotechnology, Inc.. PE labeled anti-CD34 (dilution, 1:100; cat.
no. 343604), CD44 (dilution, 1:100; cat. no. 103007), CD45
(dilution, 1:100; cat. no. 304016), CD90.2 (dilution, 1:100; cat.
no. 105314) FACS and comparisons were from BioLegend, Inc. (San
Diego, CA, USA). ECL kit was from Beyotime Institute of
Biotechnology (Shanghai, China), TRIzol Reagent was from Invitrogen
Life Technologies, RT-PCR kit, Taq polymerases and dNTPs were from
MBI Fermentas, Inc. (Burlington, ON, Canada) and IQ SYBR Green
Supermix was from Bio-Rad Laboratories, Inc. (Hercules, CA, USA).
Cartilage induction solution was from Saiye Health Research Center
(Taicang) Co., Ltd., (China). Safranin O staining was from Boster
Bio-Engineering (Wuhan, China) and Protein Quantity Assay kit was
from Boster Bio-Engineering.
Isolation and culture of BMSCs
Sprague-Dawley rats at 12 weeks were purchased from
Shanghai Laboratory Animal Center (SLAC), CAS, (Shanghai, China)
and all the animal experiments were approved by the Ethics
Committee of Nanxiang Hospital. After sacrificing the rats under
anaesthesia, we isolated bilateral limbs, cut off metaphysis and
flushed out medulla with syringe under sterile conditions. Single
cell suspension was prepared with PBS and centrifuged at 1,000 × g
for 10 min. We discarded the supernatant and suspended cells with
DMEM/F12 media. Suspended cells labeled as primary cells were
plated in 25 cm2 culture bottle and cultured in
incubators with 37°C and 5% CO2. We changed the medium
every 2 days and observed the growth and morphology under inverted
microscope (BX-42; Olympus, Tokyo, Japan). Cells were passaged with
0.25% trypsin at 1:2 when they almost merged.
Differentiation of CDs on the surface
of rat BMSCs with flow cytometry
Rat BMSCs at passage 3 were trypsinized for 2 min at
25°C and added low-glucose DMEM with 10% FBS to stop digestion.
Then cells were suspended with medium at a density of
5×105/ml after being centrifuged at 750 × g for 5 min.
PE/Cy7-conjugated anti-CD34 antibodies (10 µl), PE-conjugated
anti-CD45 antibodies (6 µl), PE-conjugated anti-CD44 antibodies (6
µl), PE-conjugated anti-CD90.2 antibodies (6 µl) and their isotype
control antibodies were added to 100 µl cell suspensions,
respectively. They were incubated at 4°C for 30 min and on the
shaker at 37°C for 60 min, then cells were washed twice with PBS
and suspended with 200 µl PBS for flow cytometry detection.
Alcian blue staining of BMSCs induced
chondroblasts microgroups
Cell groups (induced for 0, 7 and 17 days,
respectively) were fixed in 4% paraformaldehyde for 20 min and
washed for three times with 0.01M PBS (5 min each time). The washed
cells were dehydrated with graded ethanol
(30%-50%-70%-80%-90%-100%), vitrificated by dimethylbenzene and
paraffin-embedded. The cell groups went through dewaxing and
dehydration before being sliced for 5 µm. Then they were stained
with Alcian blue (pH 2.5) for 30 min, washed with running water for
5 min and dehydrated with graded ethanol, vitrificated by
dimethylbenzene, then the pieces were sealed by neutral gum.
Collagen II immunohistochemistry
staining of BMSCs induced chondroblasts microgroups
Chondroblasts (induced for 0, 7 and 17 days,
respectively) were fixed, dehydrated and embedded. BMSCs which were
not induced were considered as control group. SABC-DAB color assay
was used to detect collagen II expression.
miR-127-5p transfection in BMSCs
BMSCs at passage 3 were plated in 6-well plates and
transfected with Lipofectamine™ RNAiMAX when cells reached a
density of 50–60%. Cells were transfected with miR-127-5p analogues
(300 pmol) for the experimental group and meaningless sequence (300
pmol) for the negative control group. Thirty-six hours after
transfection, BMSCs were induced to differentiate into
chondroblasts and constructed of cartilage micro-clusters by
centrifugation.
Chondroblasts differentiation induced
by BMSCs and construction of cartilage micro-clusters
BMSCs in both experimental group and control group
were induced to differentiate. The cartilage induction solution was
composed of low-glucose DMEM medium, L-proline (40 mg/l),
indomethacin (1%), sodium pyruvate (100 mg/l), dexamethasone (100
nmol/l), FBS (1%) and ascorbic acid (50 mg/l). BMSCs at 1 week
post-induction were digested, suspended and centrifuged. The
cartilage induction solution was added shortly after supernatant
was abandoned. Cells were cultured in incubators with
5%CO2 at 37°C and changed medium every 4 days. The
formed cartilage was sliced 2 weeks later.
RT-PCR assay
BMSCs at 1 week post-induction were lysed with
TRIzol reagent and extracted RNA. After the whole RNA extraction, a
PrimeScript RT kit (Takara Bio, Dalian, China) was used for reverse
transcription. The reversed cDNA were ready for RT-PCR. Primers
were labeled with SYBR-Green I. The primers were as follows: Sox9
(forward: 5-AAAGGAAG GAAGGGAAGAAAGG-3, reverse: 5-AATATGGCATCTT
TCGATTTCTG-3); collagen II (forward: 5-CAAGTCGCTG AACAACCAGA-3,
reverse: 5-GCCCTCATCTCCACATC ATT-3); aggrecan (forward:
5-ACGGTGGGAAATGAAAG AAATG-3, reverse: 5-TCCCACTCACATGGTGTCTTCT-3);
collagen X (forward: 5-GCCCTTTTCCTCTGGCTGAT-3, reverse:
5-TTGACCAACGTCTGAACAATGG-3); GAPDH (forward:
5-TATGACTACCCACGGCAAGT-3, reverse: 5-ATACTCAGCACCAGCATCACC-3). We
extracted Ct levels when RT-PCR reaction stopped. GAPDH was used as
internal control.
Safranin O staining
The cartilage masses were fixed with 4%
paraformaldehyde, paraffin-embedded and then sliced. The slices
were differentiated with 1% ethanol hydrochloride, washed and
stained with 0.1% Safranin O for 5 min. After dehydrated and rinsed
by 95% ethanol, slices were sealed and observed.
Western blot analysis
We lysed BMSCs at 1 week post-induction with RIPA
and extracted total proteins. Protein concentration was determined
by a protein assay kit. A total of 10% SDS-PAGE was used to
separate protein, and then it was shifted to PVDF membranes
(Millipore Corp., Billerica, MA, USA). A total of 5% fat-free milk
was used to block non-specific protein interactions in TBST buffer.
The membranes loaded with proteins were incubated at 4°C with
primary antibody (rabbit anti-rat: Sox9 and Runx2) and incubated at
room temperature with secondary antibody conjugated with
horseradish peroxide (2 h). After washing these membranes in TBST
buffer, we developed the membranes using chemiluminescence to
detect antibody concentration and took GAPDH as our internal
control.
Statistical analysis
SPSS 11.0 (Chicago, IL, USA) was used to analyze our
data. Quantitative data are expressed as mean ± SD. Non-paired
t-test was used to analyzed data between groups. A P<0.05 was
considered as statistically significant.
Results
Morphological characteristics and
surface marker identification of BMSCs
Small spindle cells, adherently and scattered were
observed after BMSCs were changed into medium for 3 days and, dead
cells with high grade of refraction were also observed (Fig. 1A). After 4–7 days of culture, the
cell volume increased and grew into colonies (Fig. 1B). Fourteen days later, cells showed
uniform morphology with spiral pattern and high degree of fusion
(Fig. 1C). Five days after passage 3
cells were passaged, cells showed more uniform morphology with a
density of >80% and some manifested as wide, fat mature BMSCs
(Fig. 1D). We cultured and passaged
continually and did not observe significant morphology changes. CD
molecular phenotype of isolated BMSCs was analyzed with flow
cytometry. Results showed that CD34, CD45 (markers of hematopoietic
stem cells) were negative and CD44, CD90.2 were positive (Fig. 1E).
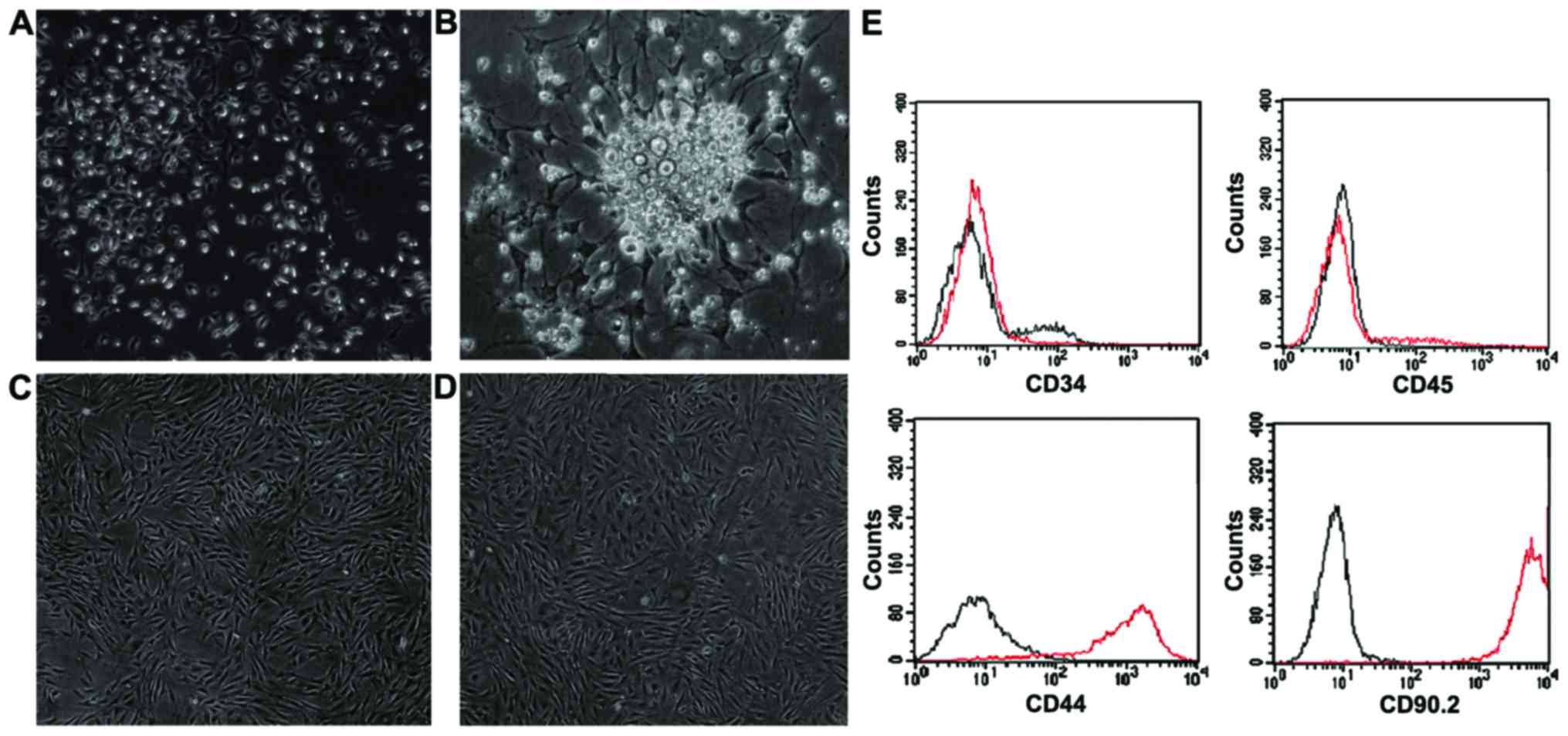 | Figure 1.Rat BMSC culture by attachment culture
method. (A) Three days after culturing of P0 BMSCs, spindle-like
cells scattered (magnification, ×100); (B) Five days after
culturing of P0 BMSCs, cells grew into colonies (magnification,
×200); (C) Fourteen days after culturing of P0 BMSCs, cells reached
a density of 90% (magnification, ×100); (D) Five days after
culturing of P3 BMSCs, cells showed more uniform morphology and
some manifested as wide, fat mature BMSCs (magnification, ×100) and
(E) Identification of CD molecules of BMSCs with flow cytometry
(black as isotype contrast). CD34 (−), CD45 (−), CD44 (+), CD99.2
(+). BMSCs, bone marrow mesenchymal stem cells. |
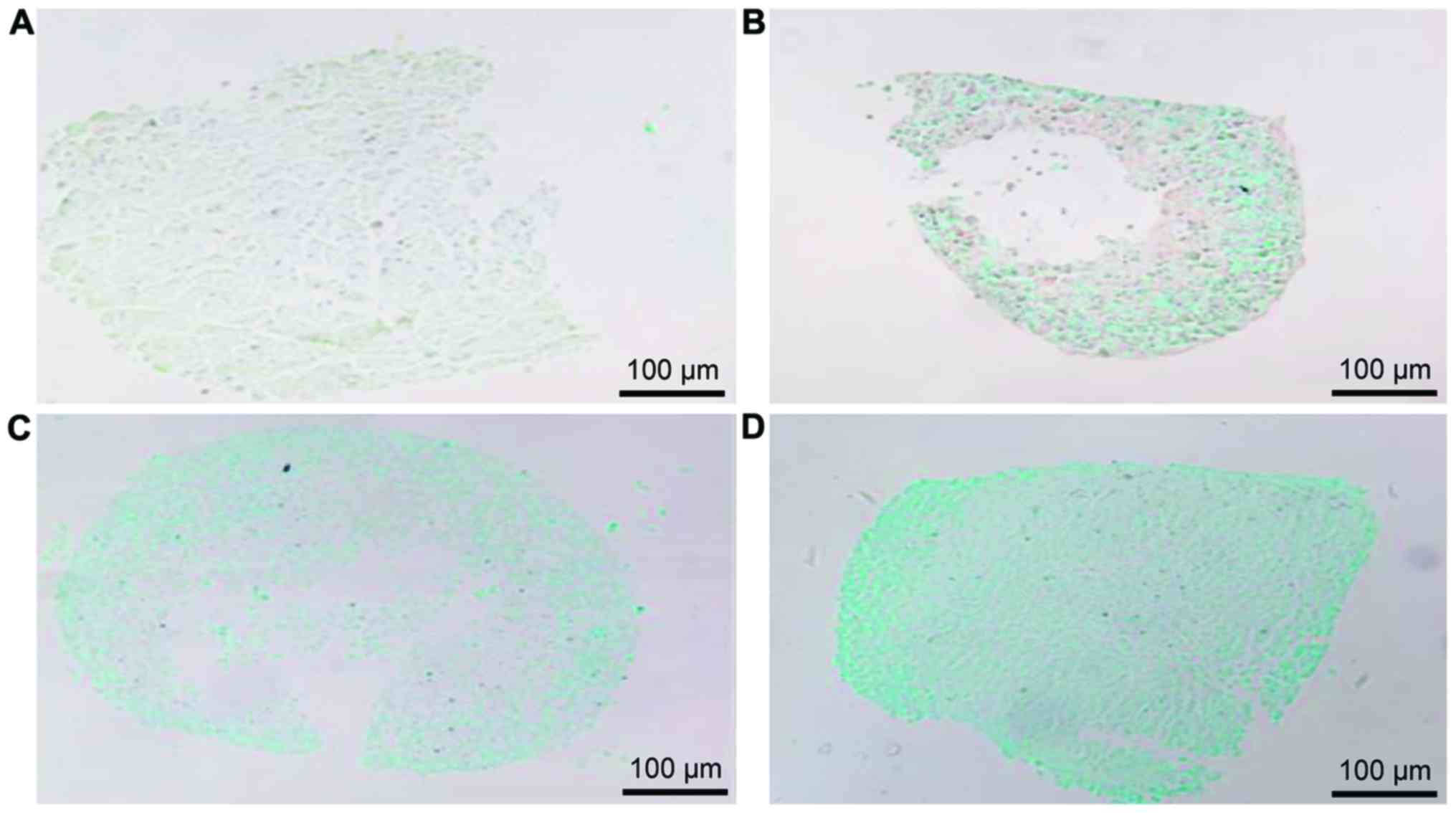
Alcian blue staining of BMSCs induced
to differentiate to chondroblasts
Alcian blue staining showed that the
glycosaminoglycan (GAGs) staining was positive in cell matrix of
induced BMSCs and the staining was obviously enhanced with the
increased induction time (Fig. 2).
It indicated that the isolated BMSCs had the potential of
differentiating into cartilage and expressing GAGs which is a
cellular phenotype of cartilage.
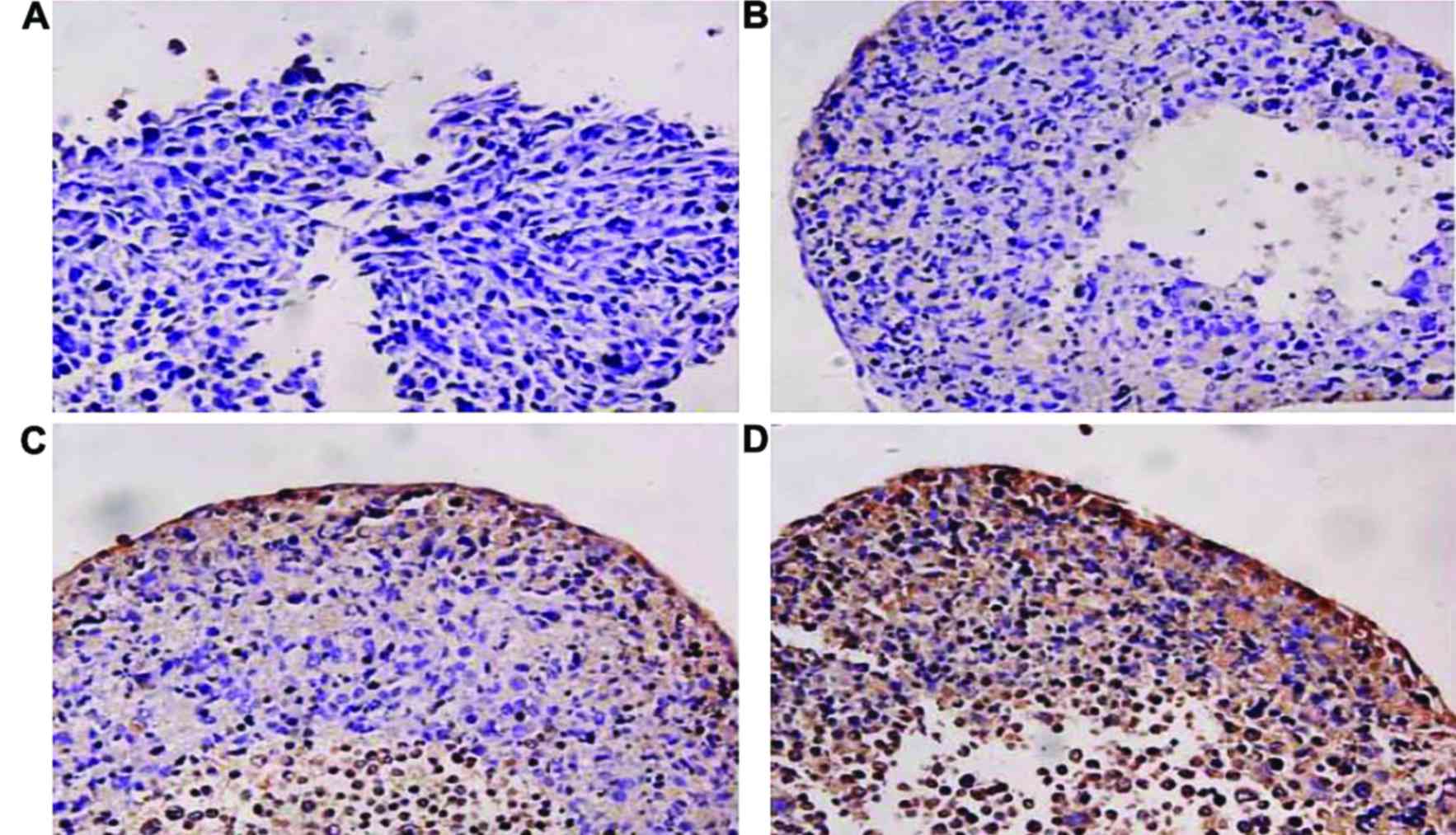
Collagen II staining of BMSCs induced
to differentiate to chondroblasts
We did not detect collagen II staining in BMSCs
induced for 1 day but the collagen II staining showed positive with
the increased induction time in which staining at 14 days was
stronger than that at 7 days (Fig.
3). It indicated that the isolated BMSCs had the potential of
differentiating into cartilage and expressing collagen II which is
a cellular phenotype of cartilage.
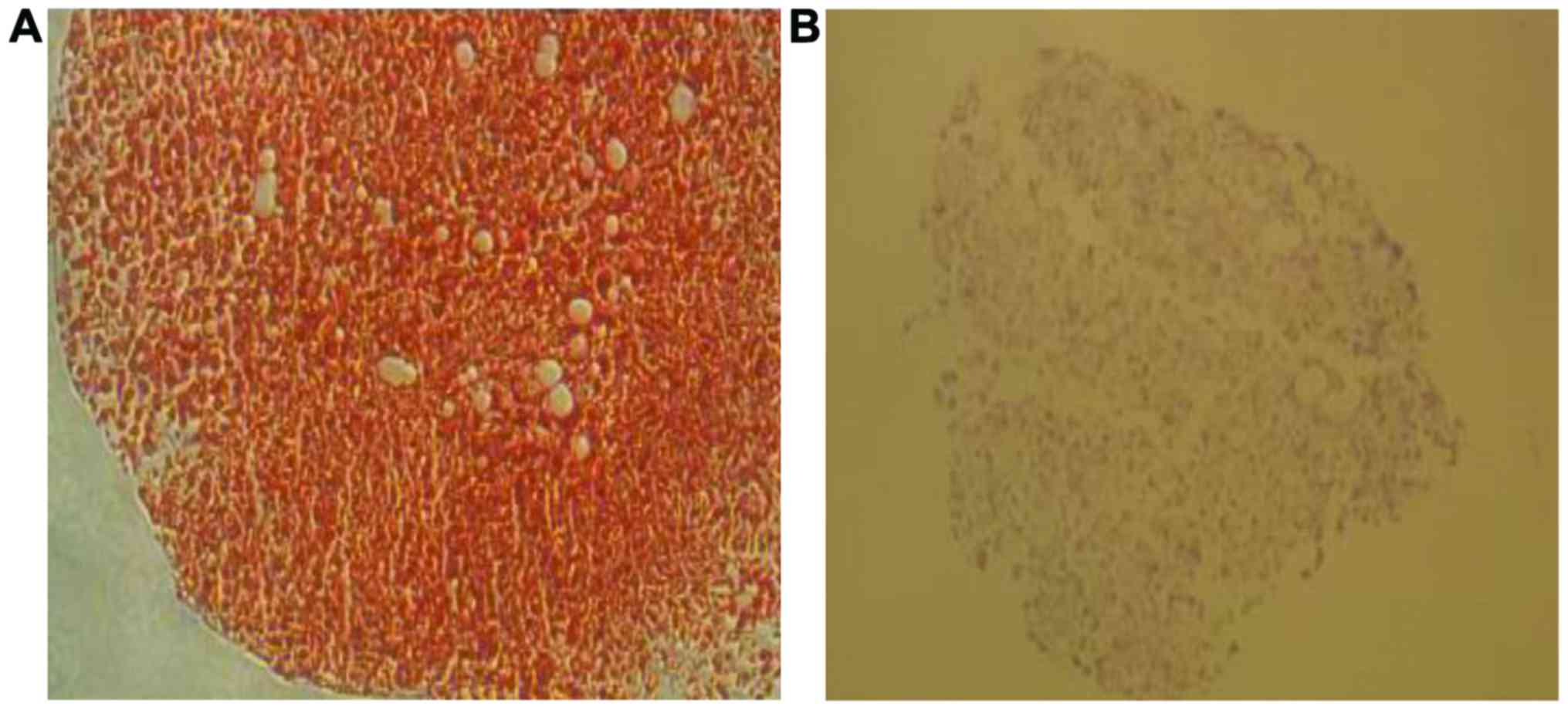
Chondroitin sulfate detection by
Safranin O staining
Safranin O staining was performed in cartilage
micro-groups transfected with miR-127-5p or not to detect
chondroitin sulfate. The transfected groups showed vivid red
staining while non-transfected groups showed very pale staining.
The results showed that miR-127-5p promoted the expression of
chondroitin sulfate in cartilage micro-groups induced by BMSCs
(Fig. 4).
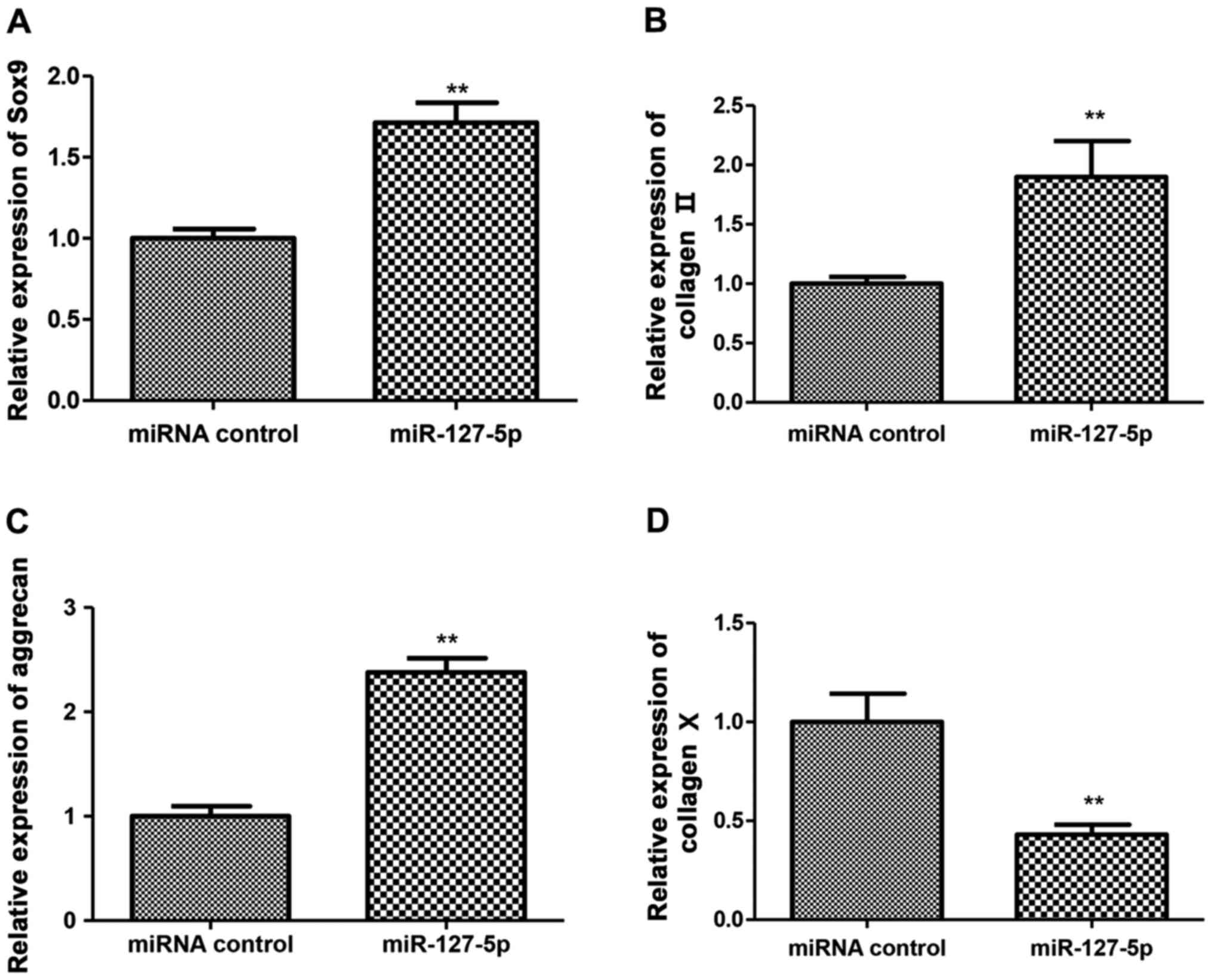
Cartilage differentiation-related gene
expression detection by RT-PCR
Cartilage differentiation-related gene expression in
BMSCs induced by induction solution at 7 days were detected by
RT-PCR. The results showed that the expression of Sox9, collagen II
and aggrecan were higher in the transfected groups than that in
control groups with significant difference (Fig. 5A-C). Another finding was that the
expression of collagen X in the transfected groups was lower than
that in control groups with significant difference (Fig. 5D).
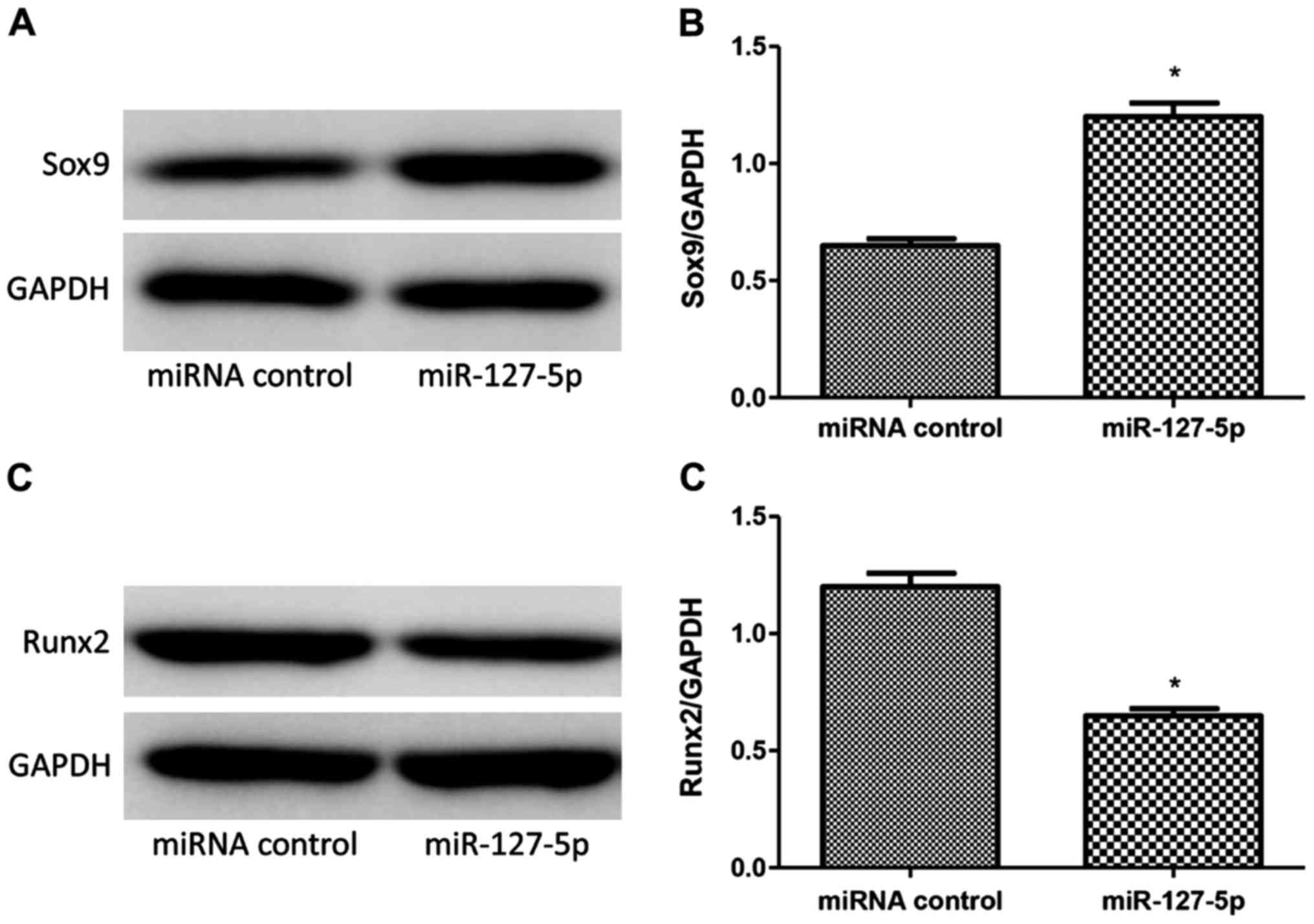
Results of western blot analysis
Sox9 and Runx2 were detected by western blot
analysis in BMSCs induced by induction solution at 7 days. Western
blot analysis showed that miR-127-5p transfection promoted the
expression of Sox9 (Fig. 6A and B)
while decreased the expression of Runx2 (Fig. 6C and D) of rat BMSCs with significant
difference.
Discussion
miRNA is a new regulator of gene expression. miRNA
is of great significance for the proliferation and self-renewal of
pluripotent stem cells. It is also very important for the
differentiation of mesenchymal stem cells and organ formation.
Recently, it was reported that miRNA plays a role in the
pathophysiological process of OA (13,14).
miR-21 could regulate the expression of GDF-5 and relieve the
inflammatory reaction of OA (15).
Park et al (12) indicated
that miR-127-5p showed obviously downregulation in OA and
downregulated MMP-13, inhibited the initiation of OA.
BMSCs, originated from bone marrow pluripotent stem
cells, could differentiate into various tissues including bone,
cartilage and adipose tissue (16).
Research has shown that BMSC differentiation into bone is a highly
programmed process and regulated by Runx2 and finally causes
calcium deposition in extracellular matrix. BMSC differentiation
into cartilage is regulated by Sox9 which induces the expression of
collagen II and chondroitin sulfate. But one important defect in
in vitro induction of BMSC differentiaton into cartilage is
that it is difficult to have a stable cartilage phenotype. In the
late stage of differentiation, Runx2 increased and hypertrophic
cartilage specific proteins expressed collagen X decreasing the
quality of engineered cartilage. Thus, the engineered cartilage
could not compare with hyaline cartilage and form good fusion
growth with surrounding cartilage when it is used to repair
articular cartilage in vivo.
As a result, downregulating Runx2 and upregulating
Sox9 may be an effective method to construct engineered cartilage
with stable phenotype in the process of BMSC differentiation into
cartilage. In the current study, miR-127-5p promoted the expression
of chondroitin sulfate in cartilage micro-groups induced by BMSCs.
miR-127-5p upregulated the expression of Sox9, collagen II and
miR-127-5p while downregulated collagen X. Western blot analysis
showed that miR-127-5p could promote the expression of Sox9 while
decreased the expression of Runx2. We inferred that miR-127-5p
promotes BMSC differentiation into cartilage and inhibits
hypertrophy by regulating core transcripts during bone and
cartilage differentiation. Our study revealed that miR-127-5p
indeed promoted the BMSC differentiation into cartilage and
inhibited its hypertrophy which provided a certain theoretical and
experimental basis for constructing engineered cartilage with a
stable phenotype through BMSCs. It is of great significance for the
treatment of OA.
References
|
1
|
Brittberg M, Lindahl A, Nilsson A, Ohlsson
C, Isaksson O and Peterson L: Treatment of deep cartilage defects
in the knee with autologous chondrocyte transplantation. N Engl J
Med. 331:889–895. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Minas T, Gomoll AH, Solhpour S,
Rosenberger R, Probst C and Bryant T: Autologous chondrocyte
implantation for joint preservation in patients with early
osteoarthritis. Clin Orthop Relat Res. 468:147–157. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
de Peppo GM, Svensson S, Lennerås M,
Synnergren J, Stenberg J, Strehl R, Hyllner J, Thomsen P and
Karlsson C: Human embryonic mesodermal progenitors highly resemble
human mesenchymal stem cells and display high potential for tissue
engineering applications. Tissue Eng Part A. 16:2161–2182. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Varnholt H, Drebber U, Schulze F,
Wedemeyer I, Schirmacher P, Dienes HP and Odenthal M: MicroRNA gene
expression profile of hepatitis C virus-associated hepatocellular
carcinoma. Hepatology. 47:1223–1232. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bhaskaran M, Wang Y, Zhang H, Weng T,
Baviskar P, Guo Y, Gou D and Liu L: MicroRNA-127 modulates fetal
lung development. Physiol Genomics. 37:268–278. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Jiang H, Jin C, Liu J, Hua D, Zhou F, Lou
X, Zhao N, Lan Q, Huang Q, Yoon JG, et al: Next generation
sequencing analysis of miRNAs: MiR-127-3p inhibits glioblastoma
proliferation and activates TGF-β signaling by targeting SKI.
OMICS. 18:196–206. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Guo LH, Li H, Wang F, Yu J and He JS: The
tumor suppressor roles of miR-433 and miR-127 in gastric cancer.
Int J Mol Sci. 14:14171–14184. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhou J, Lu S, Yang S, Chen H, Shi H, Miao
M and Jiao B: MicroRNA-127 post-transcriptionally downregulates
Sept7 and suppresses cell growth in hepatocellular carcinoma cells.
Cell Physiol Biochem. 33:1537–1546. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Tryndyak VP, Ross SA, Beland FA and
Pogribny IP: Down-regulation of the microRNAs miR-34a, miR-127, and
miR-200b in rat liver during hepatocarcinogenesis induced by a
methyl-deficient diet. Mol Carcinog. 48:479–487. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Robertus JL, Harms G, Blokzijl T, Booman
M, de Jong D, van Imhoff G, Rosati S, Schuuring E, Kluin P and van
den Berg A: Specific expression of miR-17-5p and miR-127 in
testicular and central nervous system diffuse large B-cell
lymphoma. Mod Pathol. 22:547–555. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ying H, Kang Y, Zhang H, Zhao D, Xia J, Lu
Z, Wang H, Xu F and Shi L: MiR-127 modulates macrophage
polarization and promotes lung inflammation and injury by
activating the JNK pathway. J Immunol. 194:1239–1251. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Park SJ, Cheon EJ, Lee MH and Kim HA:
MicroRNA-127-5p regulates matrix metalloproteinase 13 expression
and interleukin-1β-induced catabolic effects in human chondrocytes.
Arthritis Rheum. 65:3141–3152. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Nugent M: MicroRNAs: Exploring new
horizons in osteoarthritis. Osteoarthritis Cartilage. 24:573–580.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Cuadra VM Borgonio, González-Huerta NC,
Romero-Córdoba S, Hidalgo-Miranda A and Miranda-Duarte A: Altered
expression of circulating microRNA in plasma of patients with
primary osteoarthritis and in silico analysis of their pathways.
PLoS One. 9:e976902014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhang Y, Jia J, Yang S, Liu X, Ye S and
Tian H: MicroRNA-21 controls the development of osteoarthritis by
targeting GDF-5 in chondrocytes. Exp Mol Med. 46:e792014.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Panetta NJ, Gupta DM and Longaker MT: Bone
regeneration and repair. Curr Stem Cell Res Ther. 5:122–128. 2010.
View Article : Google Scholar : PubMed/NCBI
|