Introduction
Electric shock treatment (EST) is a psychotherapy
that may be used alone or in combination with drug therapy, and has
demonstrated positive curative effects in the treatment of mental
disorders (1,2) However, there are a considerable number
of patients, known as ‘refractory’ patients, who are immune to
these clinical interventions and show little possibility of
recovery (3). Refractory mental
illnesses contribute greatly to disability worldwide; therefore
identifying effective alternative therapies may make a huge
difference for such patients.
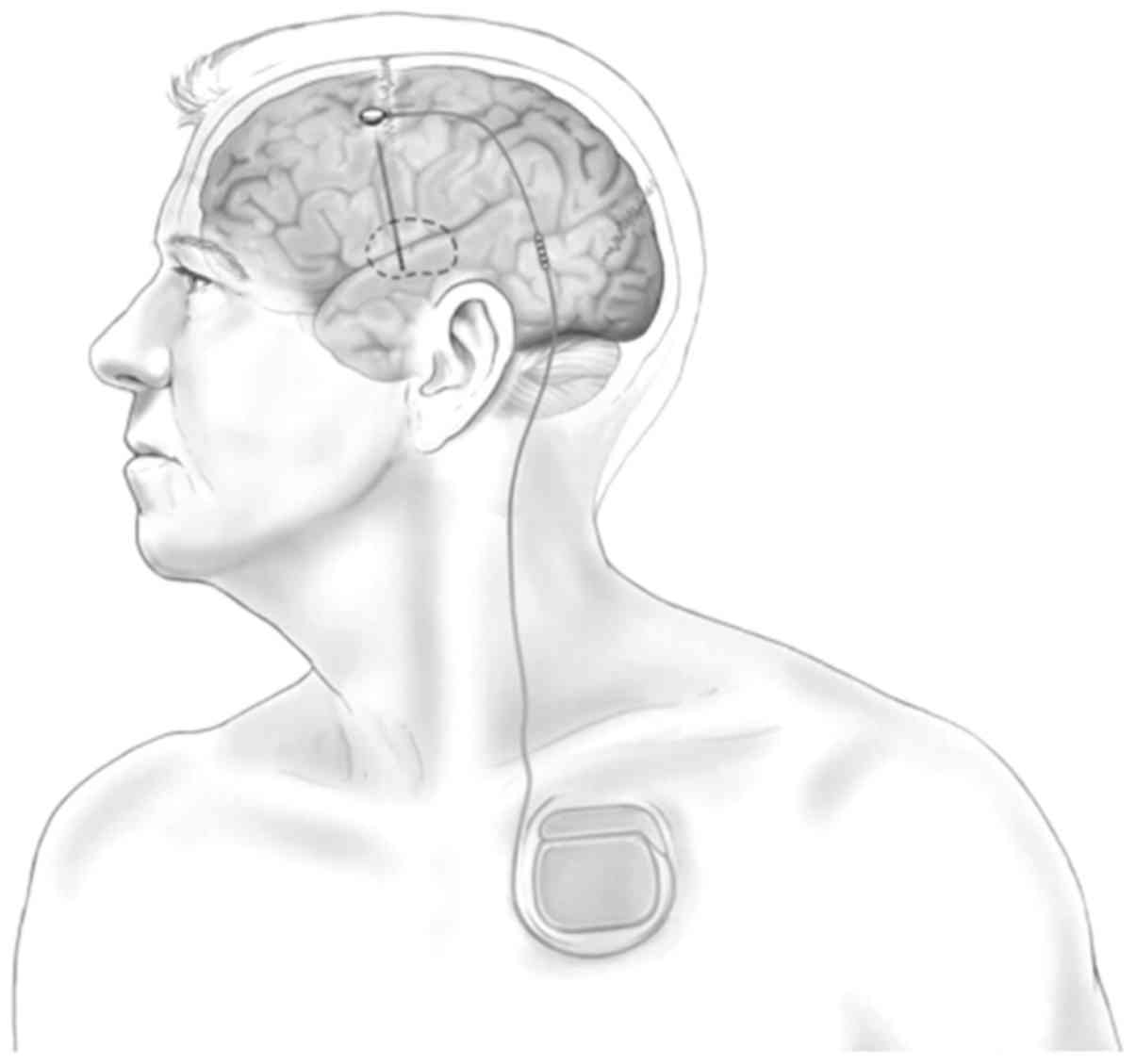
Deep brain stimulation (DBS) is accomplished via a
nerve stimulator implanted in the body and supplied by a battery
source, commonly known as a brain pacemaker (4). Typically, a pulse generator supplied
with a lithium battery is placed under the skin in the chest area,
with one or two wires attaching it to an implanted electrode that
is oriented to the target region for brain stimulation (inserted
using the stereotactic technique) (5). The pulse stimulation is conducted from
the generator to the electrode in the location of interest in the
deep brain (Fig. 1) (6). DBS has been used as a treatment for
mental diseases since 1987 (7). DBS
as a treatment for chronic diseases was first employed by Benabid
et al to treat dyskinesia (7)
and at present, is primarily used to treat tremors caused by
Parkinson's disease, chronic pain and dysmyotonia. Attempts have
been made to develop its use in the treatment of mental disorders
(8).
The first mention of using DBS to treat mental
diseases in literature was in a report published in the Lancet
journal in 2002 discussing the therapy as a possible treatment for
obsessive-compulsive disorder (OCD) and transient tic disorder
(8). The first report suggesting
that DBS could treat depressive disorder was published in 2005
(9). DBS has been authorized for the
treatment of epilepsy and OCD in Europe, and for the treatment of
refractory OCD treatment in the USA (10). This approval has increased research
and promoted the development of DBS, particularly regarding the
mechanisms of vagus nerve stimulation (11).
Side effects are uncommon following treatment with
DBS, and only mild side effects have been observed when it is used
as a treatment for dyskinesia (12).
This highlights the advantages of DBS technology; it has good
specificity, and the inhibitory effect of deep brain stimulation on
the motor nucleus of the thalamus disappeared after the cessation
of stimulation (13). Such
advantages are particularly important considering that at present,
there are no effective therapies available to treat the majority of
mentally handicapped individuals. To treat conditions where it is
unknown whether the therapy will have beneficial effects, the moral
compulsory principle of ‘no harm’ makes DBS an attractive option.
Unlike neurosurgical treatments, DBS is fully reversible, providing
the potential means to identify therapeutic targets for the
treatment of mentally handicapped individuals without risking
permanent damage (Fig. 2) (14).
The current review summarizes the selection and
relative benefits of different therapeutic targets of DBS therapy,
including refractory depression, OCD, habituation, Tourette's
syndrome (TS), anorexia nervosa (AN) and Alzheimer's disease.
Mechanisms of DBS
The neurobiological mechanisms by which DBS
regulates brain function are not yet fully understood (15). The effect of DBS on the cerebral
nuclei target region is either excitatory or inhibitory, depending
on the properties of internuncial neurons and the afferent neurons
in the target region (16). It has
been proposed that high-frequency DBS may induce functional damage
to areas surrounding the lesion, including closure of
current-dependent ion channels (17)
and blocking of depolarization via exhaustion of the
neurotransmitter (18). The
mechanism by which this damage occurs is synapse inhibition and it
is also known as neural activation in the stimulated region
(19,20).
Many scholars conclude that the influence of DBS on
the neurological network is more complicated than simply damaged
surgery and that DBS therapy may affect the neuronal somas and the
two-way activation function of axons (21,22).
Previous studies have reported that various neurotransmitters,
including glutamic acid and dopamine, are released following DBS
(23,24). Functional neuroimaging data also
indicate that DBS alters the brain activity beyond the target area
to a large extent, suggesting that DBS may have a sophisticated
neural network control function (9,25–27).
Benabid et al (7) suggested
that DBS therapy may exert synthetic action involving a variety of
mechanisms.
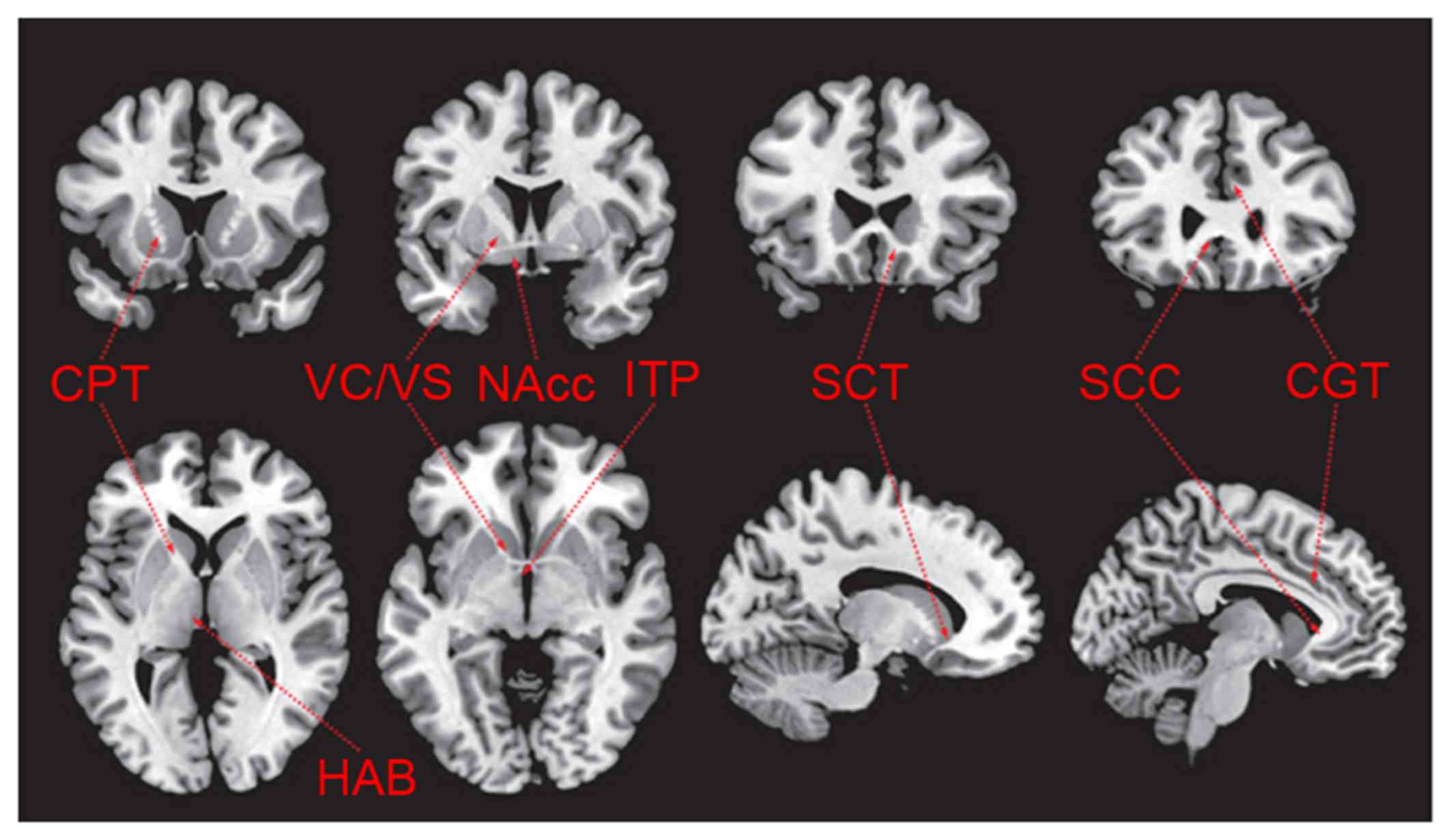
The selection of different targets for DBS
in treating mental diseases
The foundation of DBS research involves searching
for the target region by theoretical derivation (28). The target chosen by theoretical
derivation in mental disease therapy is derived from clinical
experience, results from brain imaging and the pathophysiological
knowledge of various diseases (29).
Mental diseases typically do not result from simple pathological
changes in a single brain structure, it is thought that certain
brain structures may serve different roles in the progression of
disease and its relative symptoms. Targets with similar anatomical
structures or functional relationships (neural networks) may
generate a superposition effect; therefore, different targets may
be able to regulate the same pathological network on different
nodes (30). It has been determined
that the targets discussed below are able to remit psychosis
symptoms (Fig. 3) (6).
Depression targets
DBS technology has been used to stimulate Brodmann
region cg25 under the cingulate cortex, which serves a pivotal role
in regulating negative emotions (9).
It has been demonstrated that stimulating this region may have an
antidepressant effect (9). Lozano
et al (31) demonstrated that
it had 55% efficacy, with a follow-up record of 20 patients who
underwent the surgery 3–6 years prior. In another group of patients
with depression, the anterior limb of the internal capsule was
regarded as the target of DBS therapy (32). One month post-surgery, the depressive
symptoms of patients remitted were evaluated using the
Montgomery-Asberg Depression Rating Scale (MADRS) (33). This result remained stable as
indicated by the rates (53% after 12 months and 71% at last
follow-up which ranged from 14 to 67 months). The response
criterion indicated a minimum of 50% reduction in MADRS in this
study; however, some patients experienced motor fluctuations for
>6 months (30).
The nucleus accumbens septi (NAcc) is the DBS
treatment target for depression and is able to effectively improve
the core symptom of depression, (loss of interest in daily
activities), due to the effects of the NAcc on the reward system
(14). Bewernick et al
(32) followed up 11 NAcc-DBS
patients for 4 years and 5 patients (45%) experienced lasting
beneficial effects.
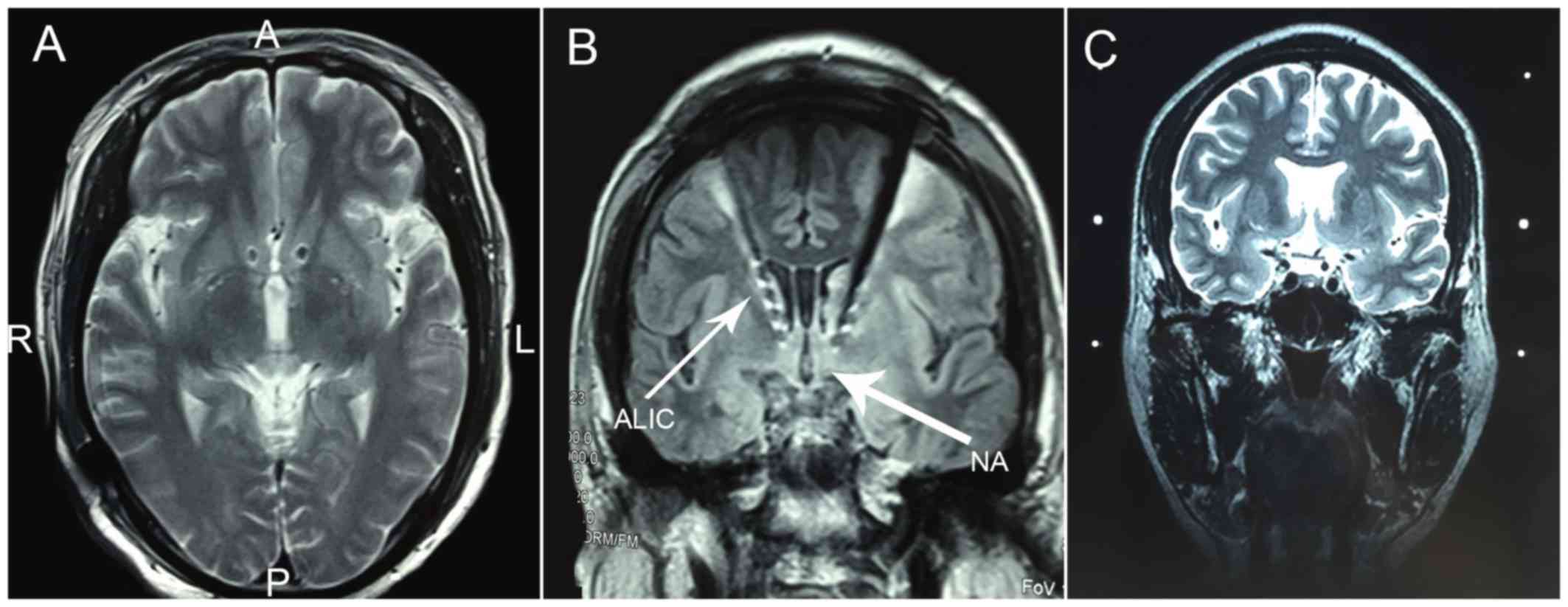
The ventral capsula interna/ventral striatum (VC/VS)
are also regarded as targets for the treatment of depression and
may be associated with the regulation of the reward system neural
network (34). Malone et al
(35) reported that 15 patients with
chronic, serious, refractory depression were treated with DBS on
the VC/VS and that 40% of these patients reported positive curative
effects 6 months post-surgery.
Other potential functional targets of depression
were identified on the basis of animal models and neuroimaging
research. Sartorius and Henn (36)
first proposed the habenula as a target of DBS in the treatment of
depression. This region controls the serotonin-activating nerve
fibers dominating the nucleus raphes dorsalis and noradrenergic
nerve governing the nucleus ceruleus (37). Sartorius and Henn (36) infer that excessive activation of the
habenula nucleus is associated with depression, and the lower part
of the thalamus neck connects the non-specific thalamic system and
the orbitofrontal cortex. The dysfunction of the non-specific
thalamic system appears to serve an important role in the
development of depression (36).
Stimulating the lower part of the bilateral thalamus neck may also
remit depression Hamilton Depression Rating Scale (38) grade decreases from 42 to 10) and the
positive curative effect is maintained for up to 24 months
(36). The use of the blinding
method (39) to interrupt the
stimulus results in a deterioration of the patient's condition.
These targets of depression have only been discussed in case
reports so far and require further validation in clinical
trials.
Overall, DBS targets for the treatment of depression
are being developed continuously. The most effective targets are
yet to be identified due to the relatively small sample size that
exists at present. However, studies have identified a number of DBS
targets that have a persistent anti-depressive effect including the
epiphysis frenum, NAcc and the VC/VS (Table I) (9,32,34–36,39,40).
 | Table I.Targets of deep brain stimulation in
the treatment of depression. |
Table I.
Targets of deep brain stimulation in
the treatment of depression.
| Authors, year | Targets | Hypothesis | Hypothesis
basis | Maximum sample
number (n) | Effectiveness
evaluation | (Refs.) |
|---|
| Mayberg et al,
2005; Connolly and Thase, 2011 | Gyrus Cinguli
(Brodmann region Cg25) | Anabiosis of
inactivating Cg25 | Neuroimaging
discovery | 20 | Persistent
effectiveness in 55% subjects over 3–6 years follow-up | (9,40) |
| Malone et al,
2009 | Anterior limb of
internal capsule | Inactivation of
abnormal neural network connections | Clinically
effective intervening measure of OCD and depression | 15 | 40–45% effective
rate | (35) |
| Bewernick et al,
2012 | NAcc | Regulates a central
reward system enhancing the pleasure response | Clinical experience
in neurobiology of the reward system | 11 | Persistent
effectiveness in 45% subjects over 4-year follow-up | (32) |
| Greenberg, et al,
2010 | VC/VS | Regulates and
improves the mood and increases motivation | Clinical experience
in neurobiology of the reward system | 15 | Valid in the sixth
month in 40% of subjects | (34) |
| Sartorius and Henn,
2007 | Epiphysis
frenum | Inhibition of
lateral habenula activating the serotonin and
noradrenaline-dopamine system, reducing the HPA axis | Neuroimaging
discovery of function in animal research | 1 | N/A | (36) |
| Jiménez et al,
2005 | Thalamus | Dysfunction of
depression thalamus system and orbital frontal cortex | Neuroimaging
discovery of function in animal research | 1 | N/A | (39) |
Targets of DBS for OCD
OCD has various targets, as the orbital frontal
cortex and anterior cingulate cortex are both part of the OCD
circuit. Unfortunately, these regions are very large; thus the size
of cortex region that needs to be modulated would be too large
(39,41). The majority of studies suggested that
unilateral or bilateral stimulation of the anterior limb of the
internal capsule as the target, and these studies have reported
promising results, from partial amelioration to complete remission
(42–47). In terms of side effects, several
researchers identified that hypomania, which may occur with direct
stimulation, disappeared completely following a decrease in
stimulation strength (41,45,46). The
acies thalamus optic-zona incerta has also been investigated in
three patients with Parkinson's disease and OCD, and the
corresponding reports indicate an improvement in
obsessive-compulsive symptoms (8,48). One
patient with OCD and comorbid depression entered remission
following DBS treatment targeting the NAcc and the caudate nucleus
(8,49). A total of 14 electrode-implanted
patients reported highly therapeutic effects following unilateral
stimulation of the NAcc and VC/VS stimulation improved the
condition of 50% of patients (41).
Side effects, including transient hypomania and anxiety induced by
DBS treatment, are typically eliminated by changing the parameters,
including stimulus frequency, pulse widths, stimulus duration and
voltage (Table II) (34,49–52).
 | Table II.Targets of DBS in the treatment of
OCD. |
Table II.
Targets of DBS in the treatment of
OCD.
| Authors, year | Targets | Hypothesis | Hypothesis
basis | Maximum sample
number (n) | Effectiveness
evaluation | (Refs.) |
|---|
| Goodman et al,
2010 | VC/anterior limb of
the internal capsule | Transfer between
the cortex and the thalamus | Imaging research of
the anterior limb of the interior capsule | 6 | Acute reason in 66%
of patients | (51) |
| Greenberg et al,
2010 | VC/VS | Interrupt or
regulate neural network access | Imaging
research | 26 | Effective in 50% of
patients at 3–36 month follow-ups | (34) |
| Aouizerate et al,
2004 | Caudate
nucleus | Direct and indirect
regulation of OCD pathways | Amputation of
caudate nuclei beam, metabolic studies, imaging and volume
research | 1 | Delayed remission
of OCD at 12–15 month follow-ups | (49) |
| Mallet et al,
2008 | Subthalamic
nucleus | Direct and indirect
regulation of OCD pathways | Imaging
research | 16 | Effective in 25–75%
of patients at 3 month follow-up | (52) |
| Denys et al,
2010 | NAcc | Direct pathway
activity causes the symptoms of OCD | Imaging
research | 16 | Effective in one
side with low efficiency | (50) |
Various targets of DBS for the treatment of OCD have
been verified with encouraging results; however identifying a
universal satisfactory target is not yet possible due to small
sample sizes. Furthermore, OCD is a heterogeneous disease and
patients with different symptom groups may have different ideal
targets.
Targets of DBS habituation
The application of DBS to treat habituation was
reported in a previous case report (15). Studies involving animal models and
imaging research are able to further increase understanding
regarding the mechanisms and safety of the treatment. Previous
studies have reported that there may be a specific ideal target of
DBS treatment for habituation (Table
III) (53–57). However, it should be noted that the
potential targets are not mutually independent and selection of the
ideal target requires more support from clinical and research data
due to potentially overlapping mechanisms and functions. It is
worth noting that the NAcc may be identified as the ideal target
for DBS treatment in patients with refractory habituation. A total
of 5 clinical studies involving 18 patients have already identified
curative effects with no reported side effects (24,57–60). A
meta-analysis indicated that the success rate of DBS in the
treatment of drug addiction is up to 49% (61).
 | Table III.Targets of DBS in the treatment of
habituation. |
Table III.
Targets of DBS in the treatment of
habituation.
| Authors, year | Targets | Hypothesis | Hypothesis
basis | Maximum sample
number (n) | Effectiveness
evaluation | (Refs.) |
|---|
| Levy et al,
2007 | ACC | Participate in the
reward system function | Neuroimaging
findings, animal experiment | 1 | Efficient in animal
experiment | (55) |
| Müller et al,
2009 | NAcc | The regulation of
NAcc, blocking the selective preference location of morphine | Clinical experience
of reward systems neurobiology | 3 | 2 patients with
good curative effect, 1 patient remitted | (57) |
| Friedman et al,
2010 | Epiphysis | Inhibition of
lateral habenula inducing the raising of serotonin activation and
noradrenaline-dopamine system, reducing of HPA axle | Neuroimaging
findings, animal experiment | 1 | Efficient in animal
experiment | (54) |
| Forget, et al,
2010 | Insular cortex | Inhibiting the
gyrus regulation and affecting the practical enteroceptive which
participates | Neuroimaging
findings, animal experiment | 1 | Efficient in animal
experiment in the coding habituation | (53) |
| Lim et al,
2009 | Subthalamic
nucleus | Key position of the
habituation | Regulating function
of dopamine | 19 | Part of patients
improved while majority invalid even exacerbation | (56) |
| Friedman et al,
2010 | LHB | Control reward
pathway of the midbrain and further effect the activation of
NAcc | Regulating the
dopamine neurons | 1 | Efficient in animal
experiment | (54) |
Targets of TS
TS is a neuropsychological disease characterized by
phonation spasm and motion spasm, with a 1% prevalence rate
(62). TS is linked to a number of
psychiatric disorders, including obsessive-compulsive disorder
(63), and some patients with TS may
present with symptoms of disability (64,65).
Standard medical treatment for TS does exist, including drug
therapy and behavioral intervention (66). Even with the best psychotropic drug
treatment and psychological behavioral therapy, only 1/3 of
patients receive complete relief; however, 30–40% of patients
experience exacerbations and ~5% of patients develop disabilities
(66,67). Therefore, it is necessary to explore
new therapies, including surgery, for patients whose condition is
difficult to control. Researchers have previously attempted to
perform nerve surgical resection in patients with TS with mixed
success; the central tract complexus region in the thalamus was
found to be the most effective target after different targets were
attempted (68). Based on results
for OCD and dyskinesia treatment, researchers have speculated that
the internal segment of globus pallidus (GPI) and the VC/VS as DBS
targets may also be effective at treating refractory TS (69).
The targeting of the thalamus DBS to treat TS has
been suggested (70). An open-label
study of 18 patients preliminarily reported positive results at 3-
and 18-month follow-ups, and a 2-year evaluation indicated that the
severity of spasms, obsessive-compulsive symptoms, anxiety and
depression had decreased remarkably (62,71). A
case report also declared that DBS of the GPI and VC/VS may have
potential curative effects in patients with severe TS (66). In 1999, Vandewalle et al
(72) reported the case of a
42-year-old patient with intractable TS whose twitch symptom was
eliminated 1 year following implantation of a double-sided
electrode in the thalamic nuclei. In the 1960s, Hassler and
Dieckmann (73) damaged the thalamic
nuclei in 3 TS subjects; since then, considerable research has been
conducted on the effectiveness of DBS treatment by implanting
electrodes in the thalamic nuclei or other targets, such as the
globus pallidus. These studies have reported significant
improvements in twitch scores, although a proportion of patients
experienced a variety of side effects, including energy loss,
fatigue, lazy speech, less movement and decreasing libido (38,74).
However, the maximum sample size investigated thus far contained
only 18 subjects and few randomized double-blind trials have been
conducted (62,75). The present evidence, however, is
enough to support large-scale randomized clinical trials of DBS
treatment for TS, with an aim to clarify the mechanism.
Targets of Alzheimer's disease
(AD)
AD is a neurodegenerative disease with a prevalence
of 1–2% in America (76). More than
10% of people >65 years old have AD and the primary therapies
available at present are drugs that slow down rather than prevent
further cognitive decline (77). One
case report described hyperamnesia in a patient with obesity
treated with fornix DBS (76). Based
on this report, Laxton et al (78) published a first phase clinical trial
involving 6 elderly patients with AD who underwent DBS of the
fornix. At a 1-year follow up, the glycometabolism in the parietal
lobe brain had improved markedly. The potential mechanisms of this
method are not yet clear; however, it is thought that activation of
the fornix axon in turn activates the downstream brain regions
involved in memory. In 2 patients who were examined using the
simple mental symptoms scale (78),
the speed of memory decline was demonstrated to have slowed down
and a clinical improvement in symptoms was observed win no evident
side effects. The effectiveness of this method of treatment for AD
further supports the use of electric stimulation therapy as a
treatment for neurodegenerative diseases including Parkinson's, AD,
myodystonia, OCD, TS and depression.
Targets of AN
AN is an eating disorder that individuals experience
to different extents, from being on a diet and deliberately
inducing weight loss to deliberately and dramatically reducing body
weight to far below healthy levels (79). The primary symptoms of AN are an
excessive attention to weight and body image, intense fear of
gaining weight, obsessive pursuit of thinness and significant
reduction in weight. This is often combined with malnutrition, as
well as metabolic and endocrine disorders, such as amenorrhea
(80). AN can be a life-threatening
condition due to excessive malnutrition, leading to cachexia and
body failure. Morbidity rate of AN is between 5–15%, with patients
typically succumbing to cardiac complications, multiple organ
failure, secondary infection or suicide (81–86).
Research into the targets of DBS to treat AN is in
the animal experiment stage at present, and the primary
experimental targets include the anterior hypothalamic nucleus
(AHN) and NAcc (87).
Research involving low frequency electric excitement
and electrolytic damage in canine and feline models identified that
the AHN controls eating behavior and the metabolism of food in the
body (88). A previous study has
reported that the ventromedial hypothalamic nucleus (VMH) and
lateral hypothalamic nucleus have an adverse function by which they
can present opposite effects with electrical excitation or
depolarization. Damage to the VMH induces overfeeding and obesity,
whereas low frequency electrical stimulation results in reduced
food intake (87). It is thought
that countering this action with high frequency electrical
stimulation may increase food intake to remedy AN; however, the
present research is only limited to animals (87).
Experimental data has also suggested that
high-frequency DBS in the NAcc region may be an effective treatment
for AN, OCD, depression and drug dependence (87). However, there are no related clinical
experimental results at present (87).
The side-effect of DBS
Approximately 80,000 individuals have undergone DBS
treatment worldwide, with a reported mortality rate of 0–0.4%
(10). The side effects associated
with DBS are categorized as acute side effects of surgery and
long-term side effects.
Acute side effects of surgery
The acute side effects of surgery observed in large
sample research studies include physical and mental side effects.
Physical side effects include intracranial hemorrhage, which has a
prevalence of 0.4–1.3% and irreversible brain damage, which has a
prevalence of 0.8% (89).
Furthermore, studies in patients who have undergone DBS have found
that the prevalence of infection, epileptic seizure and cutaneous
complications are 0.7, 1.5 and 25%, respectively (87,90,91).
One of the most harmful side effects of DBS is the
risk of inducing psychiatric symptoms that differ from the therapy.
Mental complications include transient aggressiveness, hypomania,
mania, depression, anxiety, apathy and even suicide. The most
common side effect is postoperative delirium (15.6%), followed by
depression and hypomania (92). For
DBS treatment of Parkinson's disease, for example, the most severe
side effect is an increased suicide risk, particularly when the
target region is in the subthalamic nucleus and globus pallidus
internus (87), with large sample
research reporting a suicide risk of 0.16–0.32%. It is therefore
necessary to highlight the suicide risk screening of patients
undergoing DBS.
Long-term side effects of surgery
A previous study has reported no serious side
effects or pathological damage to other brain parenchymal tissue
associated with the permanent implant, apart from cerebellar mass
cell hyperplasia surrounding the electrode observed in the
dissection results (93). Overall,
an adverse outcome may result from a combination of mental,
societal and operational factors (94). Issues with cognition have also been
reported, most notably deficiencies of language fluency (95). It should be noted that there is
currently no large-scale randomized blinded study data able to
determine the long-term impacts of DBS on personality, cognitive
function, attention and self-awareness. Overall, the side effects
of DBS are much less prevalent and less serious than those of
stereotactic surgery and there are fewer post-surgical
complications associated with DBS. Furthermore, electrodes can be
blocked to cease treatment if severe side effects manifest, to
prevent further damage occurring.
Discussion
DBS has some advantages compared with destructive
and disruptive surgical techniques; however, the treatment process
is slow and still requires invasive surgery. The popularization and
application of DBS technology is impeded by the duration limit of
the battery, regulation of stimulation parameters, selection of
optimal target, patient selection criteria and ethical arguments.
The exploitation of novel pharmacological agents and targets, more
detailed local stimulation devices and extracranial neuromodulation
devices within deeper brain structures (which are more effective
than transcranial magnetic stimulation) (96), may lead to improvements in DBS
technology. The development of other non-invasive effective
intracranial targets, including visual purple and halorhodopsin
neural circuit photosensitive manipulation, also deserve attention
(96).
The aforementioned non-invasive techniques are
unable to take the place of DBS. The tracer signal of the DBS
electrode combined with a DBS imaging map depicts the evolutionary
process of the temporal and spatial brain activities induced by
DBS, which provides more information to improve the current
research instruments and may lead to increased understanding of the
basic pathological mechanisms of brain function (30) DBS is the only neurosurgical treatment
method that can be controlled in a blinded study with the
advantages of safety, adjustability and reversibility, thus
providing a good method for investigating neurobiological
mechanisms of mental disease (30).
The scope of DBS to treat mental illnesses may
increase with in-depth knowledge of underlying pathological and
physiological mechanisms, and the progress of imaging and hardware
design technology. It should be emphasized that practitioners of
DBS must adopt compulsory ethical standards, as well as exclusion
and inclusion criteria, to prevent the practitioners from using
this technology beyond the scope of its applications.
For DBS treatment of mental illnesses, optimal
target exploration is the most important avenue of research.
Current research indicates that there may exist one or more central
targets for each mental disease based on different treatment
principles (30). Furthermore,
different syndromes and comorbidities may require different target
combinations (97). To confirm the
final potential central target, non-human primate models should be
established according to the definition of refractory mental
illness (98). Such a model should
be selected based on the ability to imitate every psychotic symptom
and pathological process entirely (97). Furthermore, a database should be
established compiling the DBS therapeutic targets for every type of
mental illness, including the mechanisms, stimulation parameters,
curative effects, side effects and postoperative imaging results.
Such a database may assist researchers from different institutions
in matching suitable patients and treatments, and to rapidly share
their study results.
In conclusion, a significant proportion of
refractory psychopathic patients may greatly benefit from novel
therapeutic methods. DBS treatment is an option that provides the
ability to control the pathology of neural networks with an
accurate and reversible treatment and promising curative effects
have been reported. However, considerably more clinical data are
required to eliminate basic ethical arguments and a universal
standard should be established for patient and target selection.
Overall, the application of DBS technology requires creative and
adventurous exploration, as well as strict and objective
standards.
References
|
1
|
Andrews JM and Nemeroff CB: Contemporary
management of depression. Am J Med. 97 Suppl:24S–32S. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Mann JJ: The medical management of
depression. N Engl J Med. 353:1819–1834. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Murray CJ and Lopez AD: Global mortality,
disability, and the contribution of risk factors: Global burden of
disease study. Lancet. 349:1436–1442. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yostos M and Murphy K: Investigation of
the therapeutic applications, neuroanatomical targets and emerging
technologies in deep brain stimulation surgery. BMC Proc. 9 Suppl
1:pp. S1–S2. 2015;
|
|
5
|
Carver R, Lee WH, Kagarise BE, Hoo BA,
Grace KP, Gleason PW and Halbert PC: Multiple configuration
surgical cutting device. 2015.
|
|
6
|
Holtzheimer PE and Mayberg HS: Deep brain
stimulation for psychiatric disorders. Annu Rev Neurosci.
34:289–307. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Benabid AL, Pollak P, Louveau A, Henry S
and de Rougemont J: Combined (thalamotomy and stimulation)
stereotactic surgery of the VIM thalamic nucleus for bilateral
Parkinson disease. Appl Neurophysiol. 50:344–346. 1987.PubMed/NCBI
|
|
8
|
Mallet L, Mesnage V, Houeto JL, Pelissolo
A, Yelnik J, Behar C, Gargiulo M, Welter ML, Bonnet AM, Pillon B,
et al: Compulsions, Parkinson's disease, and stimulation. Lancet.
360:1302–1304. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Mayberg HS, Lozano AM, Voon V, McNeely HE,
Seminowicz D, Hamani C, Schwalb JM and Kennedy SH: Deep brain
stimulation for treatment-resistant depression. Neuron. 45:651–660.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Müller UJ, Voges J, Steiner J, Galazky I,
Heinze HJ, Möller M, Pisapia J, Halpern C, Caplan A, Bogerts B and
Kuhn J: Deep brain stimulation of the nucleus accumbens for the
treatment of addiction. Ann N Y Acad Sci. 1282:119–128. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Schlaepfer TE, Frick C, Zobel A, Maier W,
Heuser I, Bajbouj M, O'Keane V, Corcoran C, Adolfsson R, Trimble M,
et al: Vagus nerve stimulation for depression: Efficacy and safety
in a European study. Psychol Med. 38:651–661. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chopra A, Klassen BT and Stead M: Current
clinical application of deep-brain stimulation for essential
tremor. Neuropsychiatr Dis Treat. 9:1859–1865. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Capelle HH and Krauss JK: Neuromodulation
in dystonia: Current aspects of deep brain stimulation.
Neuromodulation. 12:8–21. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Schlaepfer TE, Cohen MX, Frick C, Kosel M,
Brodesser D, Axmacher N, Joe AY, Kreft M, Lenartz D and Sturm V:
Deep brain stimulation to reward circuitry alleviates anhedonia in
refractory major depression. Neuropsychopharmacology. 33:368–377.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hardesty DE and Sackeim HA: Deep brain
stimulation in movement and psychiatric disorders. Biol Psychiatry.
61:831–835. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
McIntyre CC, Savasta M, Kerkerian-Le Goff
L and Vitek JL: Uncovering the mechanism(s) of action of deep brain
stimulation: Activation, inhibition, or both. Clin Neurophysiol.
115:1239–1248. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Beurrier C, Bioulac B, Audin J and Hammond
C: High-frequency stimulation produces a transient blockade of
voltage-gated currents in subthalamic neurons. J Neurophysiol.
85:1351–1356. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zucker RS and Regehr WG: Short-term
synaptic plasticity. Annu Rev Physiol. 64:355–405. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Dostrovsky JO, Levy R, Wu JP, Hutchison
WD, Tasker RR and Lozano AM: Microstimulation-induced inhibition of
neuronal firing in human globus pallidus. J Neurophysiol.
84:570–574. 2000.PubMed/NCBI
|
|
20
|
Jech R, Urgosík D, Tintera J, Nebuzelský
A, Krásenský J, Liscák R, Roth J and Růzicka E: Functional magnetic
resonance imaging during deep brain stimulation: A pilot study in
four patients with Parkinson's disease. Mov Disord. 16:1126–1132.
2001. View
Article : Google Scholar : PubMed/NCBI
|
|
21
|
McCracken CB and Grace AA: High-frequency
deep brain stimulation of the nucleus accumbens region suppresses
neuronal activity and selectively modulates afferent drive in rat
orbitofrontal cortex in vivo. J Neurosci. 27:12601–12610. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Vitek JL: Mechanisms of deep brain
stimulation: Excitation or inhibition. Mov Disord. 3 Suppl
17:S69–S72. 2002. View Article : Google Scholar
|
|
23
|
Hilker R, Voges J, Thiel A, Ghaemi M,
Herholz K, Sturm V and Heiss WD: Deep brain stimulation of the
subthalamic nucleus versus levodopa challenge in Parkinson's
disease: Measuring the on- and off-conditions with FDG-PET. J
Neural Transm (Vienna). 109:1257–1264. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Stefani A, Fedele E, Galati S, Raiteri M,
Pepicelli O, Brusa L, Pierantozzi M, Peppe A, Pisani A, Gattoni G,
et al: Deep brain stimulation in Parkinson's disease patients:
Biochemical evidence. J Neural Transm Suppl. 1–408. 2006.PubMed/NCBI
|
|
25
|
Kringelbach ML, Jenkinson N, Owen SL and
Aziz TZ: Translational principles of deep brain stimulation. Nat
Rev Neurosci. 8:623–635. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Schnitzler A and Gross J: Normal and
pathological oscillatory communication in the brain. Nat Rev
Neurosci. 6:285–296. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Stefurak T, Mikulis D, Mayberg H, Lang AE,
Hevenor S, Pahapill P, Saint-Cyr J and Lozano A: Deep brain
stimulation for Parkinson's disease dissociates mood and motor
circuits: A functional MRI case study. Mov Disord. 18:1508–1516.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Sudhyadhom A, Haq IU, Foote KD, Okun MS
and Bova FJ: A high resolution and high contrast MRI for
differentiation of subcortical structures for DBS targeting: The
fast gray matter acquisition T1 inversion recovery (FGATIR).
Neuroimage. 2 Suppl 47:T44–T52. 2009. View Article : Google Scholar
|
|
29
|
Wider C, Pollo C, Bloch J, Burkhard PR and
Vingerhoets FJ: Long-term outcome of 50 consecutive Parkinson's
disease patients treated with subthalamic deep brain stimulation.
Parkinsonism Relat Disord. 14:114–119. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Schläpfer TE and Bewernick BH: Deep brain
stimulation for psychiatric disorders-state of the art. Adv Tech
Stand Neurosurg. 34:37–57. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lozano AM, Mayberg HS, Giacobbe P, Hamani
C, Craddock RC and Kennedy SH: Subcallosal cingulate gyrus deep
brain stimulation for treatment-resistant depression. Biol
Psychiatry. 64:461–467. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Bewernick BH, Kayser S, Sturm V and
Schlaepfer TE: Long-term effects of nucleus accumbens deep brain
stimulation in treatment-resistant depression: Evidence for
sustained efficacy. Neuropsychopharmacology. 37:1975–1985. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Montgomery SA and Asberg M: A new
depression scale designed to be sensitive to change. Br J
Psychiatry. 134:382–389. 1979. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Greenberg BD, Gabriels LA, Malone DA Jr,
Rezai AR, Friehs GM, Okun MS, Shapira NA, Foote KD, Cosyns PR, Kubu
CS, et al: Deep brain stimulation of the ventral internal
capsule/ventral striatum for obsessive-compulsive disorder:
Worldwide experience. Mol Psychiatry. 15:64–79. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Malone DA Jr, Dougherty DD, Rezai AR,
Carpenter LL, Friehs GM, Eskandar EN, Rauch SL, Rasmussen SA,
Machado AG, Kubu CS, et al: Deep brain stimulation of the ventral
capsule/ventral striatum for treatment-resistant depression. Biol
Psychiatry. 65:267–275. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Sartorius A and Henn FA: Deep brain
stimulation of the lateral habenula in treatment resistant major
depression. Med Hypotheses. 69:1305–1308. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Foltynie T and Hariz MI: Surgical
management of Parkinson's disease. Expert Rev Neurother.
10:903–914. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Hamilton M: A rating scale for depression.
J Neurol Neurosurg Psychiatry. 23:56–62. 1960. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Jiménez F, Velasco F, Salin-Pascual R,
Hernández JA, Velasco M, Criales JL and Nicolini H: A patient with
a resistant major depression disorder treated with deep brain
stimulation in the inferior thalamic peduncle. Neurosurgery.
57:585–593. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Connolly KR and Thase ME: If at first you
don't succeed: A review of the evidence for antidepressant
augmentation, combination and switching strategies. Drugs.
71:43–64. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Greenberg BD, Malone DA, Friehs GM, Rezai
AR, Kubu CS, Malloy PF, Salloway SP, Okun MS, Goodman WK and
Rasmussen SA: Three-year outcomes in deep brain stimulation for
highly resistant obsessive-compulsive disorder.
Neuropsychopharmacology. 31:2384–2393. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Abelson JL, Curtis GC, Sagher O, Albucher
RC, Harrigan M, Taylor SF, Martis B and Giordani B: Deep brain
stimulation for refractory obsessive-compulsive disorder. Biol
Psychiatry. 57:510–516. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Anderson D and Ahmed A: Treatment of
patients with intractable obsessive-compulsive disorder with
anterior capsular stimulation. Case report. J Neurosurg.
98:1104–1108. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Gabriëls L, Cosyns P, Nuttin B,
Demeulemeester H and Gybels J: Deep brain stimulation for
treatment-refractory obsessive-compulsive disorder:
Psychopathological and neuropsychological outcome in three cases.
Acta Psychiatr Scand. 107:275–282. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Nuttin B, Cosyns P, Demeulemeester H,
Gybels J and Meyerson B: Electrical stimulation in anterior limbs
of internal capsules in patients with obsessive-compulsive
disorder. Lancet. 354:15261999. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Nuttin BJ, Gabriëls LA, Cosyns PR,
Meyerson BA, Andréewitch S, Sunaert SG, Maes AF, Dupont PJ, Gybels
JM, Gielen F and Demeulemeester HG: Long-term electrical capsular
stimulation in patients with obsessive-compulsive disorder.
Neurosurgery. 52:1263–1272; discussion 1272–1274. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Sturm V, Lenartz D, Koulousakis A, Treuer
H, Herholz K, Klein JC and Klosterkötter J: The nucleus accumbens:
A target for deep brain stimulation in obsessive-compulsive- and
anxiety-disorders. J Chem Neuroanat. 26:293–299. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Fontaine D, Mattei V, Borg M, von
Langsdorff D, Magnie MN, Chanalet S, Robert P and Paquis P: Effect
of subthalamic nucleus stimulation on obsessive-compulsive disorder
in a patient with Parkinson disease. Case report. J Neurosurg.
100:1084–1086. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Aouizerate B, Cuny E, Martin-Guehl C,
Guehl D, Amieva H, Benazzouz A, Fabrigoule C, Allard M, Rougier A,
Bioulac B, et al: Deep brain stimulation of the ventral caudate
nucleus in the treatment of obsessive-compulsive disorder and major
depression. Case report. J Neurosurg. 101:682–686. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Denys D, Mantione M, Figee M, van den
Munckhof P, Koerselman F, Westenberg H, Bosch A and Schuurman R:
Deep brain stimulation of the nucleus accumbens for
treatment-refractory obsessive-compulsive disorder. Arch Gen
Psychiatry. 67:1061–1068. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Goodman WK, Foote KD, Greenberg BD,
Ricciuti N, Bauer R, Ward H, Shapira NA, Wu SS, Hill CL, Rasmussen
SA and Okun MS: Deep brain stimulation for intractable obsessive
compulsive disorder: Pilot study using a blinded, staggered-onset
design. Biol Psychiatry. 67:535–542. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Mallet L, Polosan M, Jaafari N, Baup N,
Welter ML, Fontaine D, du Montcel ST, Yelnik J, Chéreau I, Arbus C,
et al: Subthalamic nucleus stimulation in severe
obsessive-compulsive disorder. N Engl J Med. 359:2121–2134. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Forget B, Pushparaj A and Le Foll B:
Granular insular cortex inactivation as a novel therapeutic
strategy for nicotine addiction. Biol Psychiatry. 68:265–271. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Friedman A, Lax E, Dikshtein Y, Abraham L,
Flaumenhaft Y, Sudai E, Ben-Tzion M, Ami-Ad L, Yaka R and Yadid G:
Electrical stimulation of the lateral habenula produces enduring
inhibitory effect on cocaine seeking behavior. Neuropharmacology.
59:452–459. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Levy D, Shabat-Simon M, Shalev U,
Barnea-Ygael N, Cooper A and Zangen A: Repeated electrical
stimulation of reward-related brain regions affects cocaine but not
‘natural’ reinforcement. J Neurosci. 27:14179–14189. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Lim SY, O'Sullivan SS, Kotschet K,
Gallagher DA, Lacey C, Lawrence AD, Lees AJ, O'Sullivan DJ, Peppard
RF, Rodrigues JP, et al: Dopamine dysregulation syndrome, impulse
control disorders and punding after deep brain stimulation surgery
for Parkinson's disease. J Clin Neurosci. 16:1148–1152. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Müller UJ, Sturm V, Voges J, Heinze HJ,
Galazky I, Heldmann M, Scheich H and Bogerts B: Successful
treatment of chronic resistant alcoholism by deep brain stimulation
of nucleus accumbens: First experience with three cases.
Pharmacopsychiatry. 42:288–291. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Valencia-Alfonso CE, Luigjes J, Smolders
R, Cohen MX, Levar N, Mazaheri A, van den Munckhof P, Schuurman PR,
van den Brink W and Denys D: Effective deep brain stimulation in
heroin addiction: A case report with complementary intracranial
electroencephalogram. Biol Psychiatry. 71:e35–e37. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Kuhn J, Gründler TO, Bauer R, Huff W,
Fischer AG, Lenartz D, Maarouf M, Bührle C, Klosterkötter J,
Ullsperger M and Sturm V: Successful deep brain stimulation of the
nucleus accumbens in severe alcohol dependence is associated with
changed performance monitoring. Addict Biol. 16:620–623. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Zhou H, Xu J and Jiang J: Deep brain
stimulation of nucleus accumbens on heroin-seeking behaviors: A
case report. Biol Psychiatry. 69:e41–e42. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Kuhn J, Huff W, Lee SH, Lenartz D, Sturm V
and Klosterkötter J: Deep brain stimulation in the treatment of
psychiatric disorders. Fortschr der Neurol Psychiatr. 75:447–457.
2007. View Article : Google Scholar
|
|
62
|
Servello D, Porta M, Sassi M, Brambilla A
and Robertson MM: Deep brain stimulation in 18 patients with severe
Gilles de la Tourette syndrome refractory to treatment: The surgery
and stimulation. J Neurol Neurosurg Psychiatry. 79:136–142. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Swain JE, Scahill L, Lombroso PJ, King RA
and Leckman JF: Tourette syndrome and tic disorders: A decade of
progress. J Am Acad Child Adolesc Psychiatry. 46:947–968. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Como PG: Neuropsychological function in
Tourette syndrome. Adv Neurol. 85:103–111. 2001.PubMed/NCBI
|
|
65
|
Mink JW, Walkup J, Frey KA, Como P, Cath
D, Delong MR, Erenberg G, Jankovic J, Juncos J, Leckman JF, et al:
Patient selection and assessment recommendations for deep brain
stimulation in Tourette syndrome. Mov Disord. 21:1831–1838. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Mink JW: Clinical review of DBS for
tourette syndrome. Front Biosci (Elite Ed). 1:72–76.
2009.PubMed/NCBI
|
|
67
|
Zhang XH, Li YJ and Zhuang P: A study on
outcome and mechanism of surgical treatment for Tourette's
syndrome. Zhonghua Wai Ke Za Zhi. 43:608–611. 2005.(In Chinese).
PubMed/NCBI
|
|
68
|
Temel Y and Visser-Vandewalle V: Surgery
in Tourette syndrome. Mov Disord. 19:3–14. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Dong S, Zhuang P, Zhang XH, Li JY and Li
YJ: Unilateral deep brain stimulation of the right globus pallidus
internus in patients with Tourette's syndrome: Two cases with
outcomes after 1 year and a brief review of the literature. J Int
Med Res. 40:2021–2028. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Plewnia C, Rzesnitzek L, Schober F,
Soekadar S, Wächter T, Gharabaghi A and Krüger R: 171. Deep brain
stimulation of the thalamus as a treatment of intractable Tourette
syndrome. Clinical Neurophysiology. 120:e722009. View Article : Google Scholar
|
|
71
|
Porta M, Brambilla A, Cavanna AE, Servello
D, Sassi M, Rickards H and Robertson MM: Thalamic deep brain
stimulation for treatment-refractory Tourette syndrome: Two-year
outcome. Neurology. 73:1375–1380. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Vandewalle V, van der Linden C,
Groenewegen HJ and Caemaert J: Stereotactic treatment of Gilles de
la Tourette syndrome by high frequency stimulation of thalamus.
Lancet. 353:7241999. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Hassler R and Dieckmann G: Stereotaxic
treatment of tics and inarticulate cries or coprolalia considered
as motor obsessional phenomena in Gilles de la Tourette's disease.
Rev Neurol (Paris). 123:89–100. 1970.(In French). PubMed/NCBI
|
|
74
|
Houeto JL, Karachi C, Mallet L, Pillon B,
Yelnik J, Mesnage V, Welter ML, Navarro S, Pelissolo A, Damier P,
et al: Tourette's syndrome and deep brain stimulation. J Neurol
Neurosurg Psychiatry. 76:992–995. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Welter ML, Mallet L, Houeto JL, Karachi C,
Czernecki V, Cornu P, Navarro S, Pidoux B, Dormont D, Bardinet E,
et al: Internal pallidal and thalamic stimulation in patients with
Tourette syndrome. Arch Neurol. 65:952–957. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Hamani C, McAndrews MP, Cohn M, Oh M,
Zumsteg D, Shapiro CM, Wennberg RA and Lozano AM: Memory
enhancement induced by hypothalamic/fornix deep brain stimulation.
Ann Neurol. 63:119–123. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Burns A and Iliffe S: Alzheimer's disease.
BMJ. 338:b1582009. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Laxton AW, Tang-Wai DF, McAndrews MP,
Zumsteg D, Wennberg R, Keren R, Wherrett J, Naglie G, Hamani C,
Smith GS and Lozano AM: A phase I trial of deep brain stimulation
of memory circuits in Alzheimer's disease. Ann Neurol. 68:521–534.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Spaulding-Barclay MA, Stern J and Mehler
PS: Cardiac changes in anorexia nervosa. Cardiol Young. 26:623–628.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Esposito R, Cieri F, di Giannantonio M and
Tartaro A: The role of body image and self-perception in anorexia
nervosa: The neuroimaging perspective. J Neuropsychol. 2016 May
25;(Epub ahead of print). View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Ulger Z, Gürses D, Ozyurek AR, Arikan C,
Levent E and Aydoğdu S: Follow-up of cardiac abnormalities in
female adolescents with anorexia nervosa after refeeding. Acta
Cardiol. 61:43–49. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Ravaldi C, Vannacci A and Ricca V: Cardiac
complications of anorexia nervosa. Recenti Prog Med. 94:267–270.
2003.(In Italian). PubMed/NCBI
|
|
83
|
McCallum K, Bermudez O, Ohlemeyer C, Tyson
E, Portilla M and Ferdman B: How should the clinician evaluate and
manage the cardiovascular complications of anorexia nervosa? Eat
Disord. 14:73–80. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Steinhausen HC: The outcome of anorexia
nervosa in the 20th century. Am J Psychiatry. 159:1284–1293. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Sullivan PF: Mortality in anorexia
nervosa. Am J Psychiatry. 152:1073–1074. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Katzman DK: Medical complications in
adolescents with anorexia nervosa: A review of the literature. Int
J Eat Disord. 37 Suppl:S52–S59; discussion S87-S89. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Benabid AL and Torres N: New targets for
DBS. Parkinsonism Relat Disord. 1 Suppl 18:S21–S23. 2012.
View Article : Google Scholar
|
|
88
|
Kishi T and Elmquist JK: Body weight is
regulated by the brain: A link between feeding and emotion. Mol
Psychiatry. 10:132–146. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Remy C, Marret E and Bonnet F: Effects of
acetaminophen on morphine side-effects and consumption after major
surgery: Meta-analysis of randomized controlled trials. Br J
Anaesth. 94:505–513. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Tykocki T, Mandat T, Kornakiewicz A,
Koziara H and Nauman P: Deep brain stimulation for refractory
epilepsy. Arch Med Sci. 8:805–816. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Voges J, Hilker R, Bötzel K, Kiening KL,
Kloss M, Kupsch A, Schnitzler A, Schneider GH, Steude U, Deuschl G
and Pinsker MO: Thirty days complication rate following surgery
performed for deep-brain-stimulation. Mov Disord. 22:1486–1489.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Houeto JL, Mesnage V, Mallet L, Pillon B,
Gargiulo M, du Moncel ST, Bonnet AM, Pidoux B, Dormont D, Cornu P
and Agid Y: Behavioural disorders, Parkinson's disease and
subthalamic stimulation. J Neurol Neurosurg Psychiatry. 72:701–707.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
DiLorenzo DJ, Jankovic J, Simpson RK,
Takei H and Powell SZ: Long-term deep brain stimulation for
essential tremor: 12-year clinicopathologic follow-up. Mov Disord.
25:232–238. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Schüpbach M, Gargiulo M, Welter ML, Mallet
L, Béhar C, Houeto JL, Maltête D, Mesnage V and Agid Y:
Neurosurgery in Parkinson disease: A distressed mind in a repaired
body? Neurology. 66:1811–1816. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Saint-Cyr JA, Trépanier LL, Kumar R,
Lozano AM and Lang AE: Neuropsychological consequences of chronic
bilateral stimulation of the subthalamic nucleus in Parkinson's
disease. Brain. 123:2091–2108. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Gradinaru V, Mogri M, Thompson KR,
Henderson JM and Deisseroth K: Optical deconstruction of
parkinsonian neural circuitry. Science. 324:354–359. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Goodman WK and Insel TR: Deep brain
stimulation in psychiatry: Concentrating on the road ahead. Biol
Psychiatry. 65:263–266. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Holtzheimer PE and Mayberg HS: Stuck in a
rut: Rethinking depression and its treatment. Trends Neurosci.
34:1–9. 2011. View Article : Google Scholar : PubMed/NCBI
|