Introduction
Tissue regeneration is a regulative process
widespread in most classes of animals that maintains or
reestablishes the normal functionality of cells, tissues, and in
some spectacular cases even major parts of organs and appendages
(1–4). Unfortunately, our own mammalian class
holds very limited regenerative potential and relies heavily on
fibrosis and scar formation following injury (3,5–10). Before regenerative therapies can
become a reality, however, our understanding of underlying
mechanisms needs to be improved and effective techniques for
monitoring the regenerative progress have to be developed. One way
of investigating regenerative phenomena is by applying the August
Krogh Principle: ‘For a large number of problems there will be some
animal of choice or a few such animals on which it can be most
conveniently studied’ (11). In
fact, there are some vertebrate species for which this statement in
a regenerative perspective applies very well: The urodele
amphibians. Urodele amphibians, salamanders and newts generally
possess a very high degree of regenerative capacity (12–16).
Cardiac tissue, intestines, liver, skeletal muscle, central and
peripheral nervous system, lens, retina, jaw, and even whole
appendages such as limbs and tail are examples of regenerative
structures of these animals (17).
Particular attention has been drawn to the endemic Mexican
salamander, the axolotl [Ambystoma mexicanum (Shaw and
Nodder, 1798)], due to its impressive regenerative potential and
easy maintenance (18,19). Axolotl limb regeneration has been
extensively studied (3,13,17,20–23) and
is characterized by a three-step regenerative process: Wound
healing, blastema formation and regrowth (3,17,23).
Within the first couple of hours following amputation of a limb the
wound is sealed with a wound epidermis by migrating cells from the
adjacent epidermis. Within 1–2 weeks, neurotrophic and epidermal
regulation induces dedifferentiation of differentiated cells
adjacent to the amputation site leading to the formation of a
structure termed a blastema containing dedifferentiated cells with
varying origin (e.g., connective tissue, muscular tissue, bone and
nerves). Finally, dedifferentiated stem cell-like blastema cells
proliferate, redifferentiate and restore the missing limb. In the
present study we chose the axolotl as animal model in order to
monitor a complete intrinsic regenerative process.
Before regenerative therapies can ever come to play,
an appropriate monitoring technology has to be developed that
ensures non-invasive follow up examinations of patients undergoing
therapy (24–26). In studies evaluating the
effectiveness of stem cell-based regenerative therapies, monitoring
has traditionally relied on histological techniques. In order to
detect the presence of cells within the region of interest, test
animals are usually sacrificed, and biopsies are collected and
evaluated using histology (27,28).
Even though these methods and techniques are valuable in a research
setting, they preclude non-invasive in vivo assessment and
longitudinal tracking of therapeutic progress.
Stem or progenitor cell fate can be monitored by an
alternative method by labeling cells of interest with non-toxic
super-paramagnetic iron oxide particles (SPIOs) that allow for
in vivo cell tracking using magnetic resonance imaging (MRI)
(24). Since SPIOs are non-toxic to
the labeled cells, this methodology is minimally invasive and
completely safe due to the harmless nature of MRI (29). SPIOs are either internalized by the
endosomal-lysosomal pathway or bind to the surface of cells, and
due to their magnetic properties, they increase the magnetic
susceptibility and decrease the MRI-measured properties of water,
especially the spin-spin (T2) and to some degree the spin-lattice
(T1) relaxation times (30). SPIO
labeling has successfully been used to track stem cell migration
and quantify the number of cells arriving in the target zone
(31,32). At present the SPIO labeling technique
has been applied in a number of preclinical studies, but to our
knowledge never in a system with true intrinsic regenerative
capacity.
The purpose of the present study was to introduce
SPIO labeling for cell tracking in a truly regenerative
environment, the regenerating limb of the axolotl. This method was
subsequently used to investigate an early homing effect of blastema
cells to a regenerative zone when applied intravascularly.
Materials and methods
Animals, husbandry and ethics
The procedures in this study were carried out in
accordance to the National and Institutional Legislation for Care
and Use of Laboratory animals. The experimental protocol was
approved by the Danish Animal Experiments Inspectorate (protocol
no. 2012-15-2934-00353). Animals used in this study were Mexican
axolotls (Ambystoma mexicanum) obtained from a commercial
breeder (Exoterra GmbH, Holzheim, Germany). Animals were housed
individually in plastic containers with a 10 cm water depth and a
930 cm2 surface area with regular water change and a
12-h light:dark cycle. They were fed every second day with
protein-enriched trout pellets. Anesthesia was obtained using 200
mg/l ethyl-4-aminobenzoate.
Nanoparticles
To increase the broadness of the experiments, two
commercially available and one custom designed SPIO, all with
similar MRI properties, were applied. First, the SPIO, Resovist
(Bayer Schering Pharma AG, Berlin, Germany), was applied for
viability testing and enhanced permeability and retention
experiments. This relatively large polycrystalline, polydisperse
carboxydextran-coated particle (core diameter: 4.2 nm, hydrodynamic
diameter: 45–60 nm), provides a high MRI relaxation enhancement (R1
relaxivity: 7.2 mM−1sec−1; R2 relaxivity: 82
mM−1sec−1; at 1.5 T and 310 K), and has been
applied for detection and characterization of small focal liver
lesion and immune cell and stem cell labeling (33). Second, an ultra-small SPIO, VSOP
C-200 (Ferropharm GmbH, Teltow, Germany), was applied for viability
testing and in situ labeling. This small citrate coated
particle (core diameter: 4 nm, hydrodynamic diameter: 7 nm) effects
T1 and T2 relaxation (R1 relaxivity: 13.97
mM−1sec−1; R2 relaxivity: 33.45
mM−1sec−1; at 1.0 T and 310 K), and has been
applied for tumor imaging and cell labeling (34,35).
Third, a custom made SPIO was synthesized as described earlier
(36) and applied for enhanced
permeability and retention experiments and testing of the homing
capabilities of blastema cells. This particle effects T2 relaxation
(R2 relaxivity: 119 mM−1sec−1; at 1.5 T and
293 K, and 92 mM−1sec−1; at 3.0 T and 293 K).
For the enhanced permeability and retention experiment the custom
made SPIO particles were coated with polyethylene glycol (PEG). A
total of 5 mg oleic acid coated particles were dissolved in 2 ml
toluene in a glass tube and 10 µl
2-methoxy(polyethyleneoxy)-propyltrimethoxysilane (Si-PEG),
Mw=460–590 (Gelest Inc., Morrisville, PA, USA) were added together
with 50 µl triethylamine (TEA) and 50 µl H2O while
stirring (161 × g). The mixture was incubated at room temperature
for 24 h. The solution was heated to 105°C for 30 min and the
particles were precipitated with pentane and the supernatant was
discarded. The particles were then re-dissolved in toluene and
precipitated with pentane. This washing procedure was repeated
three times, and the particles were resuspended in water. The
sample was centrifuged at 11,180 × g for 2 min three times to
remove any aggregates. The hydrodynamic size of the pegylated
particles was measured using dynamic light scattering (DLS) and
revealed a hydrodynamic size of 23 nm (number mean of three
measurements) hence we named this custom made particle SPIO-PEG (23
nm).
For the in situ cell labeling experiment, the
same custom made SPIO particle was coated with PEG and conjugated
to a Tide Fluor 6 (TF6) alkyne fluorophore from AAT Bioquest
allowing for dual modality imaging (MRI and optical imaging). A
total of 20 mg oleic acid coated particles were dissolved in 2 ml
toluene in a glass tube. A total of 60 mg
Azido-PEG-Si(OMe)3 (Si-PEG-N3), Mw 3,000 kDa
(Iris-Biotech) was dissolved in 2 ml toluene and added to the
particle solution together with 100 µl TEA and 100 µl
H2O during stirring (161 × g). The mixture was incubated
at room temperature for 24 h and washed as described above. The
Tide Fluor™ 6 alkyne was conjugated to the
azide-particles by a copper(I)-catalyzed Huisgen cycloaddition
reaction. A click stem solution was made from 6 mM
CuSO4, 200 mM sodium-ascorbate, 50 mM
tris-[1-(3-hydroxypropyl)-triazol-4-ylmethyl]amine ligand (THTA)
and H2O. The click stem solution was mixed with 200 µg
azide-particles and 0.012 mg Tide Fluor™ 6 alkyne. The click stem
solution made up a fourth of the total volume of this reaction
solution. After 2 h at room temperature and 28 × g, the particles
were put into dialysis bags (Spectra/PORR Dialysis
membrane, MWCO 12–14000; Spectrum Laboratories, Inc., Rancho
Dominguez, CA, USA) and dialysed against PBS with 10 shifts. The
hydrodynamic size measured with DLS was 47 nm (number mean of three
measurements); hence we named this particle SPIO-PEG-TF6 (47
nm).
Viability and SPIO labeling of
cultured blastema cells
Early bud blastema cells were harvested from 7 large
axolotls [Weight (W), 95.75±15.5 g; Length (L), 22.7±1.0 cm],
dissociated as described earlier (37), distributed into 55 wells (96-well
plates) and incubated in growth medium (50% Leibovitz's L-15, 15%
fetal bovine serum, and 35% phosphate-buffered saline) with either
0 mg Fe/L [C1 (n=11), C2 (n=11)] or 50 mg Fe/L from VSOP (n=11),
Resovist (n=11) or Resovist and the transfection agent
poly-l-lysine (PLL) (n=11) for 24 h at room temperature and
atmospheric air. After incubation with SPIO, cells were washed and
transferred to growth medium. Blastema cell viability was
investigated using a PicoGreen assay (Invitrogen; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) after 1 week (C1) and 3 weeks
(C2, VSOP, Resovist, Resovist/PLL) of culture.
In situ labeling
Twelve small axolotls (W, 7.5±2.1 g; L, 10.2±1.1 cm)
were anaesthetized, and a 50 µl bolus of either 50 mg Fe/L VSOP
(n=6) or saline (n=6) was injected intramuscularly in the muscles
lining the femur. To increase labeling success, animals were kept
under light anesthesia (20 mg/l ethyl p-aminobenzoate) and cooled
to 5°C overnight. Animals were subjected to MRI (1.5 T Siemens
Magnetom Avanto; gradient-echo sequence; TR, 10.7 msec; TE, 4.42
msec; θ, 25°; spatial resolution, 0.3×0.3×0.4 mm3; FOV
depending on sample size), and imaging was performed at 0 (pre
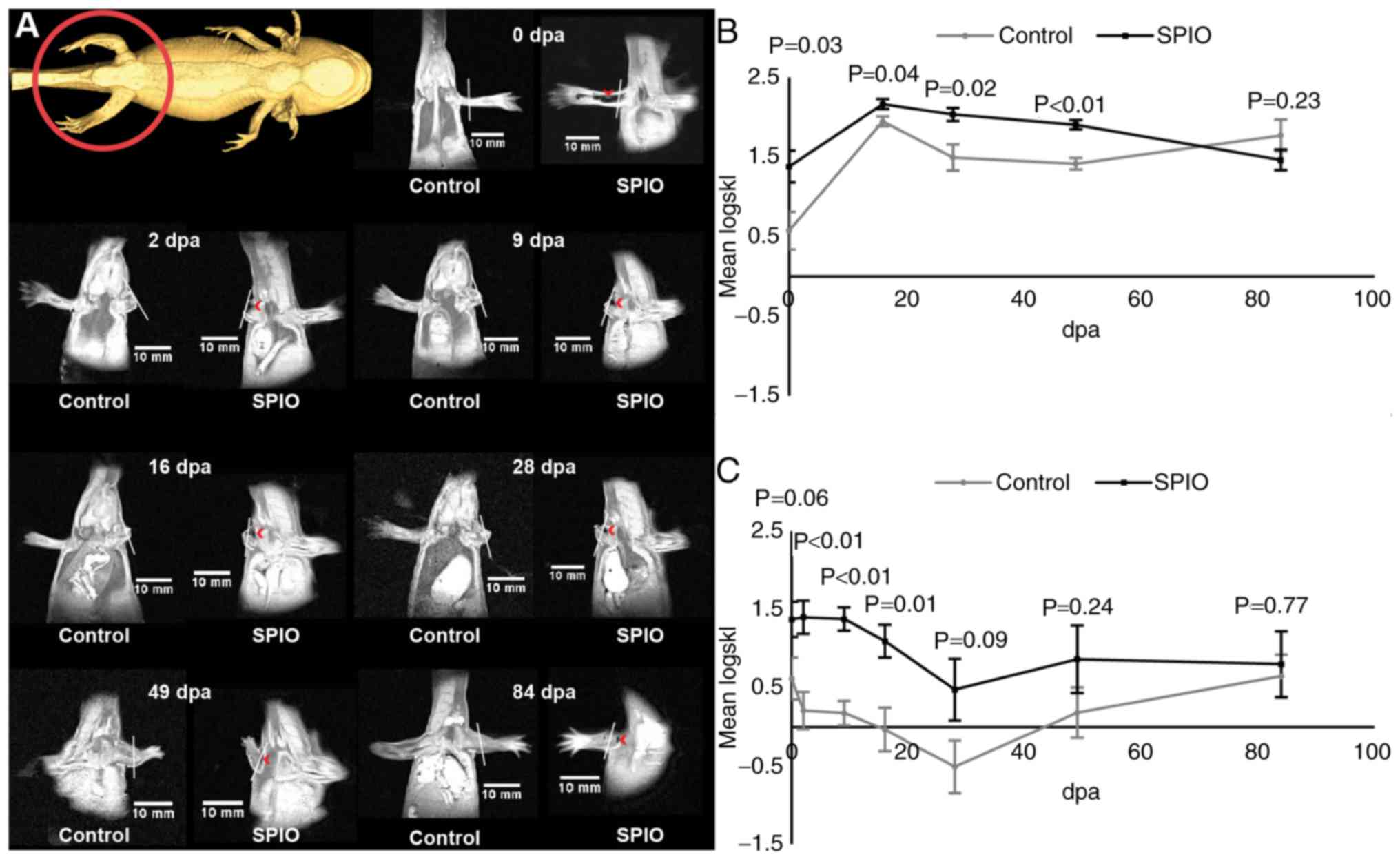
amputation), and 2, 9, 16, 28, 49, 84 days post amputation (dpa).
The regenerating blastema appeared at 16 dpa.
Presence of SPIOs resulted in decreased signal
intensity in labeled areas easily recognizable by visual inspection
of acquired MR images. To objectively compare pixel distributions
the Kullback-Leibler divergence test was applied (38). A region-of-interest (ROI) within the
limb stump and a ROI within the protruding blastema (only after 16
dpa) were selected for comparison with similar sized regions within
the contralateral unlabeled limb. Signal intensities were converted
to 8-bit grayscale and histograms were generated displaying
normalized pixel distributions. The Kullback-Leibler divergence can
be regarded as a dissimilarity measure between two arbitrary
probability distributions (39).
Given the two distributions P and Q the
Kullback-Leibler divergence (KL) is defined as:
KL(P,Q)=∫–∞∞p(x)logp(x)q(x)dx
Always non-negative the Kullback-Leibler divergence
is 0 if and only if P=Q. As a result of this, as the
signal intensity in the labeled limb theoretically approaches that
of the contralateral unlabeled limb over time due to SPIO dilution,
the Kullback-Leibler divergence will approach 0. Between each
histogram pair (P and Q) the symmetric Kullback-Leibler divergence
(SKL) defined as SKL(P,Q)=½ KL(P,Q)+½ KL(Q,P)
was calculated. As log10 (SKL) can be approximately
treated as normally distributed, a one-way ANOVA was applied to the
log10 (SKL) of each MRI data collection point.
The fully regenerated blastema (84 dpa) was
sectioned and histologically examined with H&E and Prussian
blue staining to investigate the presence of any remaining
SPIOs.
Enhanced permeability and
retention
Amputations were performed on 9 small animals (W,
8.1±0.7 g; L, 11.2±0.6 cm) to evaluate the enhanced permeability
and retention (EPR) effect during axolotl regeneration. At early
bud stage (13 dpa), animals underwent MRI and T2 maps were produced
(1.5 T Siemens Magnetom Avanto; Multiple spin-echo sequence; TR,
14,720 msec; TE, 39, 207 and 337 msec; θ, 150º; spatial resolution,
0.625×0.625×0.18 mm3; FOV depending on sample size).
Subsequently, intracardial injections were performed with 50 µl
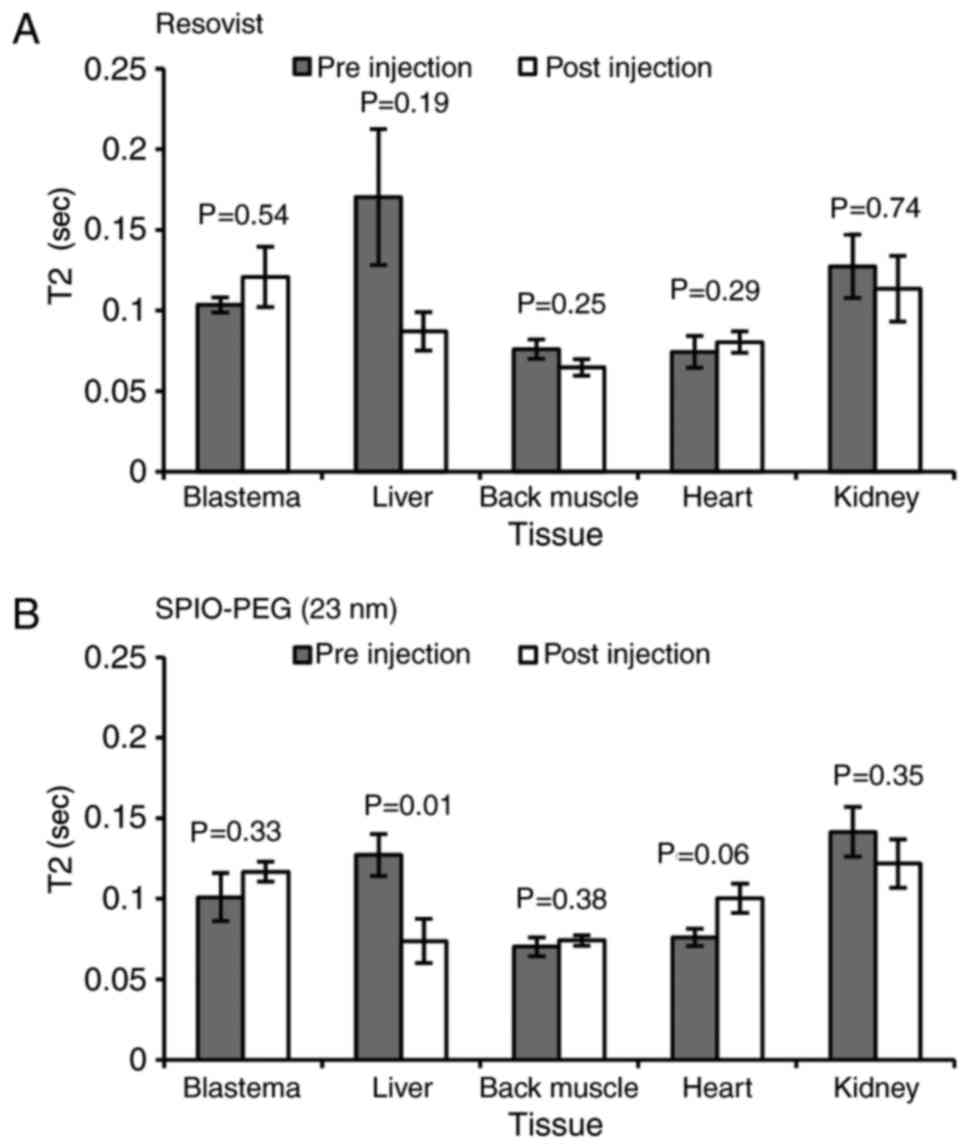
(~12.5% of blood volume) of either 5 mg Fe/(kg body weight)
Resovist (n=3) or 5 mg Fe/(kg body weight) SPIO-PEG (23 nm) (n=6).
MRI was repeated 24 h after injection of contrast agent at 14 dpa.
T2 was measured in ROIs placed in the blastema, liver, heart, a
back muscle, and kidney.
Homing of blastema cells
Amputations were performed on 12 large donor animals
(W, 48.3±5.7 g; L, 19.7±1.1 cm) and 12 small receiver animals (W,
7.8±0.9 g; L, 11.2±0.5 cm) to investigate whether blastema cells
possess the ability to home at the regenerative zone. At early bud
stage, cells were harvested from donor animal blastemas and
dissociated as described above, separated into two vials and
incubated for 24 h with 50 mg Fe/L SPIO-PEG-TF6 (47 nm) or without
SPIO respectively. Labeling success was evaluated with Prussian
blue staining of histologically sectioned coagel single cell
suspensions of labeled and unlabeled cells. SPIO-PEG-TF6 (47 nm)
labeled and unlabeled cells were administered to the receiver
animals through intracardial injections of 120,000 unlabeled cells
pr. control animal (n=6) and 120,000 SPIO labeled cells pr. SPIO
treated animal (n=6). Twenty four h after injections, animals
underwent MRI (3.0 T Siemens Magnetom Skyra; Multiple spin-echo
sequence; TR, 6.130 msec; TE, 25, 50, 75, 100 125, 150, 175, 200,
225, 250, 275, 300, 325, 350, 375 and 400 msec; θ, 180º; spatial
resolution, 0.417×0.417×0.8 mm3; FOV depending on sample
size). Additionally, animals were optically scanned using an in
vivo imaging system (IVIS Spectrum pre-clinical in vivo
imaging system, PerkinElmer). A spectral unmixing epi-illumination
protocol with multiple emission and excitation wavelengths was
applied (Em/Ex: 780/675, 760/675, 740/675, 720/675, 720/640,
700/640, 780/605, 760/605, 740/605, 720/605, 700/605, 680/605,
660/605 nm).
An additional non-MRI cell tracking experiment
designed to test the potential up-concentration of injected
blastema cells on a gross anatomical level was carried out in 10
large axolotls (W, 53.8±13.9 g; l, 19.2±1.9 cm). Amputation was
induced in three globally green fluorescent protein (GFP)
expressing donor axolotls (GFP+), two non-GFP
(GFP−) donor animals, and five GFP− receiver
animals. Blastema cells were harvested and dissociated as described
above at the early bud stage and administered to the five
GFP− receiver animals through intracardial injections of
120,000 cells each (GFP+: n=3, GFP−: n=2).
After 24 h, receiver animals were sacrificed, and the blastema,
liver, back muscle, heart, kidney, and lungs were resected and
inspected for presence of GFP+ cells with
microscopy.
To make sure that an early bud blastema is
adequately supplied by blood to allow for vascularly injected
blastema cells to reach the blastema, ultrasound examination of 5
animals (W, 47.6±6.8 g; L, 18.8±0.9 cm) was carried out as the
early bud blastema stage was reached using a 40–50 MHz ultrasound
system (VisualSonics Vevo 2100; Transducer: MS700; Fujifilm
VisualSonics, Inc., Toronto, ON, Canada). Color Doppler imaging was
applied to visualize overall blood flow and quadratic averaging was
performed on frames acquired in B-mode to visulaze small vessels
supplying the regenerating limb. Blood flow in the main artery
supplying the blastema was measured using pulsed wave Doppler
ultrasound.
Statistical analysis
Data were analyzed using statistical software Stata
12 (StataCorp LP, College Station, Texas, USA) using Students
t-test and one-way ANOVA when appropriate, significance level
α<0.05. Bonferroni correction was applied for multiple
comparisons. A custom made Matlab (Matlab R2012b; Mathworks,
Natick, MA, USA) function was applied for Kullback-Leibler
analysis.
Results
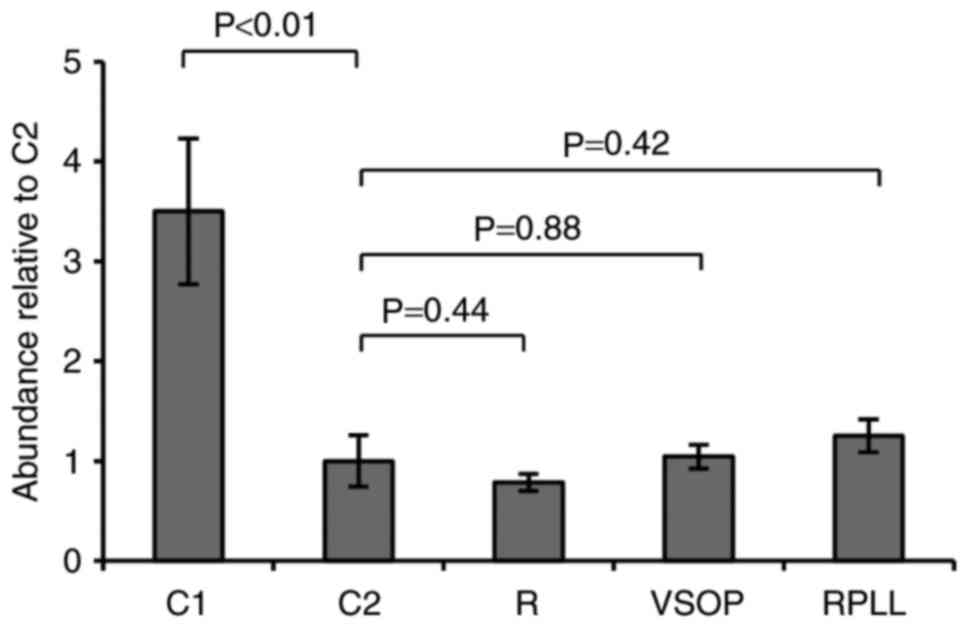
Viability of SPIO labeled blastema
cells
Blastema cells do not tolerate culturing outside
their natural milieu for extended periods very well (18). A significant decline in cell
abundance was observed in all cultures from one to three weeks of
culturing (Fig. 1). However, no
excessive mortality was observed in cultures labeled with SPIO
compared to the control after three weeks (Fig. 1). Thus, the data support no adverse
effect on cell viability in vitro following incubation and
labeling with either, VSOP, Resovist, or Resovist in conjugation
with the transfection agent PLL.
In situ labeling
VSOP-labeled tissue in the complete non-amputated
limb was clearly visible as hypointense areas after the injection
procedure (Fig. 2A, 0 dpa), as well
as in the stump and the emerging blastema following amputation
(Fig. 2A, 2–84 dpa). To allow for a
quantitative analysis the symmetric Kullback-Leibler divergence was
calculated from ROI's originating from the labeled limb stump and
blastema relative to the contralateral limb. The logarithmic
transformed symmetric Kullback-Leibler divergence approximated a
normal distribution. The signal intensity distribution of the SPIO
labeled regenerating blastema was significantly different from that
of the unlabeled control blastema for the first 48 days of
regeneration (Fig. 2B). Initially,
the signal intensity distribution of the SPIO labeled limb stump
was not significantly different (P=0.062) from that of the
unlabeled control limb, however the signal intensity distribution
was significantly different between 2 dpa and 16 dpa (Fig. 2C). From day 28 until the end of the
experiment, the signal intensity distribution of the limb stump of
labeled and unlabeled animals were no longer significantly
different (Fig. 2C).
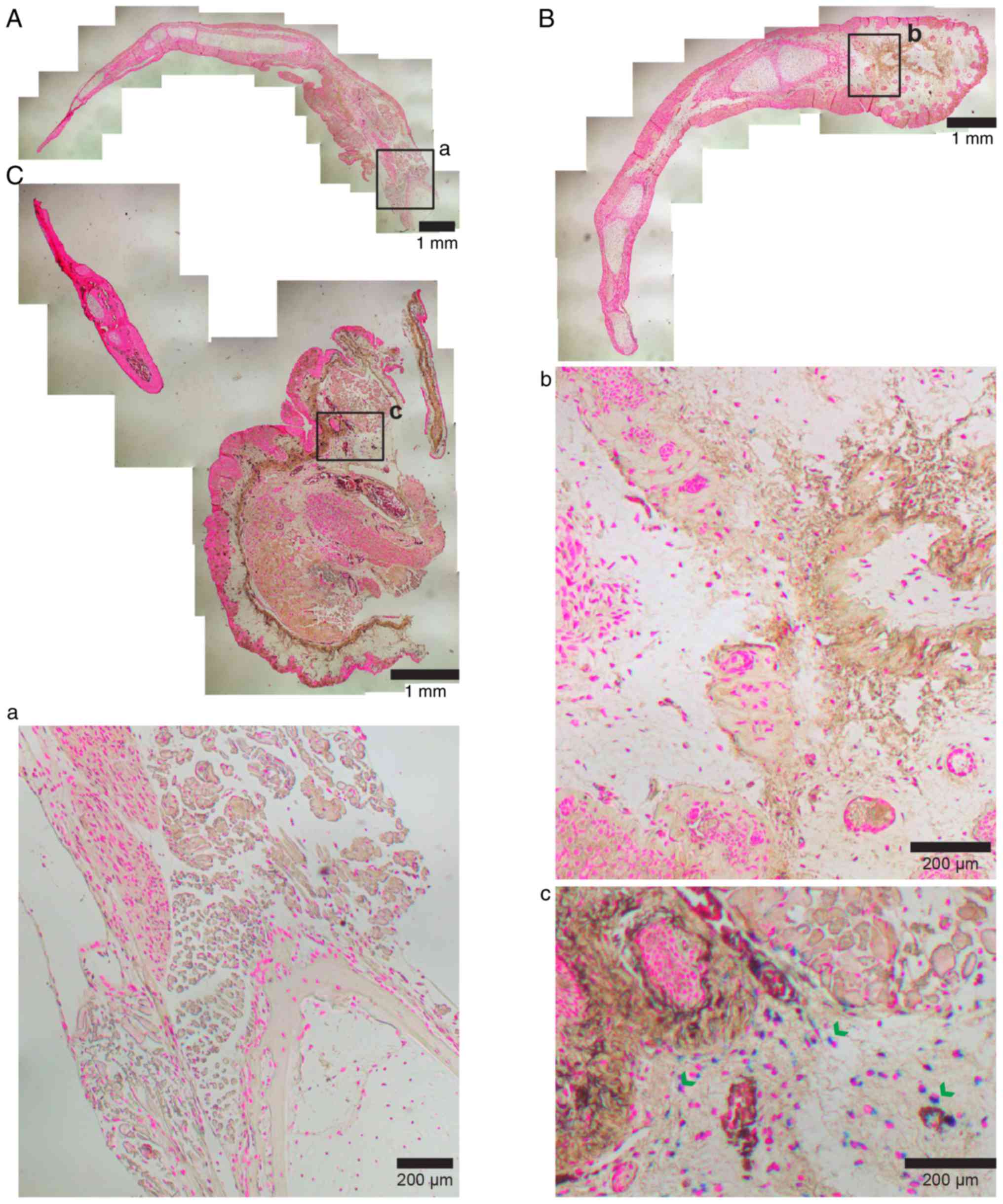
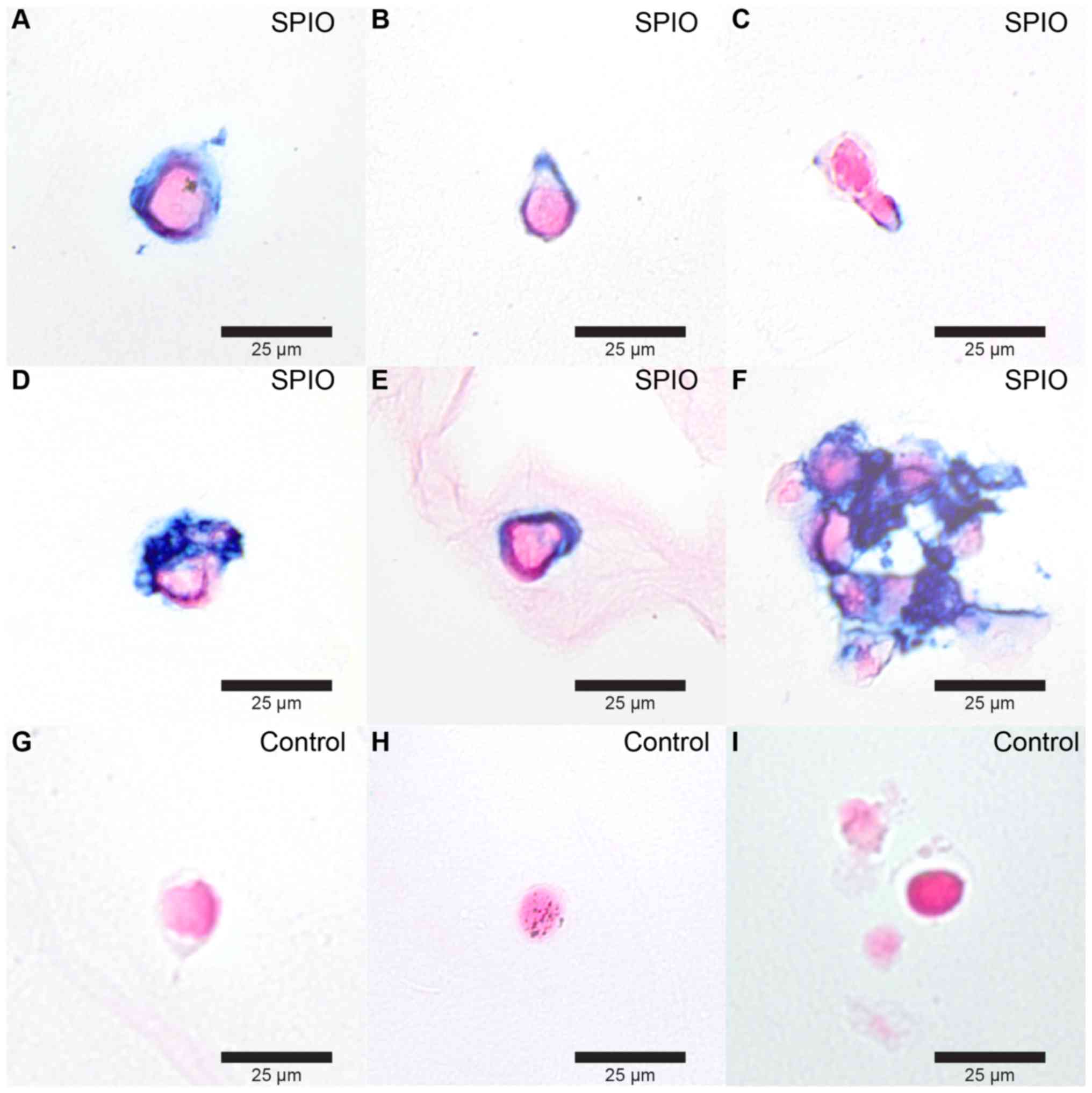
Histological examinations at the end of the
experiment showed remaining iron particles in 33% of the fully
regenerated SPIO labeled limbs all residing proximal to the
amputation plane (Fig. 3).
Enhanced permeability and
retention
No EPR effects were observed for either Resovist
(Fig. 4A) or SPIO-PEG (23 nm)
(Fig. 4B) except in the liver of
SPIO-PEG (23 nm) treated animals (Fig.
4B).
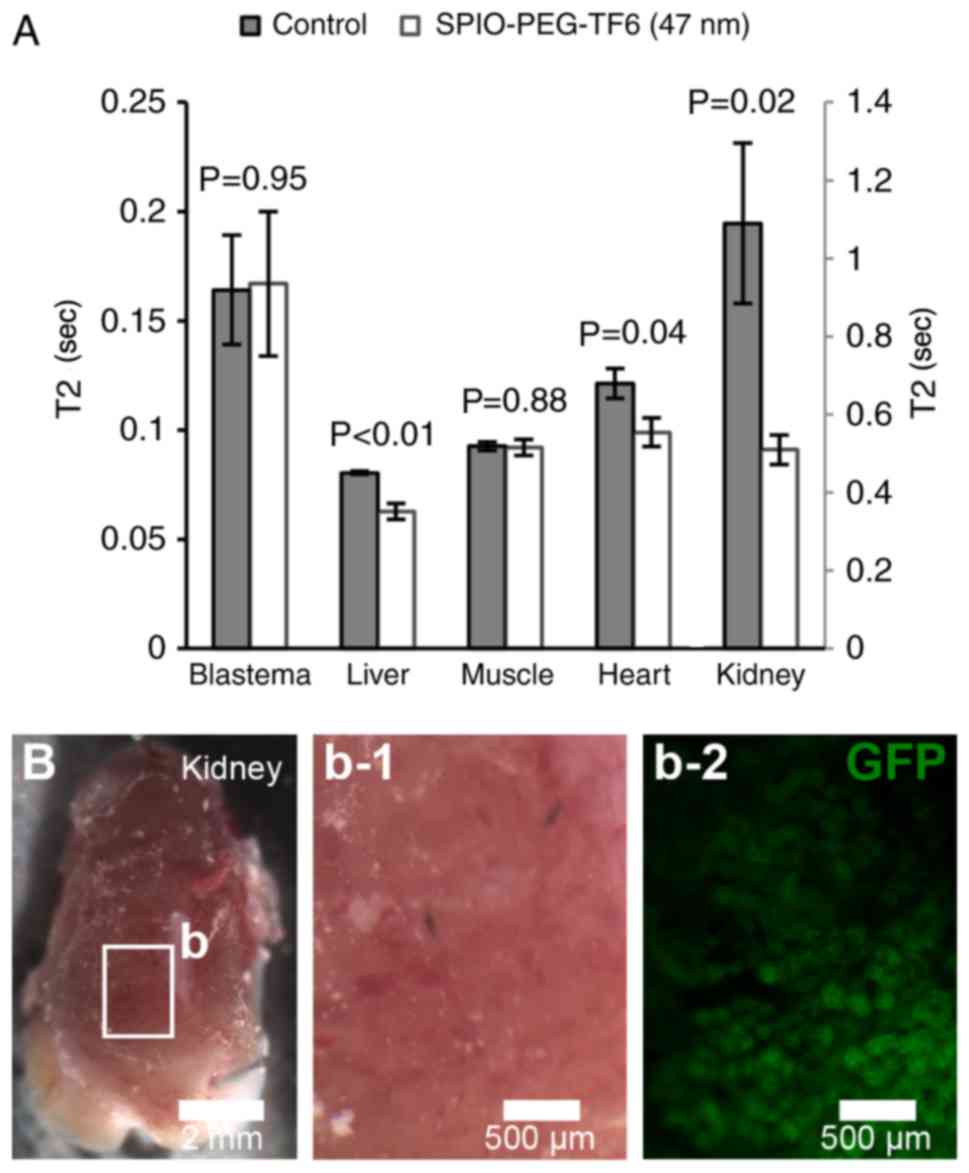
Homing of blastema cells
Incubating blastema cells for 24 h with SPIO-PEG-TF6
(47 nm) resulted in a clear uptake of iron in the cytoplasm of
labeled cells (Fig. 5) with a
labeling success of 94%. MRI revealed no significant change in T2
in the blastema of SPIO-PEG-TF6 (47 nm) treated animals relative to
control animals 24 h after cell injection (Fig. 6A). Instead, a significant decrease in
T2 was observed in the liver, heart, and kidney of transplanted
animals receiving SPIO-PEG-TF6 (47 nm) labeled cells (Fig. 6A). Injection of GFP+ cells
in GFP− hosts resulted in detectable GFP+
cells only in the kidneys of the GFP− hosts 24 h post
injection (Fig. 6B).
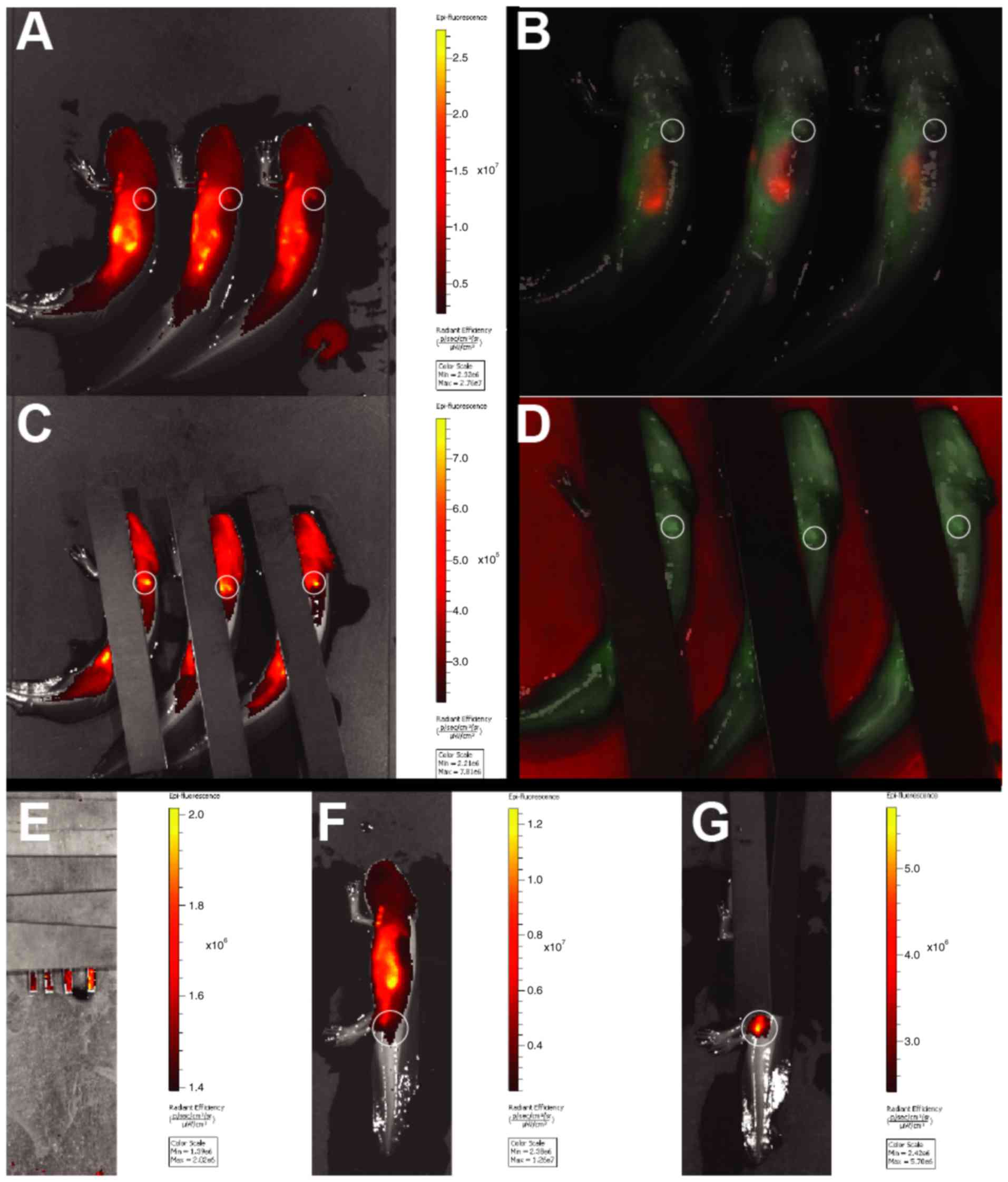
IVIS initially revealed a strong autofluorescent
signal from the stomach of both SPIO-PEG-TF6 (47 nm) labeled and
unlabeled animals (Fig. 7A and B),
possible resulting from the fodder. To avoid premature image
saturation this autofluorescent area was covered by black sheets of
plastic, however no signal difference was observed between
SPIO-PEG-TF6 (47 nm) treated or control animals from either the
blastema or any other underlying organs (Fig. 7C and D). An injection series of
increasing concentrations of SPIO-PEG-TF6 (47 nm) labeled cells was
performed subdermally in the tail of previously unlabeled animals,
and a concentration as low as 50 cells/µl allowed for detection of
the fluorescent signal (Fig. 7E and
G).
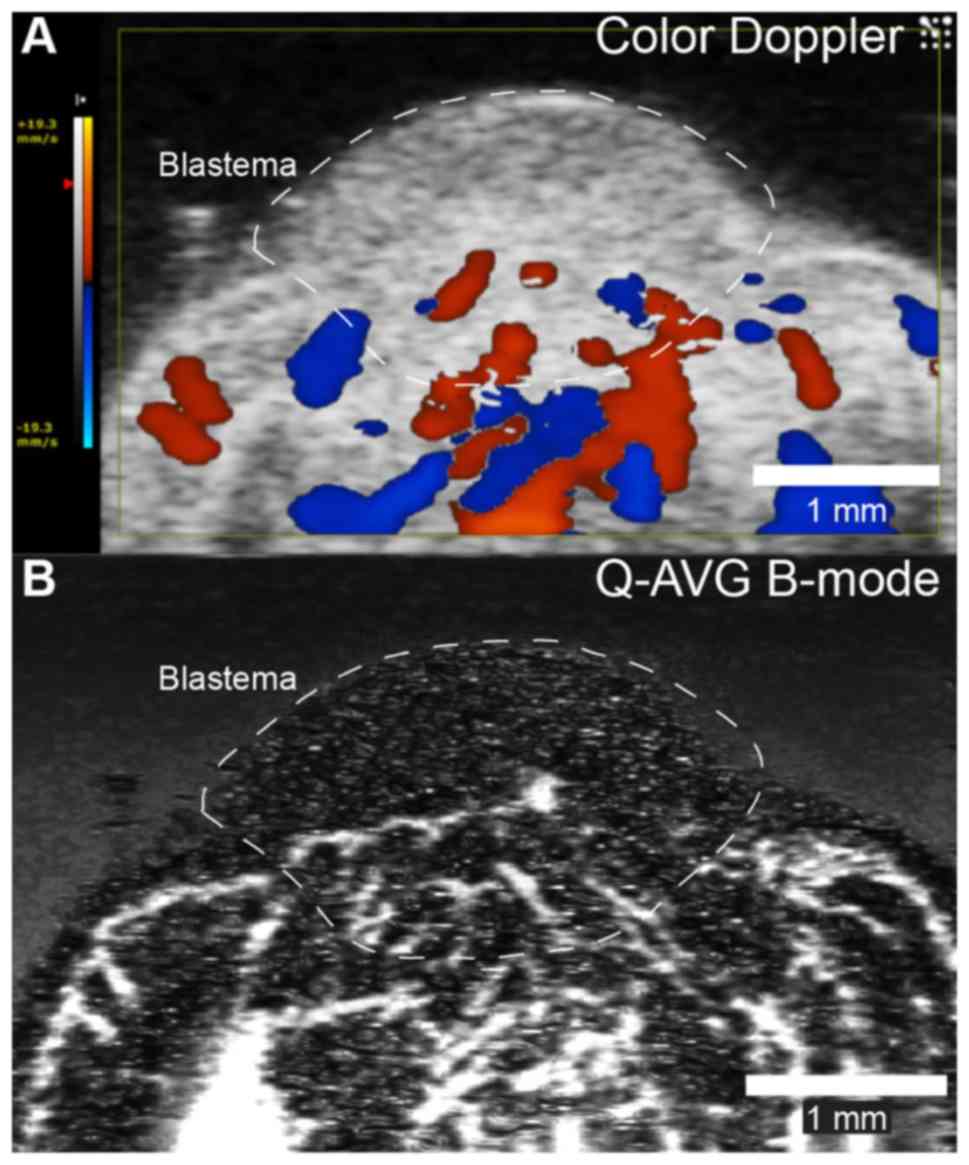
Both color Doppler and quadratic averaged B-mode
ultrasonographic imaging revealed vascular supply to the proximal
part of the early bud blastema (Fig.
8) and a blood flow of 27.2±5.8 µl/min in the main artery
supplying the blastema.
Discussion
Over the past 20–30 years, stem cell approaches in
regenerative medicine have gained ever increasing interest
facilitated by the discovery of induced pluripotent stem cells
(40). Nonetheless, with the
exception of hematopoietic stem cell transplantations for
hematopoietic disorders, most stem cell therapies remain
experimental and limited therapies are clinically available today
(41). A thorough understanding of
the route of delivery and subsequent migration of administered stem
cells is paramount in the achievement of developing future stem
cell therapies in addition to an understanding of cell-cell
signaling and requirements for the cellular milieu. This study
addresses the use on non-toxic SPIOs to track administered cells
non-invasively in an intrinsic regenerative environment, the
regenerating limb of the axolotl. Overall, we found that SPIOs are
applicable for cell tracking in this animal model, and this was
used to demonstrate the lack of an early homing mechanism of
blastema cells to the zone of regeneration.
Dissociated axolotl blastema cells do not tolerate
extended periods of culturing outside their natural environment
well (18). Therefore, a general
decline in cell numbers of 3 weeks old cultures relative to 1 week
old cultures is anticipated. However, culturing blastema cells with
either of two commercially available SPIOs and the transfection
agent PLL did not affect viability (Fig.
1). Also, no malformations or decrease in regenerative rate
were observed throughout the experiments involving SPIOs. Concerns
have been raised that loading cells with huge amounts of even
non-toxic metals such as iron can affect gene expression (34). We did not test such effects in the
present study, and cannot rule out transcriptomic effects of SPIOs
in axolotl blastema cells, although we observed no effects on
viability and phenotypic regeneration to imply this and it is
noteworthy that the majority of studies applying SPIOs for cell
labeling report of no or very little toxicity of these particles
(24–26).
To test the feasibility and longevity of SPIO
labeling in the axolotl, we initially performed in situ
labeling, by injecting a small amount of highly concentrated
particles in the limb, followed by amputation, and subsequently
monitored the regenerative process using MRI. The applied SPIOs
proved effective for labeling cells in situ for the initial
phases of regeneration (Fig. 2).
Kullback-Leibler analysis was able to detect significant
differences in signal distribution of the regenerating blastema all
the way through the regeneration of the miniature limb until 48
days after amputation, and the presence of SPIO labeled cells
proximal to the amputation zone was detectable with MRI until 16
days after amputation. A decrease in signal of SPIO labeled
proliferating cells is to be expected due the dilution of iron in
each cell as the cytoplasm is divided between new daughter cells
(42). In the regrowth period
between day 48 and day 84 post amputation all limb elements are
already in place, but the high degree of hyperplasia needed to
restore the normal sized limb may explain the dilution of SPIO to a
level below the detection limit in the blastema in this period.
Iron nanoparticles have been described to ultimately become
metabolized in the body (30,42,43)
and thus we anticipated a general decrease of concentration i.e.,
MRI signal over time, especially in tissues were macrophages
congregate such as in the limb stump after amputation (44).
Limb regeneration in the salamander relies on the
proliferative capacity of differentiated cells from various
lineages residing local to the site of amputation rather than a
source of circulating stem cells that find their way to injury
sites (13). It has been suggested
that a positional coding of primarily fibroblast enables the
regenerative environment to restore the exact anatomy of the
injured extremity, however the complete mechanisms are still not
fully understood (20). We were
interested in the capacity of blastema cells to recognize a site of
injury and hypothesized that blastema cells injected in the blood
pool would relatively rapidly congregate at an injury site given
that an adequate supply of blood vessels was restored. This
hypothesis was addressed by injecting a large quantity of SPIO
labeled blastema cells at early bud stage. At this time a capillary
network is established supporting the budding limb (Fig. 8), and based on the flow measurements
in the main artery supplying the blastema, the total blood mass (an
estimated 5% of body mass) passes through these vessels in every
1.5 h. Nevertheless, we were not able to detect any signal change
as a result of an up-concentration of fluorophore conjugated SPIOs
with neither of our two in vivo techniques (MRI and IVIS).
It is worth noting that potentially homing blastema cells would
only need to penetrate through the capillary endothelium at an
increased rate in the regeneration zone for these two techniques to
detect an accumulation here, the cells would not need to penetrate
through avascular tissue in the distal part of the blastema which
may take longer than 24 h. Instead, labeled and injected blastema
cells seem to become retained in organs normally responsible for
metabolizing administered agents as part of the detoxification
processes such as the liver and the kidneys, the latter being most
prevailing in this study. This was further supported by injecting
GFP expressing blastema cells in non-GFP hosts. Although this was a
somewhat crude method based on the fluorescence of entire organs
that possibly only allows for the detection of a large number of
florescent cells, the signal from the kidneys was in fact so strong
that it could be picked up (Fig. 6B)
supporting the result of the MRI experiment. Injected cells were
also found to be caught in the heart (Fig. 6A), which seems likely due to the
spongious nature of the amphibian heart and may also be due to the
cardiac injection of SPIO labelled cells. While our data suggest
that there is no short term homing effect of circulating blastema
cells over the course of 24 h (16 cycles of total blood volume
through the regenerating limb), we cannot rule out potential long
term homing effects and if blastema cells that are at first caught
in the kidneys, liver and heart later migrate to the budding
limb.
Concerns have been raised that SPIOs remain to
function as contrast agents for MRI even if initially labeled cells
are dead and the SPIOs are ingested in other cells or concentrate
passively via the EPR effect in organs (45). We tested the potential EPR effect of
two of the SPIOs used in this study [Resovist and SPIO-PEG (23 nm)]
and found an up-concentration of one of the SPIOs [SPIO-PEG (23
nm)] in the liver. A possible explanation for this observation is
that SPIOs are known to be metabolized in the liver (30), and therefore a short term response is
seen in this organ when injected iron is rapidly accumulated and
cleared. Additionally, we observed a decrease in T2 (i.e., an
increased iron concentration) in the liver, kidneys and the
spongious heart (the latter may be due to the cardiac injection of
the particles) after injection of SPIO labeled blastema cells.
Therefore, we expect the escape rate of SPIOs out of labeled
blastema cells to be insignificant, and the up-concentration of
SPIOs in filter organs to be a result of a passive increase in the
number of labeled cells in these organs and not free SPIO
particles.
Another concern regarding the use of SPIO particles
in cell tracking experiments is the fact that the iron-oxide based
core acts as a negative contrast agent, and therefore one is
looking for a decrease in signal at the presence of SPIO particles
in a structure rather than an increase which is the case with e.g.,
gadolinium based contrast agents for MRI. This raises the concern
that the presence of SPIO particles at a specific point of interest
can be mistaken by other sources of susceptibility artifacts such
as blood clots, hemosiderin complexes, small gas bubbles etc.
Therefore, a rigid control setup, as applied in this study, in
which control animals undergo the exact same surgical and
experimental procedures as the animals receiving SPIO particles, is
essential to rule out the possibility of falsely interpreting other
sources of susceptibility artefacts as the presence of SPIOs at a
given location.
It the present study we have applied SPIO labeling
to address the biological question whether stem cell like blastema
cells in a regeneration competent animal are able to congregate at
a regeneration site (the blastema) when applied intravascularly
after 24 h of circulation in the vascular system. We found no
indications of this on the short term, instead injected cells were
captured in the kidneys, liver and the spongious heart. There is a
considerable evolutionary distance between salamanders and mammals,
and from this study it is not possible to occlude vascular
injections as a viable way for stem cell delivery in regenerative
therapies. However, we believe that it should be taking into
account when planning future research efforts involving the
injection of circulating stem cells that this is seemingly not the
important mechanism in regeneration competent organisms such as the
axolotl, or at least we have not been able to observe this over the
course of our experiment, therefore it may be more fruitful to put
emphasis on therapies acting on the local damaged environment.
In the present study we tested the applicability of
SPIO-labeling to track stem-like cells non-invasively in an
intrinsic regenerative environment, the limb of the axolotl. SPIOs
showed no effect on viability and function of labeled cells and
proved useful for non-invasively tracking the fate of vascular
injected cells. SPIOs were applied to investigate whether blastema
cells have the potential to act as circulating stem cells homing at
sites of injury. We observed no accumulation of labeled blastema
cells in the zone of regeneration which indicates that this
mechanism is not at play in the restoration of a limb in the
axolotl or that the accumulation of blastema cells at the
regenerative zone is a very slow process that falls out of the time
regime of this experiment.
Glossary
Abbreviations
Abbreviations:
|
DLS
|
dynamic light scattering
|
|
dpa
|
days post amputation
|
|
EPR
|
enhanced permeability and
retention
|
|
GFP
|
green fluorescent protein
|
|
IVIS
|
in vivo imaging system
|
|
KL
|
Kullback-Leibler divergence
|
|
L
|
length
|
|
MRI
|
magnetic resonance imaging
|
|
PEG
|
polyethylene glycol
|
|
PLL
|
poly-l-lysine
|
|
ROI
|
region-of-interest
|
|
Si-PEG
|
2-methoxy(polyethyleneoxy)-propyltrimethoxysilane
|
|
SKL
|
symmetric Kullback-Leibler
divergence
|
|
SPIO
|
super-paramagnetic iron oxide
particle
|
|
TE
|
echo time
|
|
TEA
|
triethylamine
|
|
TF6
|
Tide Fluor 6
|
|
THTA
|
tris-[1-(3-hydroxypropyl)triazol-4-ylmethyl]amine
|
|
TR
|
repetition time
|
|
W
|
weight
|
References
|
1
|
Birnbaum KD and Sánchez-Alvarado A:
Slicing across kingdoms: Regeneration in plants and animals. Cell.
132:697–710. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Edwards RG: From embryonic stem cells to
blastema and MRL mice. Reprod Biomed Online. 16:425–461. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Stocum DL: Regenerative biology and
medicine. J Musculoskelet Neuronal Interact. 2:270–273.
2002.PubMed/NCBI
|
|
4
|
Stocum DL and Zupanc GK: Stretching the
limits: Stem cells in regeneration science. Dev Dyn. 237:3648–3671.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Douglas BS: Conservative management of
guillotine amputation of the finger in children. Aust Paediatr J.
8:86–89. 1972.PubMed/NCBI
|
|
6
|
Goss RJ, Van Praagh A and Brewer P: The
mechanism of antler casting in the fallow deer. J Exp Zool.
264:429–436. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Goss RJ: Future directions in antler
research. Anat Rec. 241:291–302. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Han M, Yang X, Lee J, Allan CH and Muneoka
K: Development and regeneration of the neonatal digit tip in mice.
Dev Biol. 315:125–135. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Huh JY, Choi BH, Kim BY, Lee SH, Zhu SJ
and Jung JH: Critical size defect in the canine mandible. Oral Surg
Oral Med Oral Pathol Oral Radiol Endod. 100:296–301. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Rosenthal LJ, Reiner MA and Bleicher MA:
Nonoperative management of distal fingertip amputations in
children. Pediatrics. 64:1–3. 1979.PubMed/NCBI
|
|
11
|
Krogh A: The progress of physiology.
Science. 70:200–204. 1929. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bryant SV, Endo T and Gardiner DM:
Vertebrate limb regeneration and the origin of limb stem cells. Int
J Dev Biol. 46:887–896. 2002.PubMed/NCBI
|
|
13
|
Kragl M, Knapp D, Nacu E, Khattak S, Maden
M, Epperlein HH and Tanaka EM: Cells keep a memory of their tissue
origin during axolotl limb regeneration. Nature. 460:60–65. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Menger B, Vogt PM, Kuhbier JW and Reimers
K: Applying amphibian limb regeneration to human wound healing: A
review. Ann Plast Surg. 65:504–510. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Morrison JL, Lööf S, He P and Simon A:
Salamander limb regeneration involves the activation of a
multipotent skeletal muscle satellite cell population. J Cell Biol.
172:433–440. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Roy S and Gatien S: Regeneration in
axolotls: A model to aim for! Exp Gerontol. 43:1–973. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Stoick-Cooper CL, Moon RT and Weidinger G:
Advances in signaling in vertebrate regeneration as a prelude to
regenerative medicine. Genes Dev. 21:1292–1315. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Armstrong JB and Malacinski GM:
Developmental Biology of the Axolotl. Barnes Noble. 65:3361989.
|
|
19
|
Gresens J: An introduction to the Mexican
axolotl (Ambystoma mexicanum). Lab Anim (NY). 33:41–47. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Endo T, Bryant SV and Gardiner DM: A
stepwise model system for limb regeneration. Dev Biol. 270:135–145.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kumar A, Nevill G, Brockes JP and Forge A:
A comparative study of gland cells implicated in the nerve
dependence of salamander limb regeneration. J Anat. 217:16–25.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Satoh A, James MA and Gardiner DM: The
role of nerve signaling in limb genesis and agenesis during axolotl
limb regeneration. J Bone Joint Surg Am. 91 Suppl 4:S90–S98. 2009.
View Article : Google Scholar
|
|
23
|
Tank PW, Carlson BM and Conelly TG: A
staging system for forelimb regeneration in the axolotl, Ambystoma
mexicanum. J Morphol. 150:117–128. 1976. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Arbab AS, Jordan EK, Wilson LB, Yocum GT,
Lewis BK and Frank JA: In vivo trafficking and targeted delivery of
magnetically labelled stem cells. Hum Gene Ther. 15:351–360. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Arbab AS, Yocum GT, Kalish H, Jordan EK,
Anderson SA, Khakoo AY, Read EJ and Frank JA: Efficient magnetic
cell labelling with protamine sulfate complexed to ferumoxides for
cellular MRI. Blood. 104:1217–1223. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Saldanha KJ, Piper SL, Ainslie KM, Kim HT
and Majumdar S: Magnetic resonance imaging of iron oxide labelled
stem cells: Applications to tissue engineering based regeneration
of the intervertebral disc. Eur Cell Mater. 16:17–25. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Crevensten G, Walsh AJ, Ananthakrishnan D,
Page P, Wahba GM, Lotz JC and Berven S: Intervertebral disc cell
therapy for regeneration: Mesenchymal stem cell implantation in rat
intervertebral discs. Ann Biomed Eng. 32:430–434. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Sakai D, Mochida J, Iwashina T, Hiyama A,
Omi H, Imai M, Nakai T, Ando K and Hotta T: Regenerative effects of
transplanting mesenchymal stem cells embedded in atelocollagen to
the degenerated intervertebral disc. Biomaterials. 27:335–345.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Haacke EM, Brown RW, Thompson MR and
Vankatesan R: Magnetic Resonance Imaging. Med diag. 1999.
|
|
30
|
Ittrich H, Peldschus K, Raabe N, Kaul M
and Adam G: Superparamagnetic iron oxide nanoparticles in
biomedicine: Applications and development in diagnostics and
therapy. Rofo. 185:1149–1166. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Frank JA, Miller BR, Arbab AS, Zywicke HA,
Jordan EK, Lewis BK, Bryant LH Jr and Bulte JW: Clinically
applicable labelling of mammalian and stem cells by combining
superparamagnetic iron oxides and transfection agents. Radiology.
228:480–487. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kalish H, Arbab AS, Miller BR, Lewis BK,
Zywicke HA, Bulte JW, Bryant LH Jr and Frank JA: Combination of
transfection agents and magnetic resonance contrast agents for
cellular imaging: Relationship between relaxivities, electrostatic
forces, and chemical composition. Magn Reson Med. 50:275–282. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Yang CY, Tai MF, Lin CP, Lu CW, Wang JL,
Hsiao JK and Liu HM: Mechanism of cellular uptake and impact of
ferucarbotran on macrophage physiology. PLoS One. 6:e255242011.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Foldager CB, Pedersen M, Ringgaard S,
Bünger C and Lind M: Chondrocyte gene expression is affected by
very small iron oxide particles-labeling in long-term in vitro MRI
tracking. J Magn Reson Imaging. 33:724–730. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kafuels N, Korn R, Wagner S, Schink T,
Hamm B, Taupitz M and Schnorr J: Magnetic resonance imaging of
liver metastases: Experimental comparison of anionic and
conventional superparamagnetic iron oxide particles with a
hepatobiliary contrast medium during dynamic and uptake phases.
Invest Radiol. 43:496–503. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Hansen L, Larsen EK, Nielsen EH, Ivesen F,
Liu Z, Thomsen K, Pedersen M, Skrydstrup T, Nielsen NC, Ploug M and
Kjems J: Targeting of peptide conjugated magnetic nanoparticles to
urokinase plasminogen activator receptor (uPAR) expressing cells.
Nanoscale. 5:8192–8201. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kumar A and Godwin JW: Preparation and
culture of limb blastema stem cells from regenerating larval and
adult salamanders. Cold Spring Harb Protoc. 2010:pdb.prot53672010.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Cosden RS, Lattermann C, Romine S, Gao J,
Voss SR and MacLeod JN: Intrinsic repair of full-thickness
articular cartilage defects in the axolotl salamander.
Osteoarthritis Cartilage. 19:200–205. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Kullback S and Leibler RA: On information
and sufficiency. Ann Math Stat. 22:79–86. 1951. View Article : Google Scholar
|
|
40
|
Takahashi K and Yamanaka S: Induction of
pluripotent stem cells from mouse embryonic and adult fibroblast
cultures by defined factors. Cell. 126:663–676. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Ratcliffe E, Glen KE, Naing MW and
Williams DJ: Current status and perspectives on stem cell-based
therapies undergoing clinical trials for regenerative medicine:
Case studies. Br Med Bull. 108:73–94. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Qi Y, Feng G, Huang Z and Yan W: The
application of super paramagnetic iron oxide-labeled mesenchymal
stem cells in cell-based therapy. Mol Biol Rep. 40:2733–2740. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Weissleder R, Stark DD, Engelstad BL,
Bacon BR, Compton CC, White DL, Jacobs P and Lewis J:
Superparamagnetic iron oxide: Pharmacokinetics and toxicity. AJR Am
J Roentgenol. 152:167–173. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Godwin JW, Pinto AR and Rosenthal NA:
Macrophages are required for adult salamander limb regeneration.
Proc Natl Acad Sci USA. 110:pp. 9415–9420. 2013; View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Winter EM, Hogers B, van der Graaf LM,
Gittenberger-de Groot AC, Poelmann RE and van der Weerd L: Cell
tracking using iron oxide fails to distinguish dead from living
transplanted cells in the infarcted heart. Magn Reson Med.
63:817–821. 2010. View Article : Google Scholar : PubMed/NCBI
|