Introduction
Intrahepatic cholangiocarcinoma (ICC) represents the
second most common primary hepatic malignancy, accounting for 5–10%
of liver cancers (1). The incidence
rate of ICC is relatively low in most populations, including those
of the the USA and Europe, although is considerably higher in
China.
MicroRNAs (miRNA/miRs), a class of
non-protein-coding RNAs, are short ribonucleic acid molecules of
approximately 17–25 nucleotides in length that typically bind to
the 3′ untranslated regions (3′UTRs) of target mRNAs to regulate
their expression levels (2). miRNA
are important regulators of gene expression, and the expression
levels of miRNAs have been closely associated with cell growth,
proliferation, and development, as well as the occurrence and
progression of several diseases (3).
miRNAs also regulate cell apoptosis, migration, invasion,
proliferation, and tumorigenesis by regulating target genes
(4). Human miR-193-3p has been
demonstrated to be upregulated in ICC cells; however, the detailed
functional mechanisms of miR-193-3p in ICC remain unclear.
The transforming growth factor-β (TGF-β) receptor
type 3 (TGFBR3) serves as an important tumor-suppressive factor in
many cancer types. For instance, TGFBR3 has been reported to
suppresse breast cancer progression by inhibiting cell migration
and invasion, as well as angiogenesis. Additionally, loss or
reduced levels of TGFBR3 expression have been identified in many
types of human cancer, including non-small cell lung cancer
(NSCLC), ovarian, prostate, pancreatic, breast and renal cell
carcinoma, and overexpression of TGFBR3 can lead to inhibited
cancer cell migration and invasion. Therefore, it has been
conclusded that TGFBR3 might play a suppressive role in the
progression of several cancers (5–10).
However, Gatza et al (11)
reported that TGFBR3 was markedly upregulated in human colon cancer
and may advance the development of colon cancer to a certain
degree. These findings indicated that TGFBR3 might play a dual role
in the formation of tumors.
TGFBR3 is an accessory receptor that binds and
modulates the activity of TGF-β, and these two members of the TGF-β
growth factor superfamily regulate many of the functions in
reproductive biology (12).
Furthermore, a recent study showed that downregulation of TGF-β
signaling in cholangiocytes promoted cholangiocarcinoma by
increasing cholangiocyte proliferation, which suggests the role of
TGF-β signaling in cholangiocarcinoma (13).
In the present study, we explored the expression of
miR-193-3p in human ICC tissues and cells and determined whether
downregulation of miR-193-3p could exert inhibitory effects on ICC
cell proliferation, migration, and invasion. In addition,
miR-193-3p was upregulated to investigate its direct effect on
TGFBR3 levels in ICC. Thus, this study aimed to investigate the
roles of miR-193-3p in ICC, and to verify whether TGFBR3 is a
downstream target gene of miR-193-3p in ICC.
Materials and methods
ICC clinical samples and grouping
Paired ICC tissues and adjacent normal tissues were
surgically obtained from 17 ICC patients who underwent surgery at
the Department of Hepatobilliary Laparoscopic Surgery Ward
Affilated Zhongshan Hospital of Dalian University (Dalian, China)
from May 2014 to December 2016. All tissues were obtained from
patients who did not receive adjuvant treatments in order to avoid
treatment-induced changes in gene expression. The normal tissues
were sampled from ≥3 cm away from the visible edge of the tumor
tissues. After extraction, all tissue samples were immediately
frozen in liquid nitrogen and stored at −80°C until subsequent RNA
isolation. 17 cases of ICC patients with paraffin specimens,
including 10 males and 7 females, aged 41 to 73 years, the median
age of onset of 61 years old. In accordance with the 2014 American
Hepatobiliary and Pancreatic Society consensus statement: ICC
management (14), clinical staging
criteria are divided into: stage II 4 cases, III stage 7 cases, IV
stage 6 cases. In our study, all experimental protocols were
carried out in accordance to the principles of the Declaration of
Helsinki as well as approved by the Ethics Committee of Affilated
Zhongshan Hospital of Dalian University. The informed consent of
the ICC samples was obtained from all patients of the study. The
follow-up ranged from 1 to 28 months with a median of 15
months.
Cell lines and culture
The human ICC-9810 cell line and normal human
intrahepatic biliary epithelial cell (HIBEC) line were purchased
from the American Type Culture Collection (ATCC; Manassas, VA, USA)
and were maintained in 25 cm2 flasks containing DMEM
(ATCC® 30–2006™; ATCC, Shanghai, China) supplemented
with 10% fetal bovine serum (FBS; Gibco; Thermo Fisher Scientific,
Inc., Waltham, MA, USA) in an incubator at 5% CO2 and
37°C.
RNA isolation and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA was isolated from the tissue samples and
cell lines with an RNeasy mini kit (Invitrogen; Thermo Fisher
Scientific, Inc.) following the manufacturer's instructions, and
cDNA synthesis was performed with a reverse transcription kit
(Promega Corporation, Madison, WI, USA). The mRNA expression levels
of miR-193-3p, TGFBR3, TGF-β and GAPDH (internal control) were
determined with a Power SYBR-Green Real-Time PCR Master Mix Kit
(Takara Bio, Inc., Otsu, Japan). The following primers were used
for PCR: For miR-193-3p, 5′-AACTGGCCTACAAAGTCCCAGT-3′; for TGFBR3
forward, 5′-CCTTCCGTTTCCTTTCCCAGA-3′ and reverse,
5′-CACATTTGACAGACAGGGCAAT-3′ (product size, 170 bp); for TGF-β
forward, 5′-CAATTCCTGGCGATACCTCAG-3′ and reverse,
5′-GCACAACTCCGGTGACATCAA-3′ (product size, 86 bp); GAPDH forward,
5′-ACAACTTTGGTATCGTGGAAGG-3′ and reverse, 5′-GCCATCACGCCACAGTTTC-3′
(product size, 101 bp). All RT-qPCR reactions were performed in a
BIO-RAD CFX96™ system (Bio-Rad Laboratories, Inc., Hercules, CA,
USA). The relative expression levels were quantified using the
2−ΔΔCq method (15). All
RT-qPCR reactions were repeated three times.
Cell transfection and grouping
The function of miR-193-3p in ICC-9810 cells was
assessed to determine its role in the development of ICC. The cells
were transfected with miR-193-3p inhibitor or control-miR-193-3p
inhibitor (Shanghai GenePharma Co., Ltd.,. Shanghai, China) using
30 µl Lipofectamine® 2000 transfection reagent
(Invitrogen; Thermo Fisher Scientific, Inc.) according to the
manufacturers' instructions. Following incubation for 48 h, the
transfected ICC-9810 cells were subjected to RT-qPCR as above to
verify the miR-193-3p knockdown, and then used in subsequent
assays. The groups were designed as follows: HIBEC group, ICC-9810
group, negative control (NC) group, miR-193-3p inhibitor group and
miR-193-3p inhibitor control group.
MTT cell proliferation assay
The viability of ICC-9810 cells was measured with an
MTT assay. After the transfection was stabilized, ICC-9810 cells
were plated in 96-well plates at a density of 1×105
cells/well. Every 24 h between days 1 and day 3 of culture, 20 µl
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
solution (5 mg/Ml; Invitrogen; Thermo Fisher Scientific, Inc.) was
added to each experimental well, after which the plates were
returned to the incubator and maintainedat 37°C in a humidified
atmosphere of 5% CO2 for 4 h. Following aspiration of
the supernatant, 200 µl dimethyl sulfoxide (Sigma-Aldrich; Merck
KGaA, Darmstadt, Germany) was added to each well to dissolve the
formazan crystals. Cell viability was subsequently analyzed at a
wavelength of 570 nm using a micro plate reader.
Dual-luciferase reporter gene
assay
For the luciferase assays, a pGL3 firefly luciferase
reporter gene vector (30 ng/Ml; Promega Corporation) with the
3′UTR-WT or 3′UTR-MUT fragment of human TGFBR3 Cdna, containing the
putative target site for miR-193-3p, was co-transfected with 50 nM
miR-193-3p or miR-NC into ICC-9810 cells. A Renilla luciferase
construct was also co-transfected to normalize the transfection
efficiency. After 24 h, a Dual-Luciferase Reporter Assay System
(Promega Corporation) was used to detect the relative luciferase
activities, according to the manufacturer's instructions, under a
FL500 microplate fluorescence reader (Omega Bio-Tek, Inc.,
Norcross, GA, USA). More than three independent experiments were
performed.
Wound-healing migration assay
The migratory ability of ICC-9810 cells was measured
with a Wound healing assay. The ICC-9810 cells were plated into
6-well plates (25,000 cells/well) with 2 ml of cell suspension in
each well. When cell confluence reached 80%, a sterile Eppendorf
pipette tip was used to scratch the cell surface to create a wound
area. Following removal of the debris, the culture was replenished
with fresh medium and incubated under 5% CO2 at 37°C for
24 h. The migration of the ICC-9810 cells into the wound area was
detected under phase contrast objectives (×10) on a CK2 inverted
microscope (DM16000; Leica Microsystems GmbH, Wetzlar, Germany)
(16).
Transwell assay
The invasion of ICC-9810 cells was measured using a
Transwell assay. The upper membrane surface was coated with
Matrigel (BD Biosciences, Franklin Lakes, NJ, USA) and 500 µl
RPMI-1640 medium containing 10% FBS was added into the lower
chambers. Serum-free DMEM and ICC-9810 cell suspension
(1×105 cells) were placed in the upper chambers. After
culturing for 48 h, 4% paraformaldehyde and 0.05% crystal violet
were used to fix and stain the invaded cells, respectively, and a
cotton swab was used to remove the non-invaded cells in the upper
chamber. A microscope (×100) was subsequently used to count the
numbers of cells in 10 random fields in order to estimate the
relative invasiveness of cells in the different groups (16).
Western blot assay
The relative protein expression levels of TGFBR3 and
TGF-β in the tissue samples and cell lines were detected by western
blotting. The total protein content of the tissues and cells was
harvested using radio-immunoprecipitation assay buffer (RIPA
Buffer; BioVision, Inc., Milpitas, CA, USA), and protein
concentration was determined by the BCA method. Subsequently, total
protein (40 µg/sample) was separated by 10% SDS-PAGE and
transferred to polyvinylidene difluoride membranes (Thermo Fisher
Scientific, Inc.). The membranes were blocked with 10% nonfat milk
in Tris-buffered saline with Tween (TBST; Invitrogen; Thermo Fisher
Scientific, Inc.) for 2 h at room temperature, and then incubated
with primary antibodies against TGFBR3 (ab78421), TGF-β (ab220084)
and GAPDH (ab8245; all from Abcam Cambridge, UK) overnight at 4°C.
Subsenquently, the membranes were washed with TBST and incubated
with horseradish peroxidase-coujugated secondary antibodies for 2 h
at room temperature. The blots were visualized using an enhanced
chemiluminescence film kit (New England Nuclear, Boston, MA, USA)
according to the manufacturer's instructions, and Image J software
was used to analyse protein density.
Statistical analysis
All data were expressed as the mean ± standard
deviation of at least three independent experiments. All
statistical analyses was performed using SPSS version 19.0
statistical software (SPSS, Inc., Chicago, IL, USA) and GraphPad
Prism 6.0 software (GraphPad Software, Inc., La Jolla, CA, USA). A
Student's t-test was used to evaluate the statistical significance
of difference between two groups, whiledifferences between multiple
groups were assessed by one-way analysis of variance followed by
the Dunnett's post hoc test. P<0.05 was considered to indicate a
statistically significant difference.
Results
Expression of miR-193-3p, TGFBR3 and
TGF-β in the tissues of ICC patients
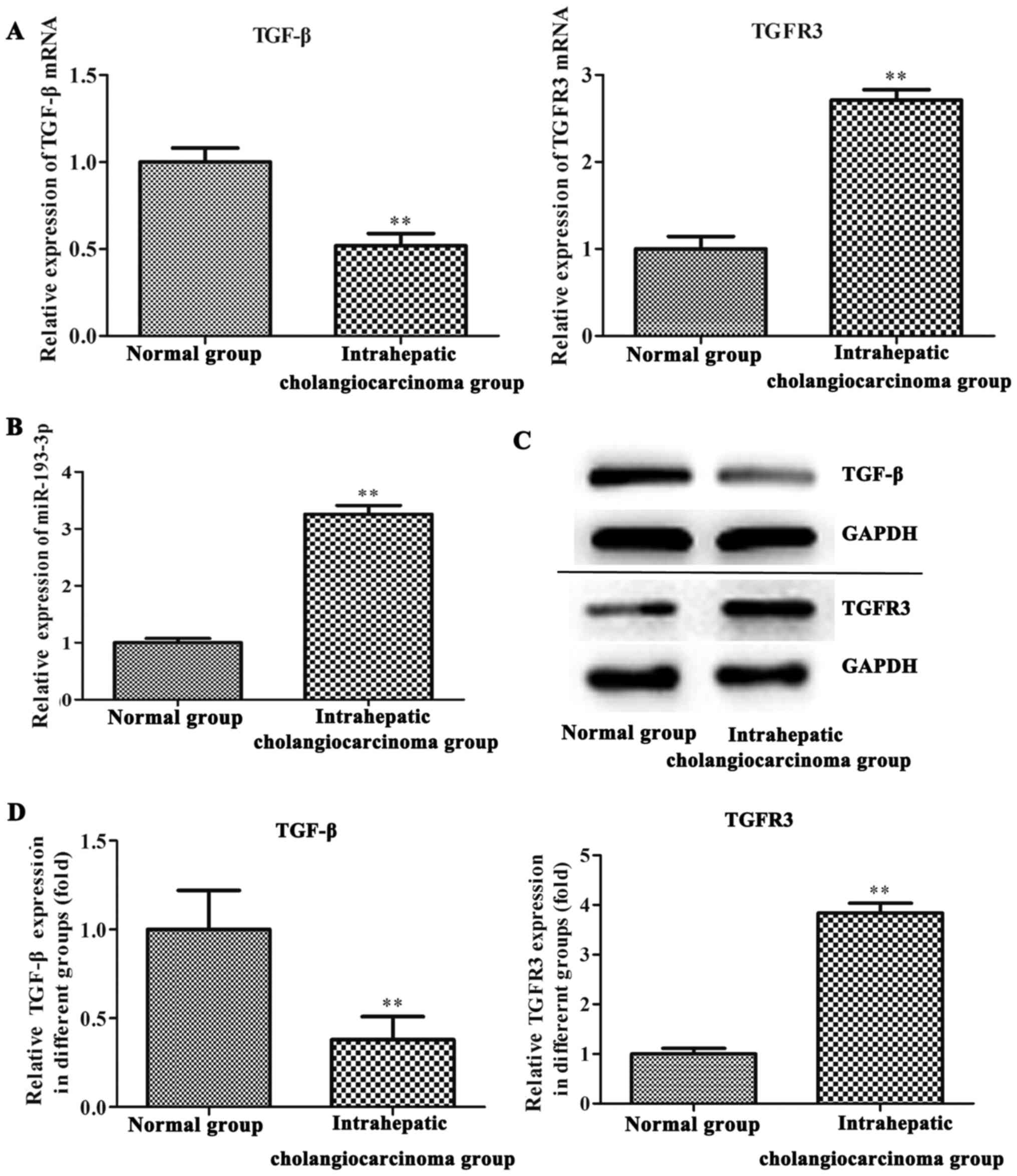
On RT-qPCR and western blot analysis, the expression
levels of miR-193-3p and TGFBR3 were found to be increased in tumor
tissue samples of ICC patients when compared with adjacent normal
tissues (P<0.01; Fig. 1). By
contrast, TGF-β was significantly downregulated in the ICC tissues
of the patients (P<0.01; Fig.
1).
miR-193-3p expression is upregulated
in the ICC-9810 cell line
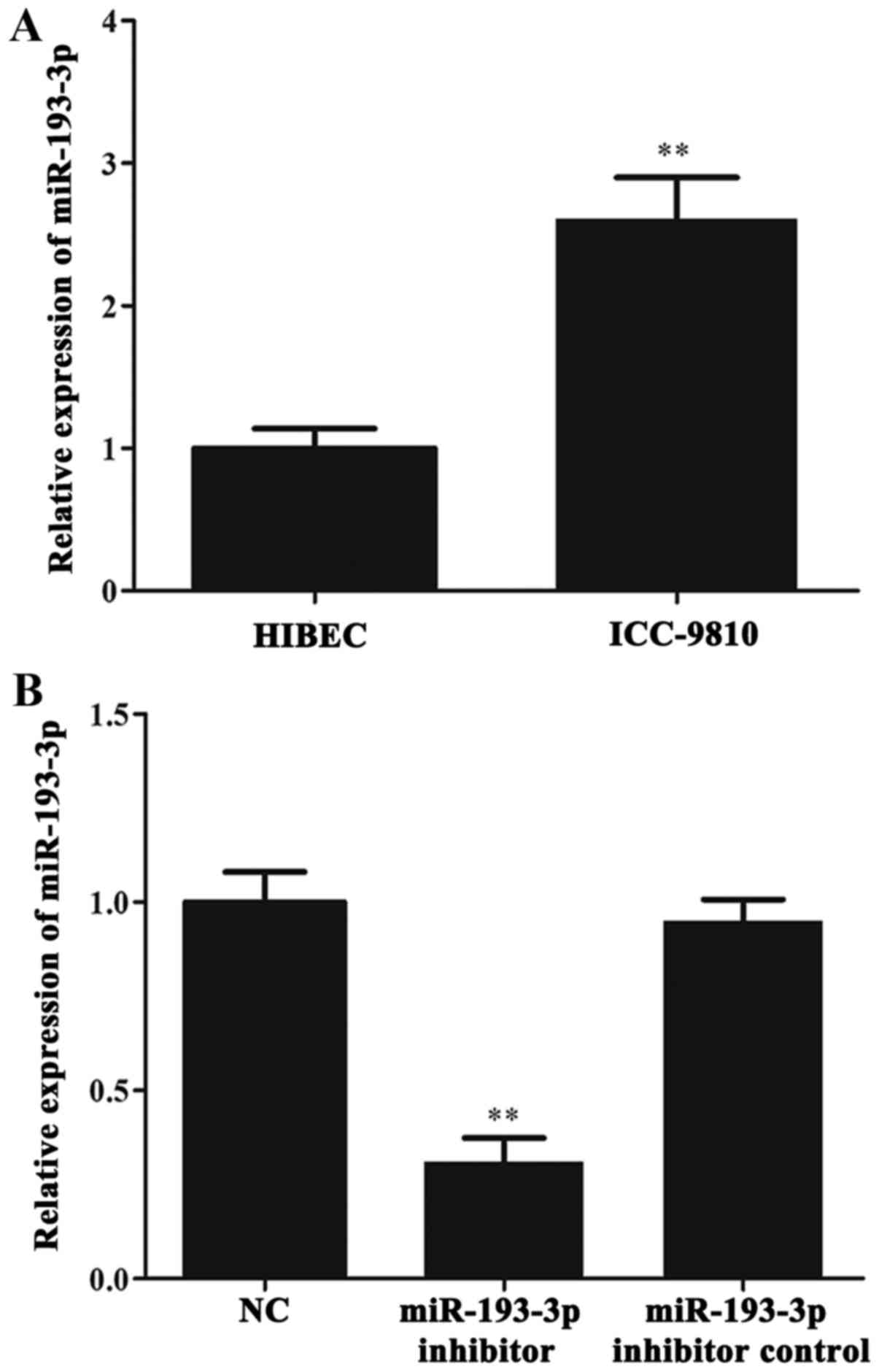
RT-qPCR was used to investigate whether miR-193-3p
was aberrantly expressed in ICC cells. First, we determined the
level of miR-193-3p in ICC-9810 and HIBEC cells. The results of
RT-qPCR showed that miR-193-3p was significantly upregulated in the
ICC-9810 cells when compared with the HIBEC cells (P < 0.01;
Fig. 2A). Subsequently, the levels
of miR-193-3p in the NC group, miR-193-3p inhibitor group and
miR-193-3p inhibitor control group were detected by RT-qPCR. The
results indicated a marked reduction in miR-193-3p levels in the
miR-193-3p inhibitor group when compared with the NC and miR-193-3p
inhibitor control groups (P<0.01; Fig. 2B). Meanwhile, there was no
significant difference in the level of miR-193-3p between the NC
and miR-193-3p inhibitor control groups.
Expression of TGFBR3 and TGF-β in the
ICC-9810 cell line
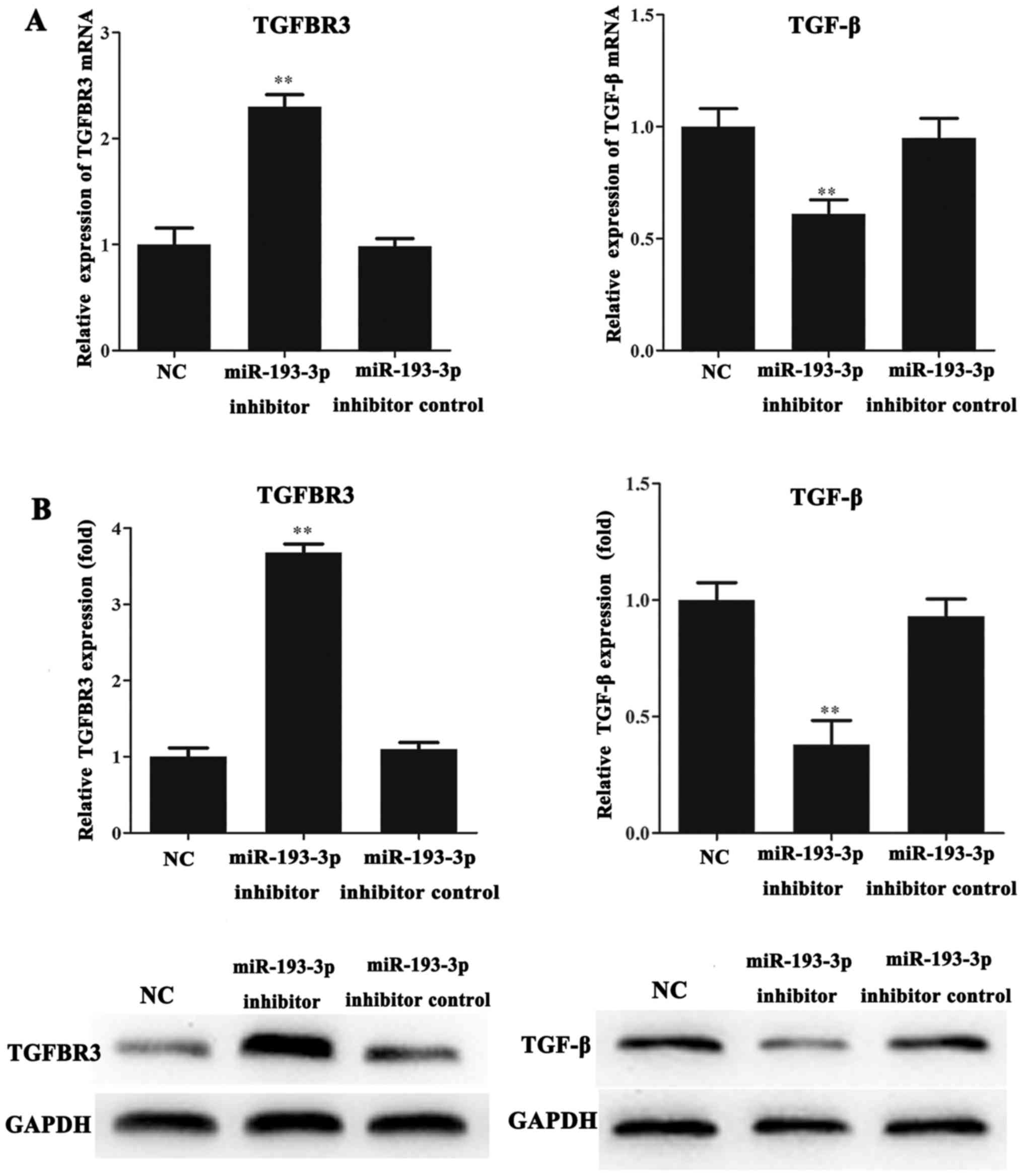
The mRNA and protein levels of TGFBR3 and TGF-β in
the ICC-9810 cells were detected by RT-qPCR and western blot
analysis. The results indicated that the mRNA and protein levels of
TGFBR3 in the miR-193-3p inhibitor group were significantly
increased compared with those in the NC and miR-193-3p inhibitor
control groups (P<0.01; Fig. 3A and
B). Conversely, we found that the mRNA and protein expression
levels of TGF-β in the miR-193-3p inhibitor group were lower than
that in the NC and miR-193-3p inhibitor control groups (P<0.01;
Fig. 3A and B).
ICC-9810 cell proliferation was
repressed following miR-193-3p inhibition
The MTT assays results showed that the viability of
ICC-9810 cells transfected with miR-193-3p inhibitor was lower than
that of cells in the NC and miR-193-3p inhibitor control groups at
48 h (P<0.05; Fig. 4A) and at 72
h (P<0.01; Fig. 4A). Thus, the
inhibition of miR-193-3p was hinder the proliferation of the
ICC-9810 cells.
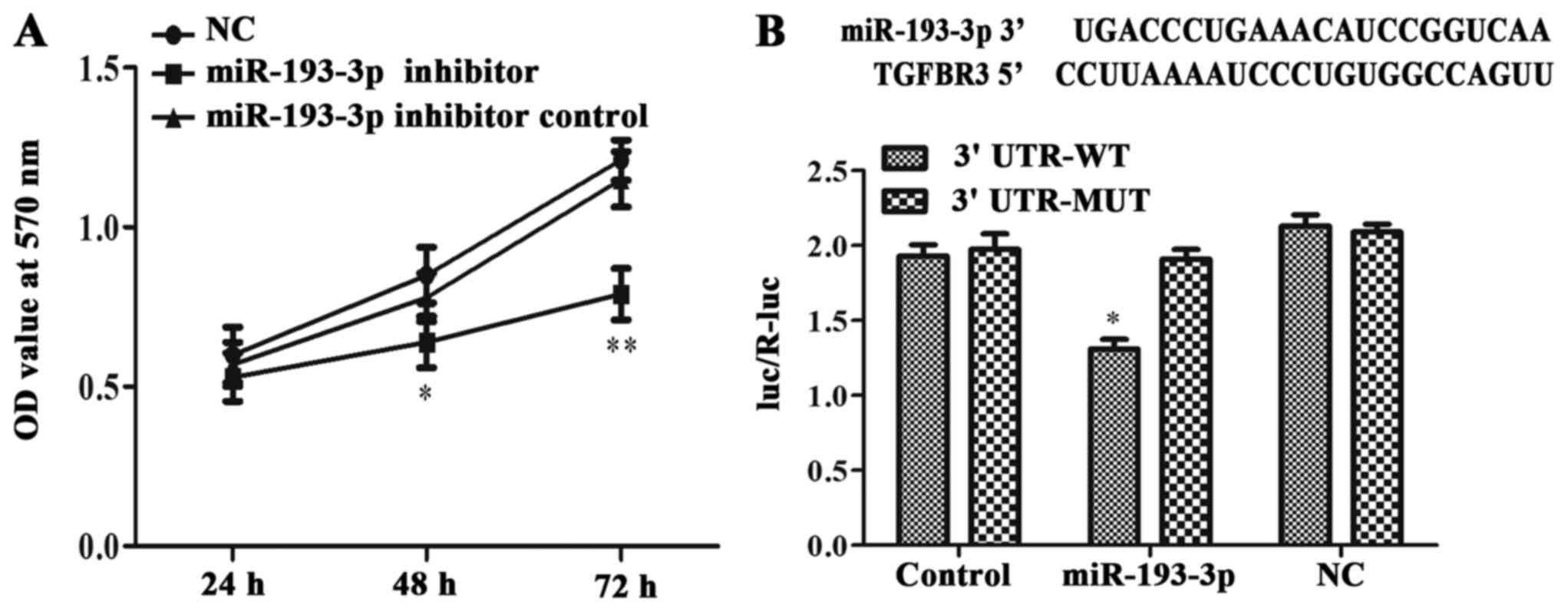 | Figure 4.miR-193-3p affected ICC-9810 cell
lines proliferation and directly targeted TGFBR3 and suppressed its
expression. (A) In ICC-9810 cells transduced with miR-193-3p
inhibitor, a three-day MTT assay was carried out to detect the
proliferation rates which has a significant drop (at 48 h, *P <
0.05 vs. miR-193-3p inhibitor group and miR-193-3p inhibitor
control group; at 72 h, **P < 0.01 vs. miR-193-3p inhibitor
group and miR-193-3p inhibitor control group). (B) The putative
binding of human miR-193-3p on human TGFBR3 gene 3′UTR was shown.
ICC-9810 cells were co-transfected with luciferase plasmids with
TGFBR3 3′UTR-WT or with TGFBR3 3′UTR-MUT. The relative luciferase
activities were measured by a dual-luciferase assay in different
groups (*P<0.05). TGFBR3, transforming growth factor-β receptor
III; TGF-β, transforming growth factor-β; ICC, intrahepatic
cholangiocarcinoma; miR, microRNA; UTR, untranslated region; WT,
wild type; MUT, mutant; NC, negative control. |
Validation of TGFBR3 as a direct
target of miR-193-3p
A dual-luciferase reporter gene assay was used to
test the binding relationship between miR-193-3p and TGFBR3. The
complimentary sequences for miR-193-3p in the 3′UTR of TGFBR3 were
obtained from the TargetScan database (Fig. 4B). Cells co-transfected with
miR-193-3p and the TGFBR3 3′UTR-WT exhibited lower intracellular
luciferase activity than those transfected TGFBR3 3′UTR-WT alone
(P<0.05; Fig. 4B). Meanwhile, the
luciferase activity of cells co-transfection with miR-193-3p and
mutant TGFBR3 3′UTR-MUT did not differ from that of the NC + TGFBR3
3′UTR-MUT or the control + TGFBR3 3′UTR-MUT groups. This date
indicated that TGFBR3 was negatively regulated by miR-193-3p,
consistently with the western blotting results, which indicated
that the inhibition of miR-193-3p could increase the expression of
TGFBR3 (Fig. 2A). Thus, we concluded
that miR-193-3p could directly target TGFBR3 and inhibit its
expression at the mRNA and protein levels.
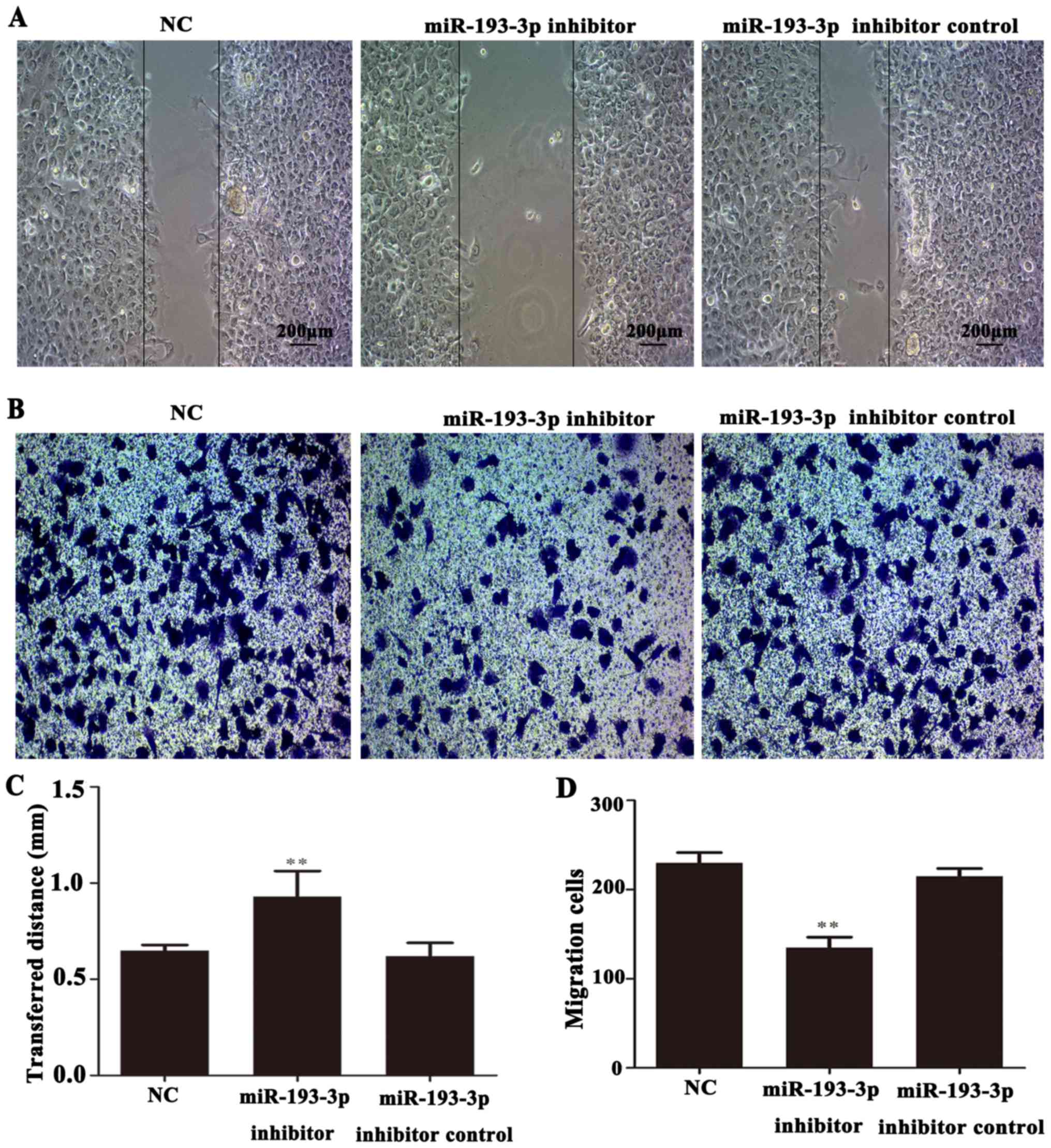
miR-193-3p downregulation or TGFBR3
overexpression suppresses the migration and invasion ability of
ICC-9810 cells
The migratory ability of ICC-9810 cells was measured
using a wound healing assay. The results showed that the migration
distance of ICC-9810 cells in the miR-193-3p inhibitor group was
markedly lower than that NC and miR-193-3p inhibitor control groups
(P<0.01; Fig. 5A and C).
Similarly, to investigate the invasive ability of ICC-9810 cells,
we measured the numbers of invaded in a Transwell assay. In the
microscope images (magnification, ×200), significantly fewer
invaded cells were observed in the miR-193-3p inhibitor group than
in the NC and miR-193-3p inhibitor control groups (P<0.01;
Fig. 5B and D). In summary, these
results indicated that the downregulation of miR-193-3p leading to
TGFBR3 overexpression could inhibit the invasion and migration of
ICC-9810 cells.
Discussion
ICC is a common primary liver tumor with increasing
incidence worldwide. The clinical outcomes of ICC are typically
worse than those for hepatocellular carcinoma due to its
non-specific presentation and detection at more advanced stages
(17). A previous study suggested
that miR-193-3p predominantly exerted an oncogenic effect in
gastric cancer (18); however,
interestingly, in human NSCLC, miR-193-3p was downregulated in the
cancer cells, indicating that miR-193-3p may act as a tumor
suppressor to inhibit the proliferation and migration of NSCLC
(19). Similarly, a previous miRNA
analysis demonstrated that miR-193-3p was significantly
downregulated in the lung tissue and serum of PAH patients
(4). In the current study, which
aimed to explore the functional significance of miR-193-3p in ICC,
we demonstrated by RT-qPCR that miR-193-3p was upregulated in
ICC-9810 cells when compared with HIBECs, and in ICC tissues
compared with adjacent normal tissues. Further data showed that
miR-193-3p enhanced the proliferation, invasion and migration of
ICC-9810 cells. Collectively these findings indicated that
miR-193-3p may act as an oncogene in ICC. Considering the multiple
functions of miR-193-3p within complex signaling pathways during
the development of different cancers, we conclude that miR-193-3p
may act as either an oncogene or a tumor suppressor gene depending
on the cancer type.
Previous research has shown that TGFBR3 may play a
dichotomous role in human bladder cancer, acting as both a tumor
suppressor and tumor promoter (20).
Based on these findings, we speculated that there may be a
potential relationship between TGFBR3 and miR-193-3p in ICC, and
found that TGFBR3 was a direct target gene of miR-193-3p in ICC.
Our studies also showed that TGFBR3 expression was upregulated by
miR-193-3p downregulation in ICC, thus indicating that TGFBR3 is
not only a tumor suppressor, but is also directly regulated by
miRNA in ICC.
In conclusion, the downregulation of miR-193-3p
directly affected TGFBR3 expression and suppressed the
proliferation, migration and invasion of ICC cells. Therefore, our
study provides that miR-193-3p may promote the invasion and
migration of ICC cells by directly targeting TGFBR3 and reducing
its expression.
To the best of our knowledge, this study is the
first to elucidate mechanistic functions of miR-193-3pregarding its
role in human ICC. Notably, we identified that TGFBR3 was closely
related to the regulatory effects of miR-193-3p in ICC. These
findings may provide a basis for therapeutic strategies in the
treatment of ICC.
References
|
1
|
Wang LJ, He CC, Sui X, Cai MJ, Zhou CY, Ma
JL, Wu L, Wang H, Han SX and Zhu Q: MiR-21 promotes intrahepatic
cholangiocarcinoma proliferation and growth in vitro and in vivo by
targeting PTPN14 and PTEN. Oncotarget. 6:5932–5946. 2015.PubMed/NCBI
|
|
2
|
Narouie B, Ziaee SAM, Basiri A and Hashemi
M: Functional polymorphism at the miR-502-binding site in the
3′untranslated region of the SETD8 gene increased the risk of
prostate cancer in a sample of Iranian population. Gene.
626:354–357. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Mandujano-Tinoco EA, García-Venzor A,
Muñoz-Galindo L, Lizarraga-Sanchez F, Favela-Orozco A,
Chavez-Gutierrez E, Krötzsch E, Salgado RM, Melendez-Zajgla J and
Maldonado V: miRNA expression profile in multicellular breast
cancer spheroids. Biochim Biophys Acta. 1864:1642–1655. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sharma S, Umar S, Potus F, Iorga A, Wong
G, Meriwether D, Breuils-Bonnet S, Mai D, Navab K, Ross D, et al:
Apolipoprotein A-I mimetic peptide 4F rescues pulmonary
hypertension by inducing microRNA-193-3p. Circulation. 130:776–785.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Cooper SJ, Zou H, Legrand SN, Marlow LA,
von Roemeling CA, Radisky DC, Wu KJ, Hempel N, Margulis V, Tun HW,
et al: Loss of type III transforming growth factor-beta receptor
expression is due to methylation silencing of the transcription
factor GATA3 in renal cell carcinoma. Oncogene. 29:2905–2915. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Finger EC, Turley RS, Dong M, How T,
Fields TA and Blobe GC: TbetaRIII suppresses non-small cell lung
cancer invasiveness and tumorigenicity. Carcinogenesis. 29:528–535.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Gordon KJ, Dong M, Chislock EM, Fields TA
and Blobe GC: Loss of type III transforming growth factor beta
receptor expression increases motility and invasiveness associated
with epithelial to mesenchymal transition during pancreatic cancer
progression. Carcinogenesis. 29:252–262. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hempel N, How T, Dong M, Murphy SK, Fields
TA and Blobe GC: Loss of betaglycan expression in ovarian cancer:
Role in motility and invasion. Cancer Res. 67:5231–5238. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Turley RS, Finger EC, Hempel N, How T,
Fields TA and Blobe GC: The type III transforming growth
factor-beta receptor as a novel tumor suppressor gene in prostate
cancer. Cancer Res. 67:1090–1098. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Dong M, How T, Kirkbride KC, Gordon KJ,
Lee JD, Hempel N, Kelly P, Moeller BJ, Marks JR and Blobe GC: The
type III TGF-beta receptor suppresses breast cancer progression. J
Clin Invest. 117:206–217. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gatza CE, Holtzhausen A, Kirkbride KC,
Gordon KJ, Lee JD, Hempel N, Kelly P, Moeller BJ, Marks JR and
Blobe GC: Type III TGF-β receptor enhances colon cancer cell
migration and anchorage-independent growth. J Clin Invest.
117:206–217. 2007.PubMed/NCBI
|
|
12
|
Sarraj MA, Chua HK, Umbers A, Loveland KL,
Findlay JK and Stenvers KL: Differential expression of TGFBR3
(betaglycan) in mouse ovary and testis during gonadogenesis. Growth
Factors. 25:334–345. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Mu X, Pradere JP, Affό S, Dapito DH,
Friedman R, Lefkovitch JH and Schwabe RF: Epithelial transforming
growth factor-β signaling does not contribute to liver fibrosis but
protects mice from cholangiocarcinoma. Gastroenterology.
150:720–733. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhou X, Zang H and Liu H L: An excerpt of
intrahepatic cholangiocarcinoma: expert consensus statement. J Clin
Hepatol. 31:1584–1587. 2015.(In Chinese).
|
|
15
|
Deng M, Jing DD and Meng XJ: Effect of
MUC1 siRNA on drug resistance of gastric cancer cells to
trastuzumab. Asian Pac J Cancer Prev. 14:127–131. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
He B, Lin X, Tian F, Yu W and Qiao B:
MiR-133a-3p inhibits oral squamous cell carcinoma (OSCC)
proliferation and invasion by suppressing COL1A1. J Cell Biochem.
Jun 1–2017.(Epub ahead of print).
|
|
17
|
Guro H, Kim JW, Choi Y, Cho JY, Yoon YS
and Han HS: Multidisciplinary management of intrahepatic
cholangiocarcinoma: Current approaches. Surg Oncol. 26:146–152.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Jian B, Li Z, Xiao D, He G, Bai L and Yang
Q: Downregulation of microRNA-193-3p inhibits tumor proliferation
migration and chemoresistance in human gastric cancer by regulating
PTEN gene. Tumor Biol. 37:8941–8949. 2016. View Article : Google Scholar
|
|
19
|
Yu T, Li J, Yan M, Liu L, Lin H, Zhao F,
Sun L, Zhang Y, Cui Y, Zhang F, et al: MicroRNA-193a-3p and −5p
suppress the metastasis of human non-small-cell lung cancer by
downregulating the ERBB4/PIK3R3/mTOR/S6K2 signaling pathway.
Oncogene. 34:413–423. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Liu XL, Xiao K, Xue B, Yang D, Lei Z, Shan
Y and Zhang HT: Dual role of TGFBR3 in bladder cancer. Oncol Rep.
30:1301–1308. 2013. View Article : Google Scholar : PubMed/NCBI
|