Introduction
Liver cancer is the sixth most prevalent type of
cancer and has the second highest tumor associated mortality
worldwide, with over half of all new cases and mortalities occuring
in China (1). The current clinical
treatments for liver cancer include surgery, radiotherapy and
chemotherapy (1). For patients who
miss the optimal timing required for surgery, chemotherapy is the
recommended therapy (2). Although
the majority of chemotherapy drugs inhibit the excessive
proliferation of liver cancer cells, the side effects are may be
severe (3–5). Novel drugs are required that have
anti-liver cancer effects with minimal side-effects (6).
Curcumol (Cur), also known as turmeric alcohol, is a
major component of essential oil (a type of oily liquid obtained by
steam distillation) of the traditional Chinese medicine root of
Rhizoma zedoariae that has been demonstrated to have
anticancer, antibacterial, anti-inflammatory and antiviral effects
(7,8). Previous studies have revealed that Cur
has significant anti-liver cancer effects and serves an important
role in liver protection (9,10). However, it is not as effective at
treating liver cancer as traditional chemotherapeutic drugs,
including fluorouracil (5-FU) and cisplatin (11,12). Cur
is poorly soluble in water and a number of toxic solubilizer, such
as Tween-80 have been previously used to enhance its solubility
(13). These restrictions have
limited the application of Cur within a clinical setting. In recent
years, different dosing methods of Cur have been investigated
(14). Cur liposomes have previously
been investigated, however to the best of our knowledge Cur active
targeting liposomes have not. To enhance the anti-liver cancer
effect of Cur and minimize the efficacy gap between Cur and
traditional chemotherapeutic drugs, active targeting liposomes may
be used to encapsulate Cur.
Liposomes are novel carriers of targeting agents
(15). The structure of liposomes is
similar to that of biofilm and their membrane material is non-toxic
in the human body (16). Liposomes
may be used to precisely target and improve the stability of drugs
within the human body without the need for complex structural
modifications to the drugs themselves (17). Due to these characteristics,
liposomes have previously been used as carriers of anticarcinogens
(18–20). Galactosylated-liposomes, which
specifically bind with the asialoglyco protein receptor (ASGPR) on
the surface of liver cells are a type of liver targeting
preparation (21). These liposomes
may be transferred to liver cells via receptor-mediated endocytosis
(RME) (22). A number of previous
studies have demonstrated that drugs may be targeted to liver cells
via galactosylated-liposomes (23,24).
In the present study, galactosylated-stearate
(Gal-s) was used as a modifier to lead liposomes encapsulating Cur
to HepG2 cells, with the aim of enhancing the anti-liver cancer
efficacy of Cur. The in vitro uptake and anti-liver cancer
efficacy of these liposomes were also investigated. The HepG2 cell
line was originally thought to be a hepatocellular carcinoma cell
line but was later revealed to derive from a hepatoblastoma
(25). The HepG2 cell line has been
previously used for the study of anti-liver cancer therapies
(26) and was therefore selected for
use in the present study to investigate liver-targeting
liposomes.
Materials and methods
Preparation of liposomes
The preparation of Gal-s was performed as previously
described (24). Briefly,
D-galactose and vinyl stearate, which were dissolved in
tetrahydrofuran, were used to synthesize galactose stearate under
the catalysis of Novozym 435 immobilized lipase. The synthetic
product was purified by silica gel column chromatography and
analyzed using mass spectrometry and proton nuclear magnetic
resonance. The content of the target product was detected using a
high-performance liquid chromatography-evaporative light scattering
detector, which was then used to confer the yield of the target
product.
A total of 1.98 mg propidium iodide (PI; purity
≥95%; cat. no. P-4170; Sigma-Aldrich; Merck KGaA, Darmstadt,
Germany) was dissolved in 100 ml PBS to obtain the PI solution.
Using the thin film dispersion method as previously described
(27), yolk lecithin (cat. no.
PC-98T; injection grade) and cholesterol (cat. no. CHO-HP;
injection grade) (both Advanced Vehicle Technology Pharmaceutical
Co., Ltd., Shanghai, China) were used at a ratio of yolk lecithin:
cholesterol, 2:1 to prepare Gal-s liposomes containing PI. The
lipid film was rehydrated using PI solution at 45°C for 50 min.
Galactosylated PI liposomes (Gal-PI-L) with different percentages
of Gal-s (0, 5, 10, 15, 20 and 25%) were prepared, which were
denoted as 0% Gal-PI-L, 5% Gal-PI-L, 10% Gal-PI-L, 15% Gal-PI-L,
20% Gal-PI-L and 25% Gal-PI-L, respectively. Normal liposomes were
free of Gal-s.
Based on previous studies (7,28), the
concentration of Cur in the liposomes used in the present study was
set as 4 g/l. Using the two-step emulsification method (29), Gal-s, Cur (purity 95%; Nanjing
Jingzhu Bio-technology Co., Ltd., Nanjing, China), yolk lecithin,
cholesterol and glycerol trioleate (purity 60%; Aladdin Shanghai
Biochemical Technology Co., Ltd., Shanghai, China) were used at a
mass ratio of yolk lecithin: cholesterol, 2:1 to prepare
galactosylated-Cur-liposomes (Gal-Cur-L). These lipids and Cur were
completely dissolved in 4 ml diethyl ether. The total concentration
of glycerol trioleate was 1 g/l. Poloxamer 188 (Shanghai Civi
Chemical Technology Co. Ltd., Shanghai, China) was used as an
emulgator in the second emulsification step. Normal Cur liposomes
(Cur-L) contained 0% Gal-s and Blank liposomes (Gal-L) were devoid
of Cur. The percentage of Gal-s in Gal-Cur-L was determined by a
liposome in vitro uptake assay. The content of Cur in
Gal-Cur-L was detected by the vanillin chromogenic method as
previously described (30). In
addition, the entrapment efficiency of these liposomes was
calculated using the petroleum ether extraction method (30). The particle diameter of these
liposomes was determined using laser scattering equipment (Delsa™
Nano Beckman Coulter, Inc., Brea, CA, USA).
Cell culture
HepG2 (hepatoblastoma), SGC-7901 (gastric cancer)
and A549 (non-small cell lung cancer) were purchased from the cell
bank of the Chinese Academy of Medical Science (Beijing, China) and
maintained in RPMI 1640 medium (Gibco; Thermo Fisher Scientific,
Inc., Waltham, MA, USA) supplemented with 10% fetal bovine serum
(FBS; Gibco; Thermo Fisher Scientific, Inc.) in the thermostat
incubator at 37°C with 5% CO2. When cells were in the
logarithmic growth phase, trypsin-EDTA solution (0.25:0.02%;
Nanjing SenBeiJia Biological Technology Co., Ltd., Nanjing, China)
was used to digest them. The cells were seeded in 12- or 96-well
plates at a density of 5×105 or 2×104
cells/well, respectively with RPMI 1640 medium and 10% FBS in a
thermostat incubator at 37°C with 5% CO2 for 24 h prior
to further experimentation.
Liposomes in vitro uptake assay
The nutrient solution in the 12-well plates was
removed and a mixture of Gal-PI-L, PBS and RPMI 1640 medium
(excluding FBS) was added. Following 20 min incubation in
thermostat incubator at 37°C with 5% CO2, the mixture
was removed and cells were washed twice with PBS. A laser confocal
inverted fluorescence microscope (LSM710; Zeiss AG, Oberkochen,
Germany) was used to capture images of the cells (magnification,
×200). The cells in the 96-well plate (fluorescence detection
plate) were handled according to the above method and a
fluorescence microplate reader (Gemini XPS; Molecular Devices, LLC,
Sunnyvale, CA, USA) was used to analyze the fluorescence intensity
of the cells. The volume of PBS, liposome, and RPMI 1640 medium
used in the 96-well plate was 10, 40 and 50 µl, respectively for
each group. The volumes used in the 12-well plate were 100, 400 and
500 µl, respectively.
Liposome in vitro anti-liver cancer
study
Cur-suspension and Cur-blank-suspension were
prepared as previously described (31). Fluorouracil (5-Fu; Shanghai Xudong
Haipu Pharmaceutical Co., Ltd., Shanghai, China), which is a
current drug used for cancer therapy was used for comparison in the
present study, was diluted to the desired concentration using
saline. An MTT reagent kit (Nanjing Jiancheng Bioengineering
Institute Co., Ltd., Nanjing, China) was used to determine the
viability of HepG2 cells. In the MTT assay dimethyl sulfoxide was
used to dissolve the purple formazan. The absorbance value of each
well was measured at 490 nm wavelength by a microplate reader. A
lactate dehydrogenase (LDH) assay kit (Nanjing Jiancheng
Bioengineering Institute Co., Ltd.) was used to analyze the LDH
release rate of HepG2 cells. These assays were performed according
to the manufacturer's protocol. The drug concentrations used in
each experimental group are listed in Table I.
 | Table I.Group of in vitro anti-liver
cancer study. |
Table I.
Group of in vitro anti-liver
cancer study.
| Group | Final concentration
of drug (mg/l) |
|---|
|
Cur-blank-suspension | 0.0 |
| Gal-L | 0.0 |
| 5-Fu | 1.7 |
| Cur-suspension | 27.0 |
| Cur-L | 27.0 |
| Gal-Cur-L low
dosage | 13.5 |
| Gal-Cur-L medium
dosage | 27.0 |
| Gal-Cur-L high
dosage | 54.0 |
Statistical analysis
Data are expressed as the mean + standard deviation.
Multiple group comparisons were performed using one-way analysis of
variance followed by Dunnett's post hoc test to detect inter-group
differences. SPSS 17.0 (SPSS, Inc., Chicago, IL, USA) was used for
statistical analysis of the data. Each experiment was repeated a
minimum of six times. P<0.05 was considered to indicate a
statistically significant difference.
Results
Characterization of liposome particle
size
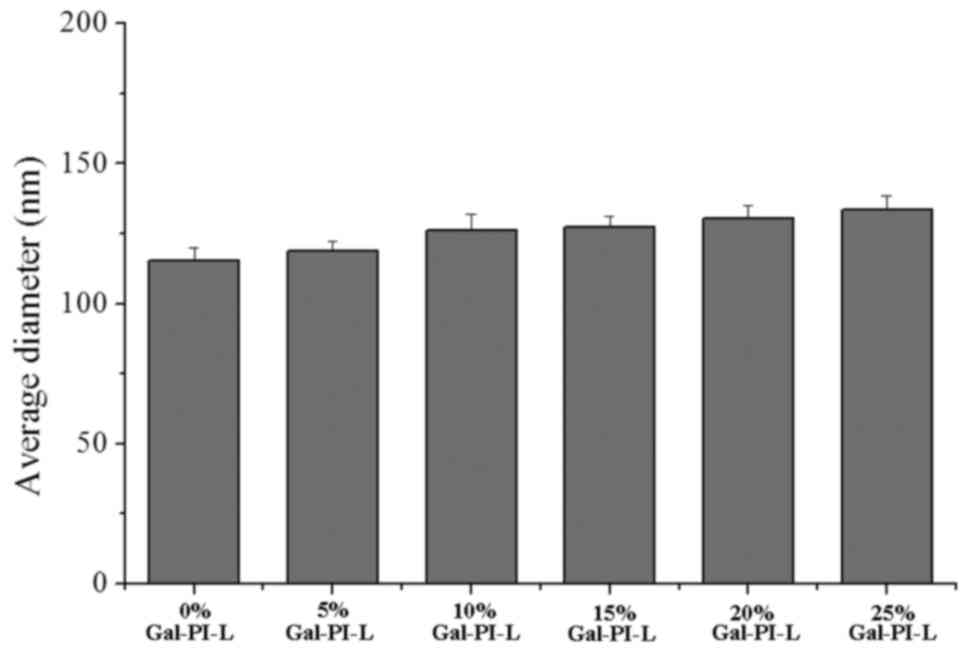
Fig. 1 indicates the
average particle diameter of the liposomes in each group. As the
percentage of Gal-s increased, the average diameter of these
liposomes also gradually increased. All liposomes were <200 nm,
which fulfilled the requirements for the present study.
Liver-targeted drug delivery enhances
PI delivery to HepG2 cells
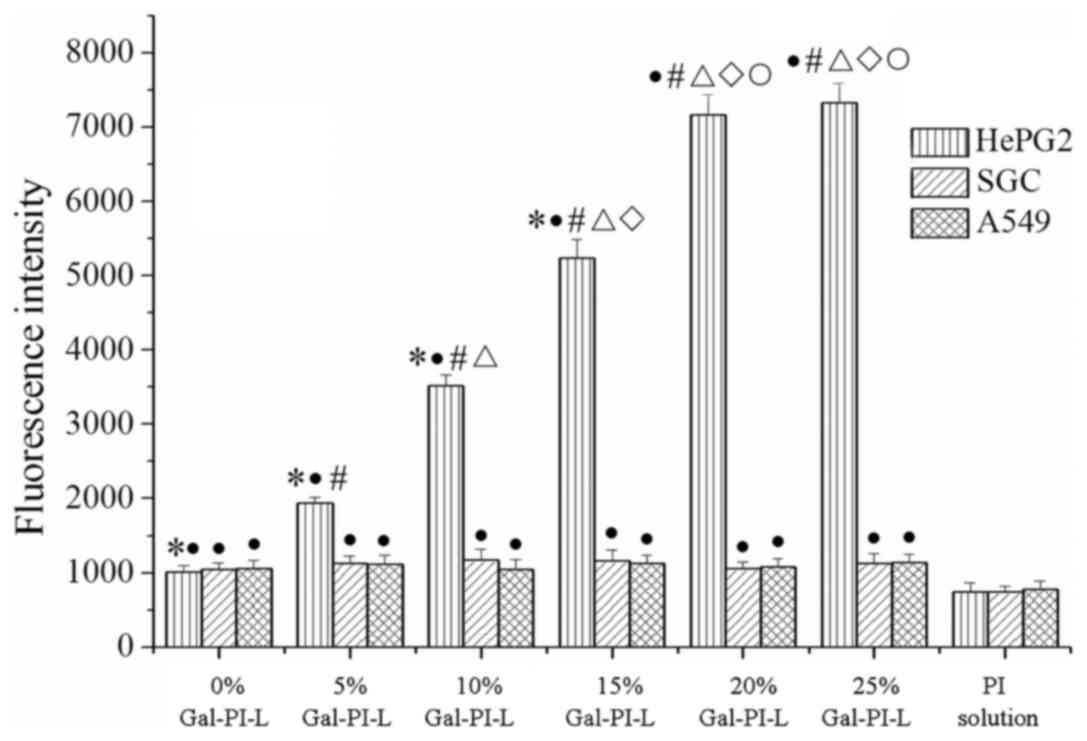
Quantitative analysis of liposome uptake was
performed in vitro using a fluorescence microplate reader
(Fig. 2). As the percentage of Gal-s
increased the fluorescence intensity of HepG2 cells signifiacntly
increased (P<0.05). No statistically significant differences
were observed between the 20% Gal-PI-L group and the 25% Gal-PI-L
group, which may indicate that the fluorescence intensity of HepG2
cells plateaued at its maximum. No significant changes in the
fluorescence intensity of the SGC-7901 and A549 cells were
observed. The fluorescence intensity of the PI solution groups was
significantly decreased compared with all other groups
(P<0.05).
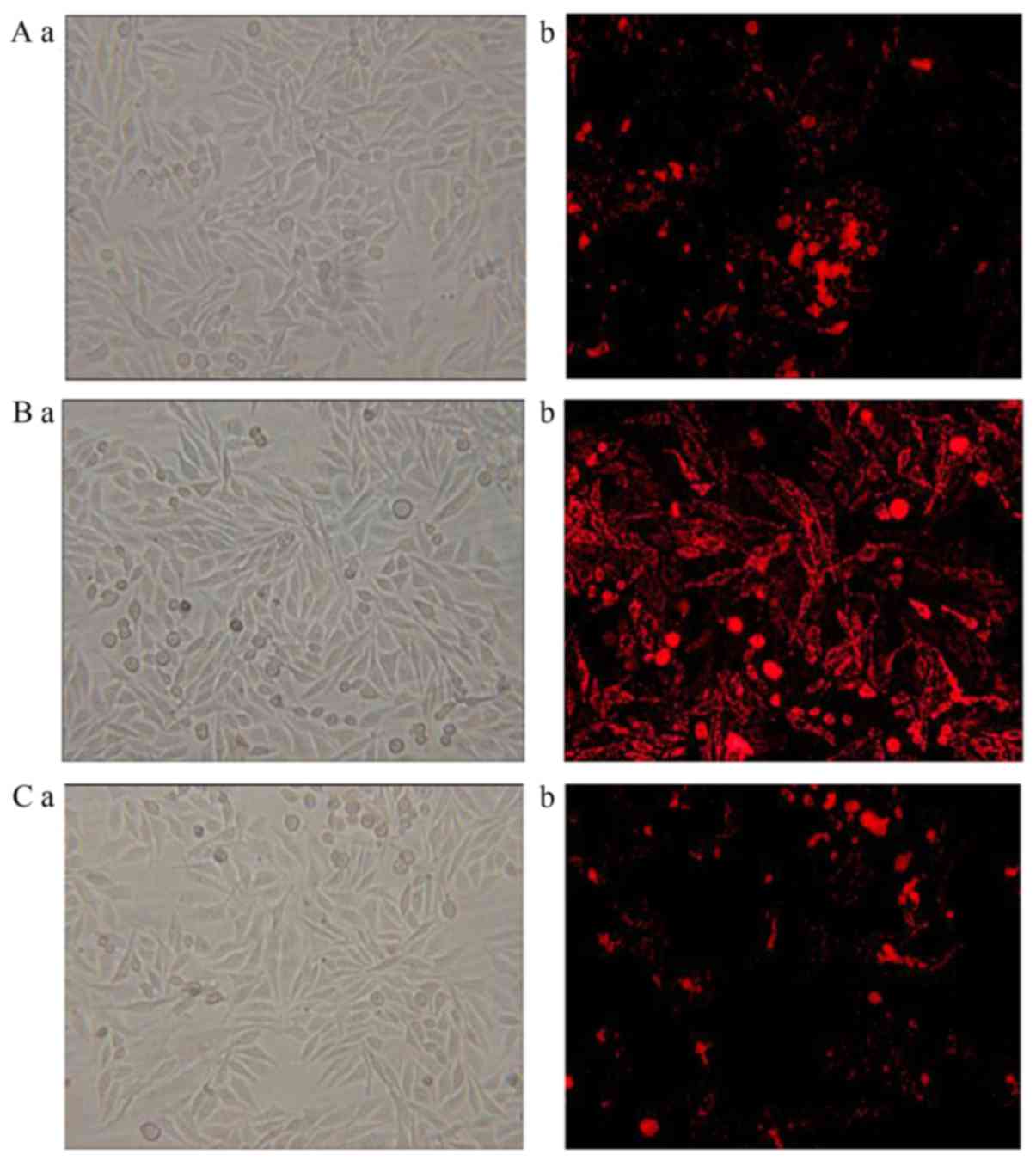
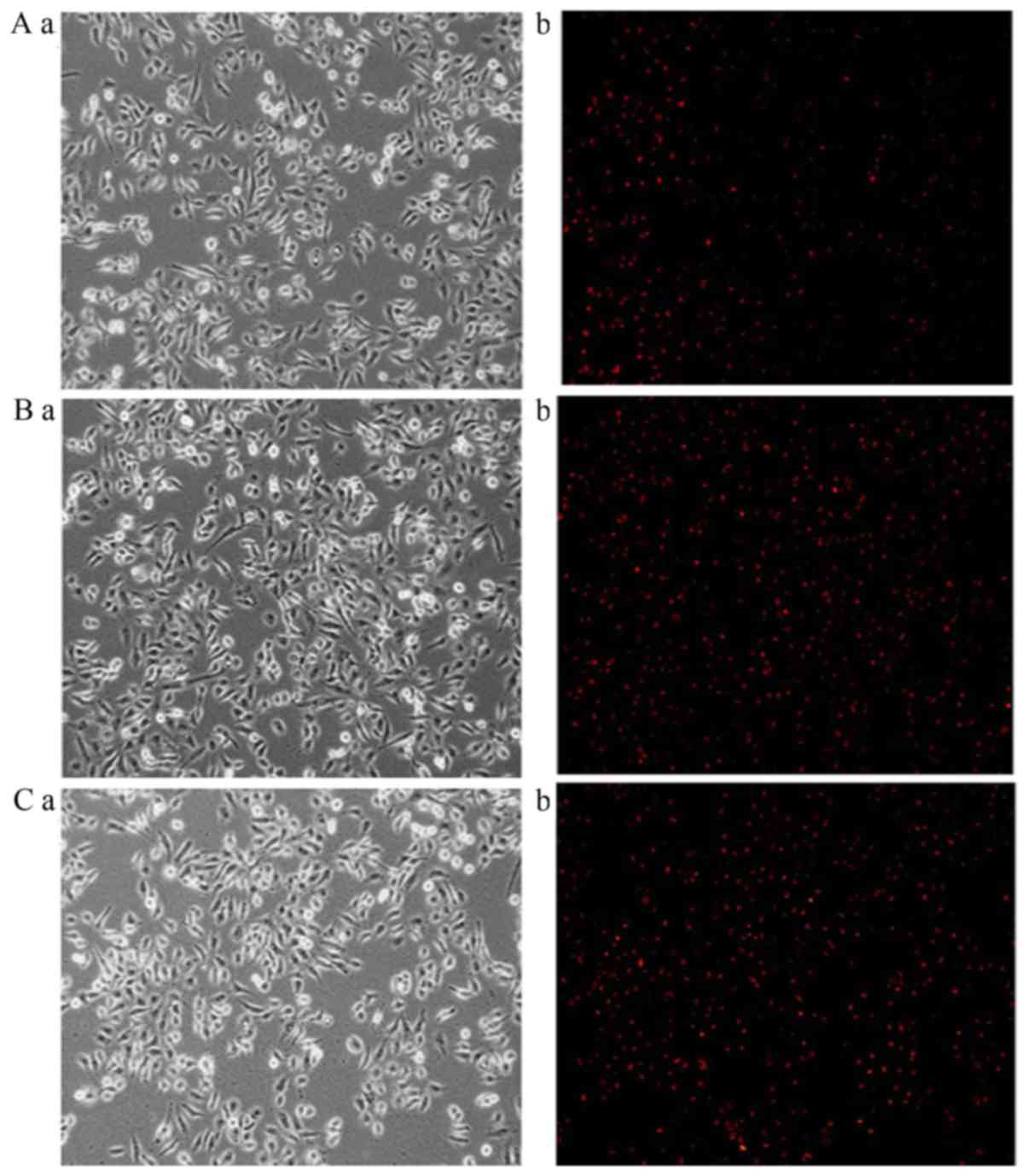
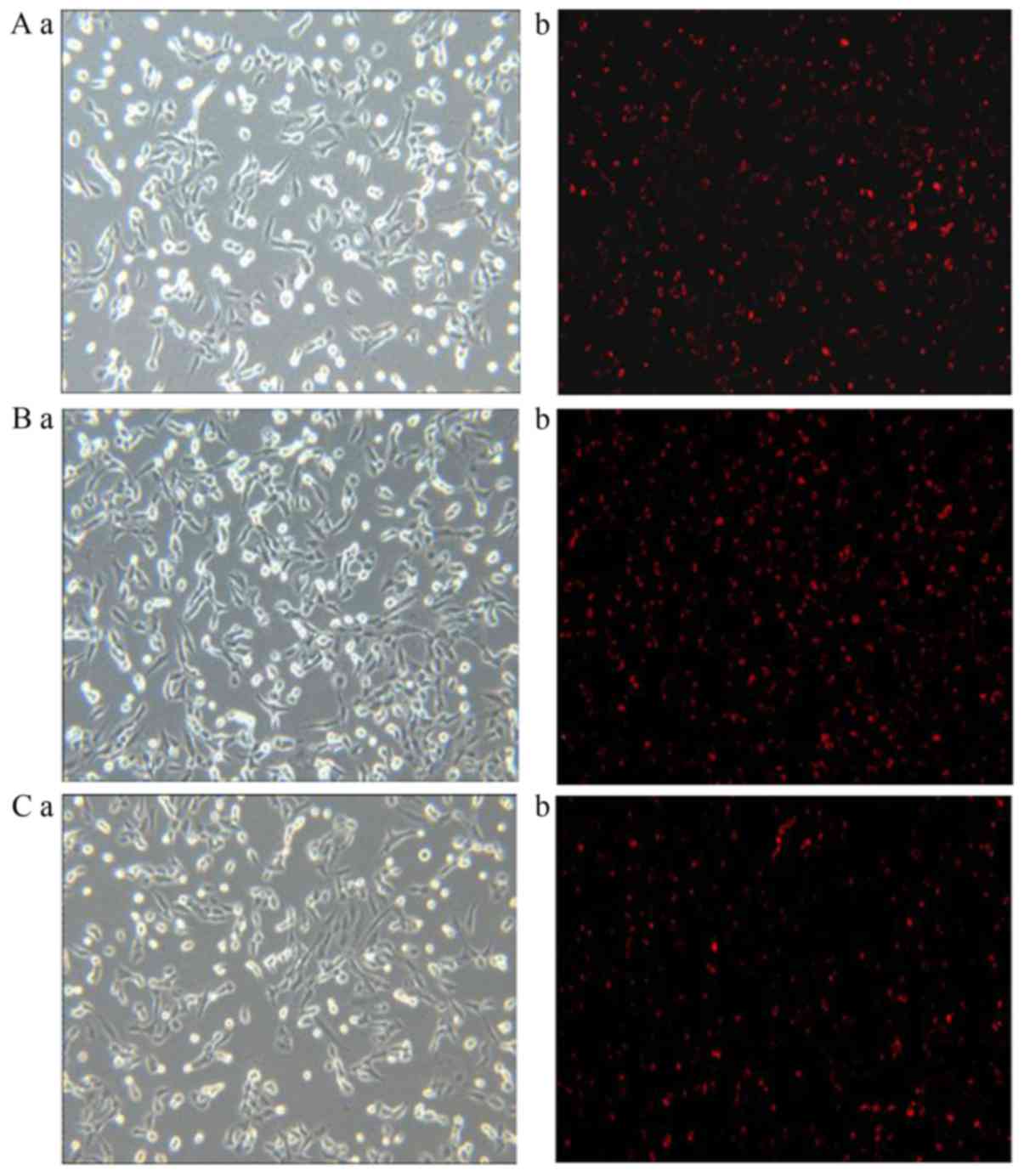
Images captured of liposome uptake in HepG2,
SGC-7901 and A549 cells following fluorescent staining are
presented in Figs. 3–5, respectively. In the 0% Gal-PI-L group, a
few dyed cells were present for all cell types. In the 20% Gal-PI-L
group, a notable increase in the number of dyed cells was observed
in the HepG2 group (Fig. 3B-b);
however, there was no notable increase in the number of dyed cells
in the SGC-7901 and A549 groups (Figs.
4B-b and 5B-b). In the PI
solution group, very few dyed cells were observed for all cell
types.
Liver-targeted drug delivery enhances
the anti-liver cancer effect of Cur in HepG2 cells
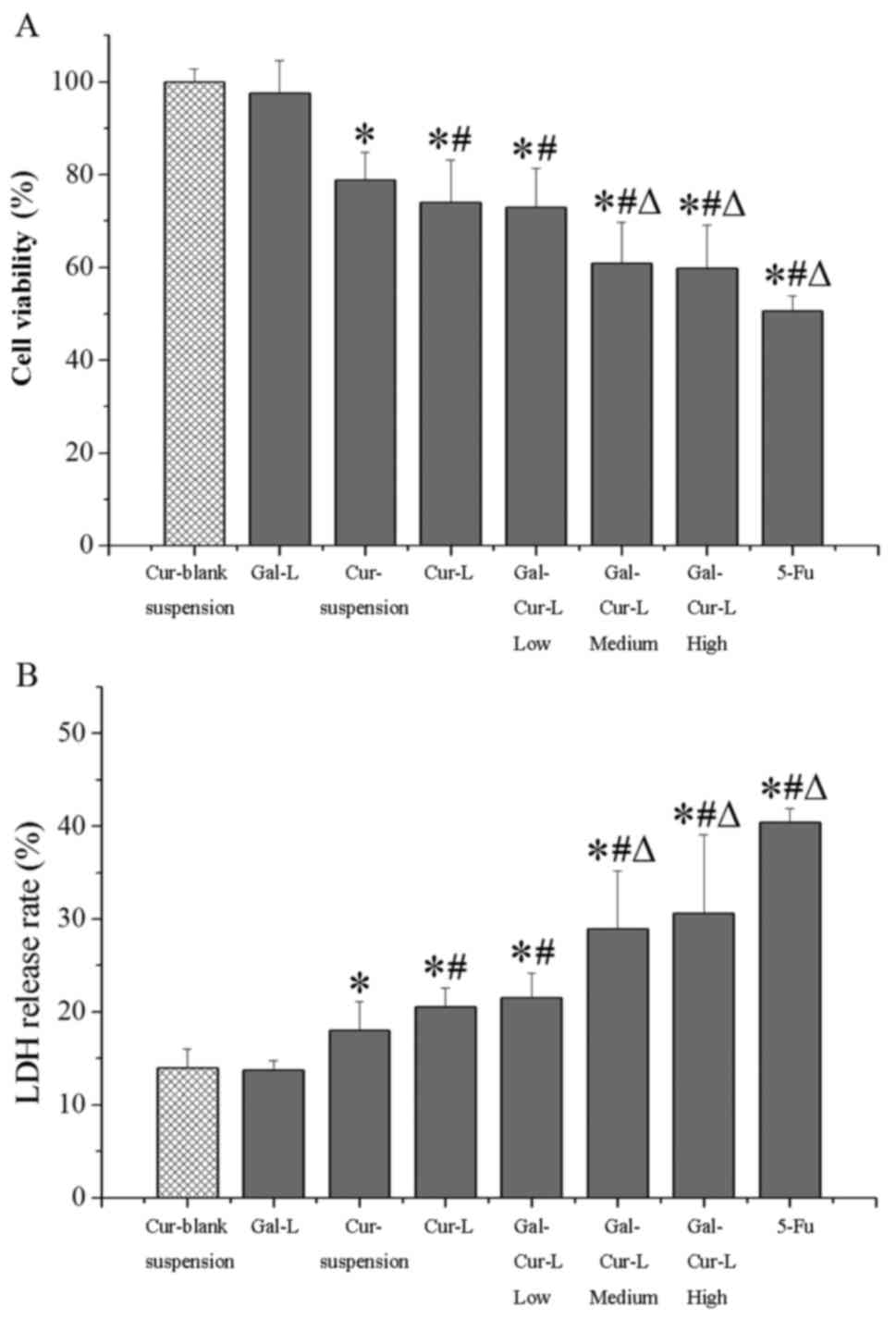
MTT and LDH assays were performed to determine the
viability and LDH release rate of each cell type (Fig. 6). The viability of the
Cur-blank-suspension group was significantly increased compared
with all other groups with the exception of Gal-L (P<0.05;
Fig. 6A). Cell viability was
significantly decreased in the Cur-L and all the Gal-Cur-L groups
compared with the Cur-suspension group (P<0.05). Cell viability
was significantly decreased in the Gal-Cur-L Med and High groups
compared with the Cur-L group (P<0.05), which indicates that Gal
was able to target drug delivery and result in cell death.
The LDH release rates were the reverse of the MTT
assay results (Fig. 6B). The LDH
release rate in all groups except the Gal-L group were
significantly increased compared with the Cur-blank-suspension
group (P<0.05). Furthermore, the LDH release rate in the
Gal-Cur-L high and Gal-Cur-L medium groups were significantly
increased compared with the Cur-L group (P<0.05). The Cur-L
group and all Gal-Cur-L groups had significantly increased LDH
release rates compared with the Cur-suspension group (P<0.05;
Fig. 6B). These results further
clarify that galactosylated-liposomes enhance the anti-liver cancer
effect of Cur.
Discussion
Fluorescent imaging of HepG2, SGC-7901 and A549
cells revealed that free PI, a type of water-soluble fluorescent
dye, did not readily enter the cells under normal growth
conditions. Only a few cells, which were in terminal apoptosis or
dead, were visible with fluorescence under normal conditions.
Gal-PI-Ls were delivered to the liver cancer cells, whereas they
were not delivered to the gastric or lung carcinoma cells. The
liver-specific delivery was regulated by galactose residues, which
specifically bind with ASGPR on the surface of liver cells
(32). Galactosylated-liposomes,
which have galactose residues, may be transferred to liver cells
through RME (33). ASGPRs are
primarily expressed on the surface of mammal liver parenchyma cells
(34). The surfaces of stomach and
lung cancer cells do not express ASGPR (34), which explains why there was no clear
targeting of galactosylated-liposomes to SGC-7901 and A549
cells.
As the phospholipid membrane shares certain
similarities with the cell membrane, a few PI-L (0% Gal-PI-L) may
penetrate the cell membrane and enter the cells. The results of
quantitative fluorescent analysis supported this suggestion. When
the percentage of Gal-s in the liposomes reached 20%, liposome
uptake appeared to plateau. Therefore, 20% was selected as the
percentage of Gal-s in galactosylated-liposomes to be used in the
present study.
A cell viability assay combined with an LDH release
rate assay comprehensively evaluated the anti-tumor activity of the
liposomes. These results revealed that Cur-suspension, Cur-L and
Gal-Cur-L all had certain in vitro anti-tumor activity,
whereas blank liposomes did minimal damage to the cells. When Cur
was encapsulated by liposomes, its anti-liver cancer efficacy was
significantly enhanced. The galactosylated-liposomes significantly
increased the anti-tumor activity compared with the normal
liposomes.
In conclusion, the results of the present study
demonstrate that liposomes modified with Gal-s may be easily and
effectively delivered to liver cancer cells. The anti-liver cancer
efficacy of Cur was significantly enhanced by the use of
galactosylated-liposomes. Future research based on the present
study should use small animals to investigate the in vivo
distribution of galactosylated-liposomes. Poly
(2-ethyl-2-oxazoline) may also be used to enhance permeability and
avoid the rapid clearance of the liposomes by macrophages (35).
Acknowledgements
Not applicable.
Funding
The present study was supported by a grant from the
Natural Science Foundation of Guangdong Province, Guangdong, China
(grant no. 2015A030310121).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
WJL, YC and HL participated in research design. YWL,
QSG, NL and WJL conducted the experiments. WXL and YBH performed
data analysis. WJL wrote the manuscript.
Ethics approval and consent to
participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors confirm that they have no competing
interests.
References
|
1
|
Jing F and Hongyang W: Precision diagnosis
and treatment of liver cancer in China. Cancer Letters.
412:283–288. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Yang H, Woo HY, Lee SK, Han JW, Jang B,
Nam HC, Lee HL, Lee SW, Song DS, Song MJ, et al: A comparative
study of sorafenib and metronomic chemotherapy for Barcelona Clinic
Liver Cancer-stage C hepatocellular carcinoma with poor liver
function. Clin Mol Hepatol. 23:128–137. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Mugada V, Ramineni H and Padala D:
5-Fluorouracil induced severe febrile neutropenia and death. J
Young Pharm. 9:133–134. 2017. View Article : Google Scholar
|
|
4
|
Nematbakhsh M, Pezeshki Z, Eshraghi Jazi
F, Mazaheri B, Moeini M, Safari T, Azarkish F, Moslemi F, Maleki M,
Rezaei A, et al: Cisplatin-induced nephrotoxicity; protective
supplements and gender differences. Asian Pac J Cancer Prev.
18:295–314. 2017.PubMed/NCBI
|
|
5
|
Oun R and Rowan E: Cisplatin induced
arrhythmia; electrolyte imbalance or disturbance of the SA node?
Eur J Pharmacol. 811:125–128. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Jia WX, Zhao HB, Chen GJ and Zhang CY:
Using drug thinking of Traditional Chinese Medicine on liver
cancer. Chin J Basic Med Tradi Chin Med. 23:121–123. 2017.(In
Chinese).
|
|
7
|
Guo P, Liang G and Yang S: Research
progresses of curcumol in medicine. China Med. 11:768–772. 2016.(In
Chinese).
|
|
8
|
Zang S, Tang Q, Dong F, Liu H, Li L, Guo
F, Pan X, Lin H, Zeng W, Cai Z, et al: Curcumol inhibits the
proliferation of gastric adenocarcinoma MGC-803 cells via
downregulation of IDH1. Oncol Report. 38:3583–3591. 2017.
|
|
9
|
Ling G, Xiaofang X, Xiaoping Y, Jian W and
Zhao J: Effects of curcumol-β-cyclodextrin inclusion compound on
proliferation and cell cycle of human hepatocarcinoma cell line
Bel-7404. China Mod Doct. 16:11–14. 2015.
|
|
10
|
Hossen MS, Tanvir EM, Prince MB, Paul S,
Saha M, Ali MY, Gan SH, Khalil MI and Karim N: Protective mechanism
of turmeric (Curcuma longa) on carbofuran-induced hematological and
hepatic toxicities in a rat model. Pharm Biol. 55:1937–1945. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Mengqing S, Yongxing D, Zongze L, Lei Y,
Hong S and Ziwen L: 5-Fluorouracil suppresses human hepatocellular
carcinoma cell growth via promoting miR-22 expression. Basic Clin
Med. 37:1368–1372. 2017.(In Chinese).
|
|
12
|
Hua L and Dong'ang Z: Matrine injection
for enhanced sensitivity to cisplatin in chemotherapy for liver
cancer. Pract J Cancer. 32:1659–1662. 2017.(In Chinese).
|
|
13
|
Zhe M, Fang W, Xin J and Yu L:
Preformulation study of curcumol. Int Pharm Res. 43:561–563.
2016.(In Chinese).
|
|
14
|
Ting W and Xiaokun L: Study on technology
of preparation and quality evaluation of curcumol liposome. Chin
Arch Tradit Chin Med. 33:2687–2689. 2015.
|
|
15
|
Darwish WM, Bayoumi NA and El-Kolaly MT:
Laser-responsive liposome for selective tumor targeting of
nitazoxanide nanoparticles. Eur J Pharm Sci. 111:526–533. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Joseph RR, Tan DWN, Ramon MRM, Natarajan
JV, Agrawal R, Wong TT and Venkatraman SS: Characterization of
liposomal carriers for the trans-scleral transport of Ranibizumab.
Sci Rep. 7:168032017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Aryasomayajula B, Salzano G and Torchilin
VP: Multifunctional Liposomes. Methods Mol Biol. 1530:41–61. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhao M, Ding XF, Shen JY, Zhang XP, Ding
XW and Xu B: Use of liposomal doxorubicin for adjuvant chemotherapy
of breast cancer in clinical practice. J Zhejiang Univ Sci B.
18:15–26. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zununi Vahed S, Salehi R, Davaran S and
Sharifi S: Liposome-based drug co-delivery systems in cancer cells.
Mater Sci Eng C Mater Biol Appl. 71:1327–1341. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Koshy ST, Cheung AS, Gu L, Graveline AR
and Mooney DJ: Liposomal delivery enhances immune activation by
STING agonists for cancer immunotherapy. Advanced Biosystems.
1:1–19. 2017. View Article : Google Scholar
|
|
21
|
Oh HR, Jo HY, Park JS, Kim DE, Cho JY, Kim
PH and Kim KS: Galactosylated liposomes for targeted co-delivery of
Doxorubicin/Vimentin siRNA to hepatocellular carcinoma.
Nanomaterials. 6:E1412016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Mbatha L, Chakravorty S, de Koning CB, van
Otterlo WA, Arbuthnot P, Ariatti M and Singh M: Spacer length: A
determining factor in the design of galactosyl ligands for hepatoma
cell-specific liposomal gene delivery. Curr Drug Deliv. 13:935–945.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Luo LH, Zheng PJ, Nie H, Chen YC, Tong D,
Chen J and Cheng Y: Pharmacokinetics and tissue distribution of
docetaxel liposome mediated by a novel galactosylated cholesterol
derivatives synthesized by lipase-catalyzed esterification in
non-aqueous phase. Drug Deliv. 23:1282–1290. 2016.PubMed/NCBI
|
|
24
|
Jiang PL, Lin HJ, Wang HW, Tsai WY, Lin
SF, Chien MY, Liang PH, Huang YY and Liu DZ: Galactosylated
liposome as a dendritic cell-targeted mucosal vaccine for inducing
protective anti-tumor immunity. Acta Biomater. 11:356–367. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
López-Terrada D, Cheung SW, Finegold MJ
and Knowles BB: HepG2 is a hepatoblastoma-derived cell line. Hum
Pathol. 40:1512–1515. 2009. View Article : Google Scholar
|
|
26
|
Cheng D, Meng M, Zhang X and Wang C: An
anti-tumor peptide from Musca Domestica Pupae (MATP) induces
apoptosis in human liver cancer cells HepG2 cells through a ROS-JNK
pathway. Internat J Pept Res Ther. 23:101–109. 2017. View Article : Google Scholar
|
|
27
|
Cui H, Li W and Lin L: Antibacterial
activity of liposome containing curry plant essential oil against
Bacillus cereus in rice. J Food Saf. 37:1–8. 2017. View Article : Google Scholar
|
|
28
|
Ping S, Chunhui Z, Jing H and Dongbin H:
The curcumol inhibitory effect on HNE1 cell of nasopharyngeal
carcinoma in tumor-bearing mouse mode. Lab Anim Sci. 34:18–22.
2017.
|
|
29
|
Zhang L, Ding L, Tang C, Li Y and Yang L:
Liraglutide-loaded multivesicular liposome as a sustained-delivery
reduces blood glucose in SD rats with diabetes. Drug Deliv.
23:3358–3363. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wenjie L, Hui L, Qiuqiang Y, Juxiang Z,
Zhenbin J and Yi C: Rapid determination of entrapment efficiency of
zedoary turmeric oil liposomes by extraction-chromogenic method. J
N Pharm. 10:3–6. 2013.
|
|
31
|
Xiao Z: Research of curcumenol intravenous
submicron emulsion. Peking Union Med Coll. 2008
|
|
32
|
Ma Y, Chen H, Su S, Wang T, Zhang C, Fida
G, Cui S, Zhao J and Gu Y: Galactose as Broad ligand for multiple
tumor imaging and therapy. J Cancer. 6:658–670. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Pathak PO, Nagarsenker MS, Barhate CR,
Padhye SG, Dhawan VV, Bhattacharyya D, Viswanathan CL, Steiniger F
and Fahr A: Cholesterol anchored arabinogalactan for
asialoglycoprotein receptor targeting: Synthesis, characterization,
and proof of concept of hepatospecific delivery. Carbohydr Res.
408:33–43. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Li Y, Huang G, Diakur J and Wiebe LI:
Targeted delivery of macromolecular drugs: Asialoglycoprotein
receptor (ASGPR) expression by selected hepatoma cell Lines used in
antiviral drug development. Curr Drug Deliv. 5:299–302. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Xu H, Hu M, Yu X, Li Y, Fu Y, Zhou X,
Zhang D and Li J: Design and evaluation of pH-sensitive liposomes
constructed by poly (2-ethyl-2-oxazoline)-cholesterol hemisuccinate
for doxorubicin delivery. Eur J Biopharm. 91:66–74. 2015.
View Article : Google Scholar
|