Introduction
Astrocytes are important cells in the central
nervous system (CNS) that serve a key role in the inflammatory
response. Astrocytes exhibit complex functions in ischemic injury
associated with the CNS due to the secretion of large volumes of
anti-inflammatory and pro-inflammatory cytokines in response to
changes in the cellular microenvironment (1). In the early stages of ischemic injury
associated with the CNS, astrocytes may be activated to secrete
anti-inflammatory and neuroprotective cytokines. Furthermore, as
ischemic injury progresses and neuroinflammation increases,
astrocytes may be damaged and subsequently release pro-inflammatory
cytokines, including tumor necrosis factor-α (TNF-α), interleukin
(IL)-1β and IL-6 (2).
In the present study, hypoxia-ischemia conditions
were induced via oxygen-glucose deprivation (OGD) in vitro.
Hypoxia-inducible factor-1 (HIF-1), an important regulator of
oxygen homeostasis, is a heterodimeric transcription factor
comprising HIF-1α and HIF-1β. HIF-1α may only be detected at low
levels due to its continuous degradation; however, HIF-1β remains
stable constitutively (3). Formation
of the active HIF-1 complex is induced by the stabilization and
accumulation of HIF-1α protein under hypoxic conditions. Following
HIF-1 activation, the transcription of hypoxia-inducible genes
associated with angiogenesis, vasodilation and cell survival is
increased (2). HIF-1 also serves an
important role in inflammatory and immune responses.
Immunomodulatory cytokines, including TNF-α and IL-1, upregulate
HIF-1-dependent gene expression (4).
HIF-1 activation is associated with the phosphoinsitide-3-kinase
(PI3K) and mitogen-activated protein kinase (MAPK) signaling
pathways. Furthermore, HIF-1 subunits interact with heat shock
proteins and other co-factors (4).
HIF-1 upregulates a number of proteins that increase inflammation
and blood flow, including nitric oxide synthase (NOS),
cyclooxygenase-2 (COX-2), vascular endothelial growth factor and
heme oxygenase-1 (4). In addition,
HIF-1 activation serves a crucial role in inflammatory responses
(4). However, the exact role of
HIF-1 in neuroinflammation has not yet been determined.
Acetylpuerarin is a novel modified isoflavone
derivative of puerarin. Puerarin is an important active isoflavone
glycoside extracted from the roots of Pueraria lobata and
has been widely used in China for the treatment of ischemic strokes
(5). The effect of acetylpuerarin on
signaling pathways associated with HIF-1-regulated inflammation in
astrocytes remains unclear. The present study therefore aimed to
investigate the effect of acetylpuerarin and HIF-1 regulation on
OGD-induced inflammation in astrocytes.
Materials and methods
Primary astrocyte extraction and
culture
Primary astrocytes were isolated from 1-day-old
neonatal Wistar rats as previously described (6). All experimental animals were obtained
from the Laboratory Animal Center of Shandong University (Jinan,
China). Cortical tissues were mechanically dissociated in PBS, and
astrocytes were seeded at 1×106 cells/ml in Dulbecco's
modified Eagle's medium (DMEM; Gibco; Thermo Fisher Scientific,
Inc., Waltham, MA, USA) supplemented with 10% fetal bovine serum
(FBS; Gibco; Thermo Fisher Scientific, Inc.). Cells were incubated
with serum-free DMEM at 37°C for 1 h prior to further
experimentation. Primary astrocytes were divided into three groups:
Acetylpuerarin (AP)+OGD, OGD and control groups. The current study
was approved by the Scientific Research Ethics Committee of Qilu
Hospital of Shandong University (Jinan, China).
OGD-induced inflammation of
astrocytes
Astrocytes were seeded in 6-well plates at a density
of 5×104 cells/well with 1 ml DMEM containing 10% FBS.
The medium was subsequently replaced with fresh serum-free medium
and plates were incubated at 37°C for 24 h. Astrocytes were
pre-treated with or without acetylpuerarin (1.6 µM) for 24 h, as
previously described (7). Cells in
the AP+OGD and OGD groups were incubated with OGD stimulation at
37°C for 2 h in a hypoxic incubator containing 94% N2,
1% O2 and 5% CO2 (8) with the following isotonic OGD solution
(1 ml; pH 7.4): 0 mM glucose, 21 mM NaHCO3, 120 mM NaCl,
5.36 mM KCl, 0.33 mM Na2HPO4, 0.44 mM
KH2PO4, 1.27 mM CaCl2 and 0.81 mM
MgSO4 (9). Normoxic
control cells were incubated in 5% CO2 and atmospheric
air in an isotonic control solution for 2 h at 37°C.
Immunocytochemistry of astrocytes
Astrocytes were collected and subcultured on sterile
glass coverslips at 37°C for 12–16 h. Following fixation with 4%
formaldehyde for 15 min at room temperature and permeabilization
using Triton-X-100 for 20 min, all astrocytes were incubated with
primary antibodies (rabbit anti-GFAP antibody; cat. no. ab7260;
1:1,000; Abcam, Cambridge, MA, USA) at 4°C for 12 h. PBS was used
to wash the cells, which were subsequently incubated with secondary
antibodies (goat anti-rabbit secondary antibodies; cat. no.
A-21094; Alexa Fluor 633; 1:30,000; Invitrogen; Thermo Fisher
Scientific, Inc.) for 40 min at 37°C. Images were captured at a
magnification of ×200 using a Nikon Eclipse 80i fluorescence
microscope (Nikon Corportaion, Tokyo, Japan) and analyzed using
ImageJ 2X software (National Institutes of Health, Bethesda, MD,
USA).
MTT and lactate dehydrogenase (LDH)
release assay
A 3-[4,
5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide (MTT) assay
was used to investigate astrocyte viability. Astrocytes were
pre-treated with acetylpuerarin for 24 h and then subjected to OGD
stimulation for 2 h. MTT solution (5 mg/ml; Sigma-Aldrich; Merck
KGaA, Darmstadt, Germany) was added to each well at 37°C for 4 h.
After removing the supernatant, 150 µl DMSO was added to each well
to dissolve the purple formazan for 10 min. A Varioskan Flash
Multimode Reader (Thermo Fisher Scientific, Inc.) was used to
measure absorbance at 490 nm. In the LDH release assay, astrocytes
were pre-treated with acetylpuerarin for 24 h and subjected to OGD
stimulation for 2 h. Cell damage was detected using an
LDH-Cytotoxicity Assay kit (Roche Applied Science, Mannheim,
Germany) according to the manufacturer's protocol. A Varioskan
Flash Multimode Reader was used to determine the absorbance at 450
nm.
Western blot analysis
The immunoreactivity of HIF-1α and nuclear factor
(NF)-κB p65 was investigated using western blotting post-OGD
stimulation for 2 h. Cells were washed using PBS and collected in a
radioimmunoprecipitation lysis buffer (Beyotime Institute of
Biotechnology, Haimen, China) containing 1 mM PMSF. Nuclear
extraction was performed as previously described (10). Protein extraction was performed using
a BCA kit (Beyotime Institute of Biotechnology) and adjusted to the
same concentration. Protein (20 µg) was loaded per lane and
polyvinylidene membranes were used for protein transfer. Primary
antibodies (anti-HIF-1α; cat. no. 36169; 1:1,000) and anti-NF-κB
(cat. no. 8242; 1:1,000; each, Cell Signaling Technology, Inc.,
Danvers, MA, USA) and secondary antibodies (mouse anti-rabbit IgG
mAb #5127, 1:30,000 dilution, Cell Signaling Technology, Inc.,
Danvers, MA, USA) were used. β-actin and histone H1 (anti-β-actin,
cat. no. ab8226; 1:1,000; anti-histone H1, cat. no. ab203337;
1:1,000; Abcam) were used as loading control. 5% BSA was used for
blocking at room temperature for 1 h. Immunoblots were visualized
using a FluorChem E Chemiluminescent Western Blot Imaging system
(ProteinSimple, San Jose, CA, USA) and ImageJ 2X (National
Institutes of Health) was used to quantify the results. The
relative expression of target proteins was determined relative to a
control (β-actin or histone H1).
ELISA
Following OGD stimulation for 2 h, the
immunoreactivity and expression of IL-1β and TNF-α in the astrocyte
culture medium were determined using ELISA kits (Rat IL-1β/IL-1F2
Quantikine ELISA kit, cat no. RLB00C; Rat TNF-α Quantikine ELISA
kit, cat. no. RTA00; each, R&D Systems Inc., Minneapolis, MN,
USA). A Varioskan Flash Multimode Reader was used to detect optical
density values at 450 nm.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Astrocytes were pre-treated with or without
acetylpuerarin for 24 h, followed by OGD induction for 2 h. Total
RNA was extracted by using TRIzol Plus RNA Purification kit (cat.
no. 12183555; Invitrogen, Thermo Fisher Scientific, Inc.).
2−ΔΔCq was used to quantify the levels of mRNA
expression following a protocol as previously described (7). PCR conditions and primers used for
amplification were performed according to the manufacturer's
protocol using a SYBR GreenER qPCR SuperMix (cat. no. 11761500;
Invitrogen; Thermo Fisher Scientific, Inc.) as previously described
(7). Lightcycler Software version
4.0 (Roche Applied Science) was used for quantitative data
analysis. The relative expression of target genes was normalized to
β-actin expression. Experiments were performed in triplicate.
Statistical analysis
All quantitative data are presented as the mean ±
standard deviation. Statistical analysis was performed using SPSS
17.0 software (SPSS, Inc., Chicago, IL, USA). A Student's t-test
was performed for pairwise comparisons. Variance between multiple
groups was analyzed using one-way analysis of vairance followed by
a Dunnett's test for comparisons. P<0.05 was considered to
indicate a statistically significant difference.
Results
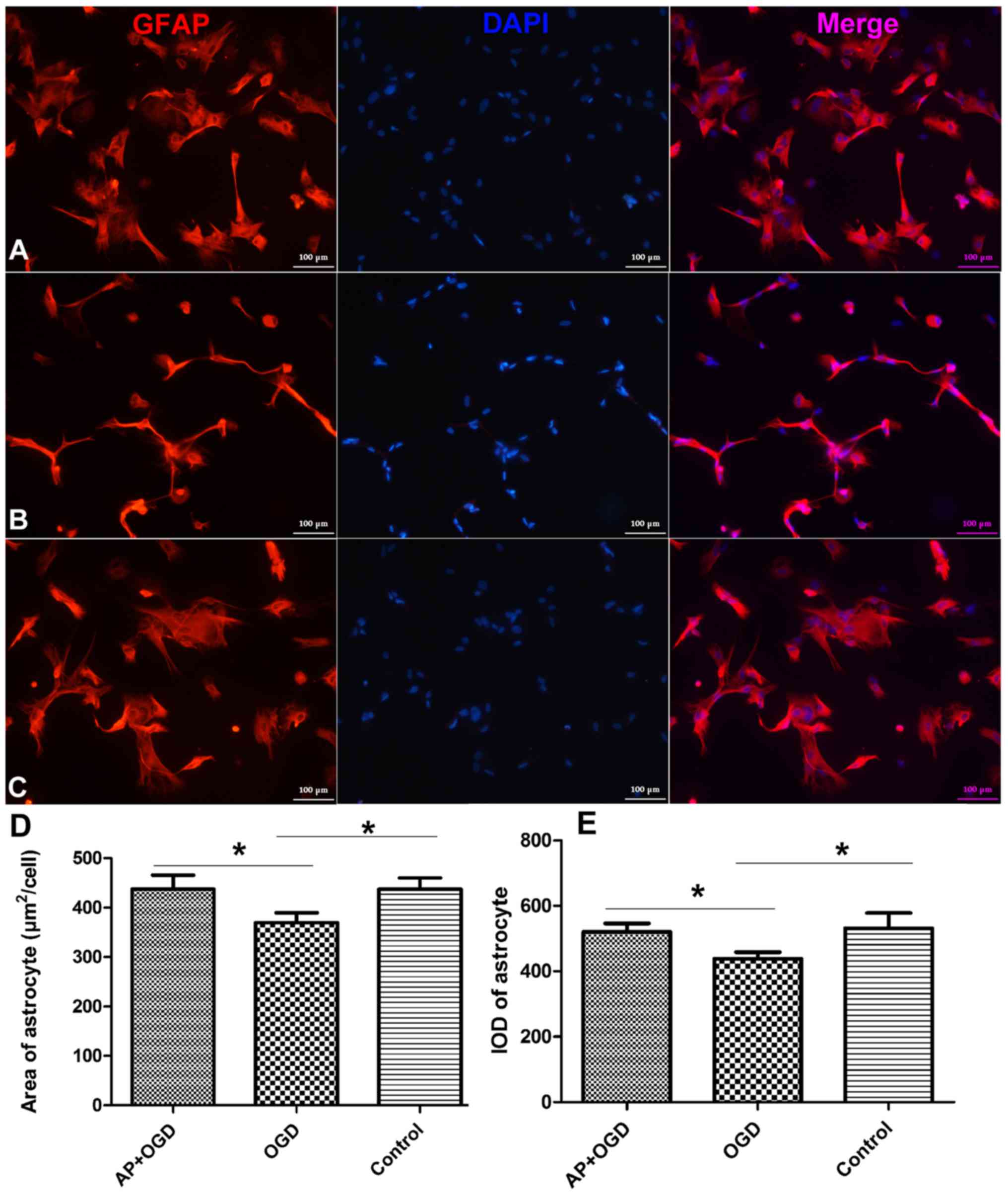
Acetylpuerarin rescues astrocyte
morphology under OGD stress
Following OGD induction for 24 h, the outline and
appearance of astrocytes were observed to be thinner, and the
surface area of astrocytes (µm2/cell) was significantly
decreased (369.52±16.67) compared with the control group
(442.28±18.68; Fig. 1A-D;
P<0.05). In addition, the integrated optical density (IOD) of
glial fibrillary acidic protein (GFAP) was revealed to be
significantly decreased in the OGD group (438.60±16.37) compared
with the control group (531.35±38.41; P<0.05; Fig. 1E). However, the morphology of
astrocytes was markedly improved in the AP+OGD group compared with
the OGD group (Fig. 1A-C).
Furthermore, the area of astrocytes significantly increased to
437.09±23.66 (Fig. 1D) and the IOD
of GFAP increased significantly to 520.26±21.33 following treatment
with acetylpuerarin compared with the OGD group (P<0.05;
Fig. 1E).
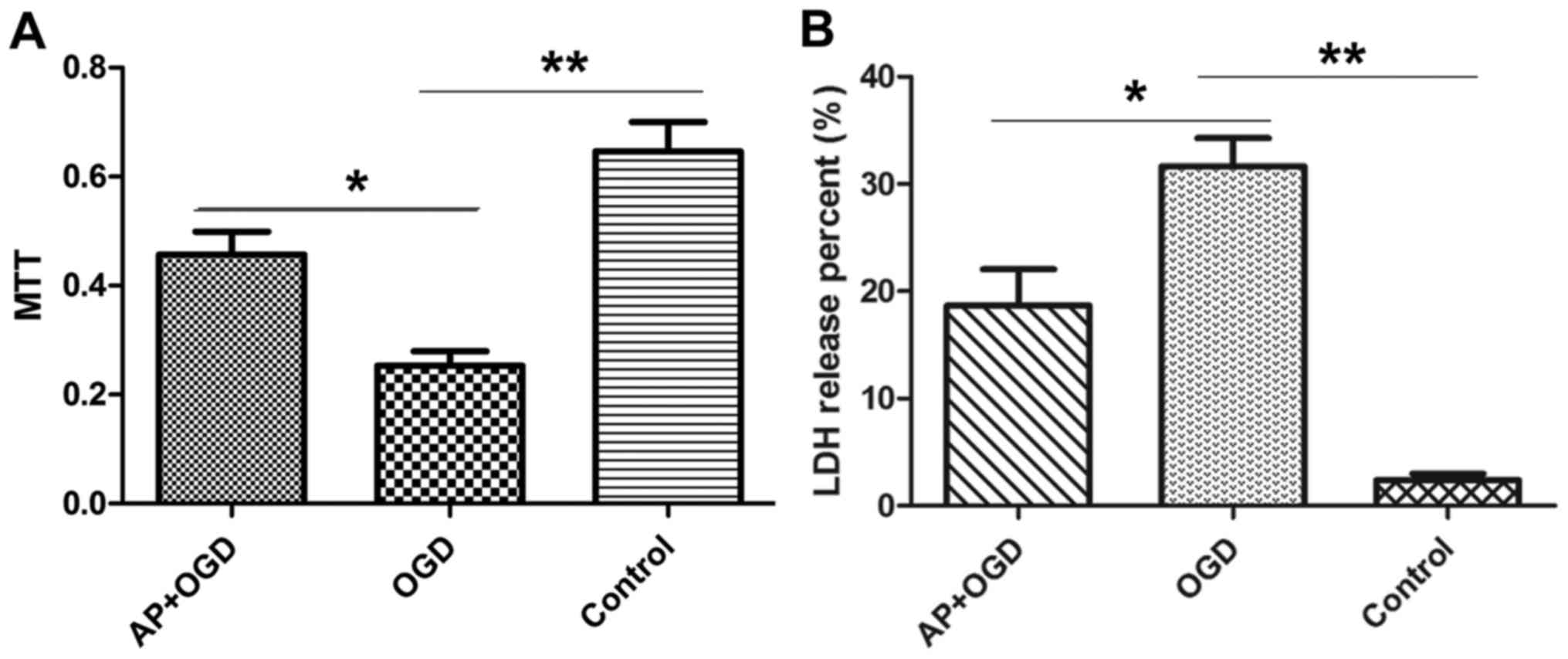
Acetylpuerarin attenuates OGD-induced astrocyte
damage. The effect of acetylpuerarin on OGD-induced astrocytes was
investigated using MTT and LDH release assays. The results of the
MTT assay demonstrated that astrocyte viability was significantly
decreased following OGD induction (0.25±0.04) compared with the
control group (0.65±0.08; P<0.01); however, pre-treatment with
acetylpuerarin was revealed to significantly attenuate this effect
(0.46±0.06; P<0.05; Fig. 2A).
LDH release assays were performed to investigate
cell damage in the different groups. The percentage of LDH released
by the control group was 2.39±0.81%; whereas in the OGD group this
value was 31.66±3.71% (P<0.01 vs. control group), suggesting
that OGD induced cell damage in astrocytes. However, treatment with
acetylpuerarin significantly reduced the amount of LDH released in
the AP+OGD group (18.67±4.8; P<0.05 vs. OGD group), suggesting
that acetylpuerarin suppresses OGD-induced cell damage in
astrocytes (Fig. 2B).
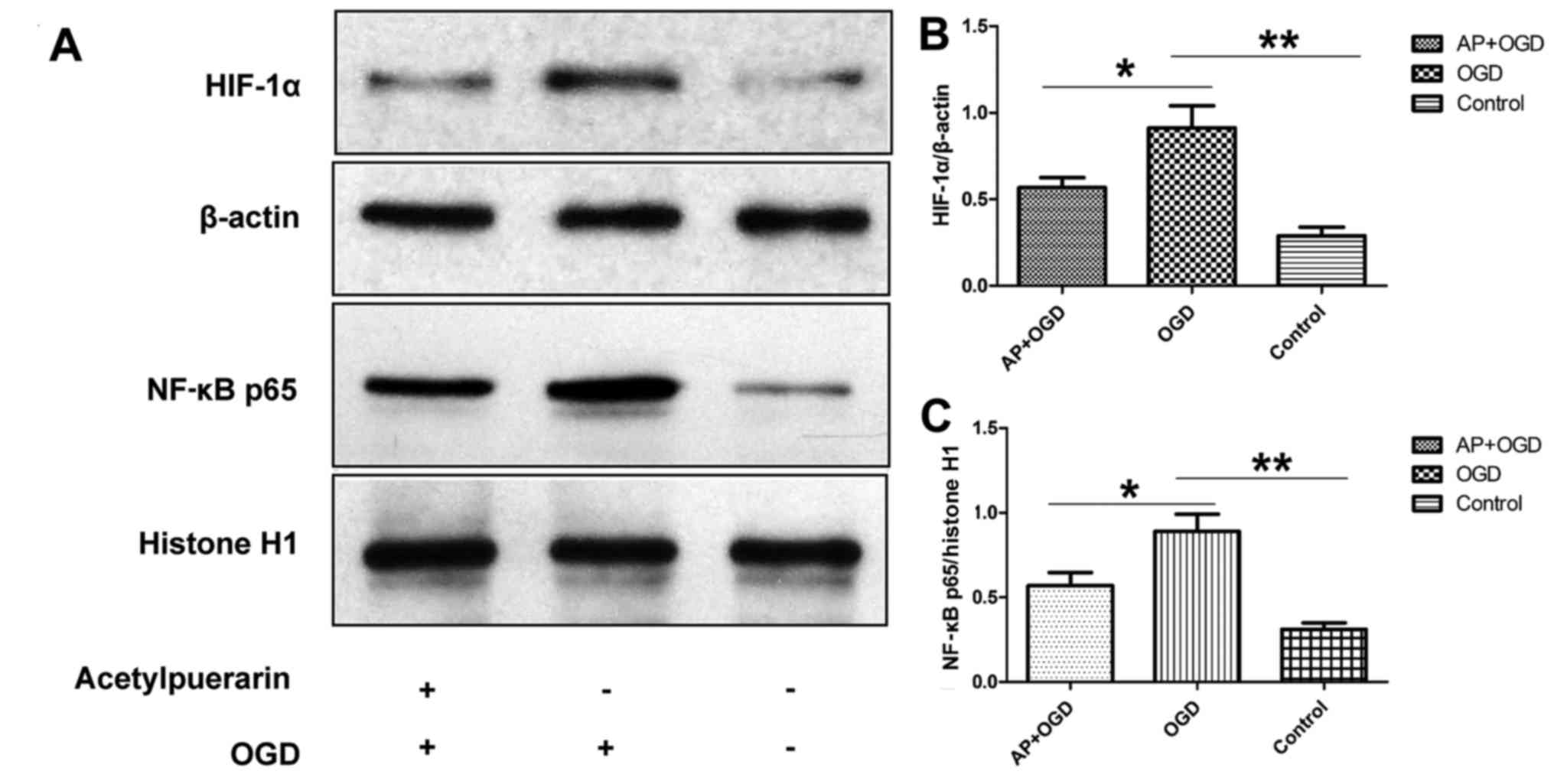
Acetylpuerarin decreases HIF-1α and
NF-κB expression in OGD-induced astrocytes
To investigate the effect of acetylpuerarin on
OGD-induced neuroinflammation, HIF-1α and NF-κB p65 expression in
OGD-induced astrocytes was determined following pre-treatment with
acetylpuerarin using western blot analysis. The results
demonstrated that HIF-1α was significantly upregulated in the OGD
group (0.91±0.13) compared with the control group (0.29±0.05;
P<0.01; Fig. 3A and B). However,
when cells were pre-treated with acetylpuerarin, HIF-1α was
significantly downregulated in the AP+OGD group (0.57±0.06)
compared with the OGD group (P<0.05; Fig. 3A and B). Furthermore, NF-κB p65 was
upregulated in OGD-induced astrocytes (0.89±0.10) compared with the
control (0.32±0.04; P<0.01), and this effect was significantly
attenuated in the AP+OGD group (0.57±0.08) compared with the OGD
group (P<0.05; Fig. 3A and C).
These results suggest that acetylpuerarin decreases the activation
of NF-κB, an important regulator of pro-inflammatory pathways, via
suppressing HIF-1α activation in OGD-induced astrocytes.
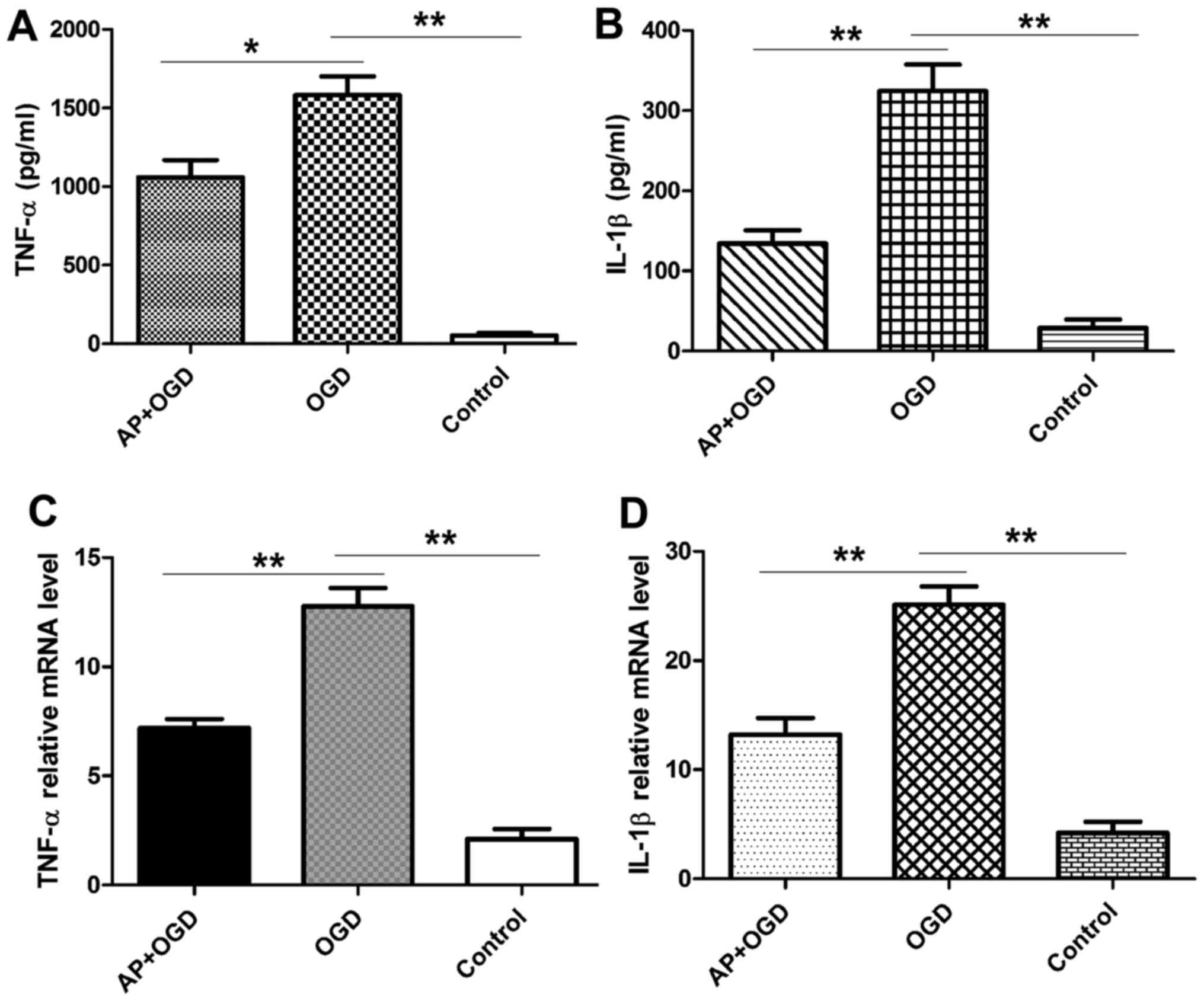
Acetylpuerarin attenuates inflammatory cytokine
secretion in astrocytes induced by OGD. TNF-α and IL-1β expression
in the astrocyte culture medium was measured using ELISA following
2 h of OGD induction. The results revealed that OGD treatment
significantly increased the expression of TNF-α in astrocytes
(1,582.96±169.14 pg/ml) compared with the control group
(52.88±20.81 pg/ml; P<0.01), and that this was significantly
attenuated in acetylpuerarin pre-treated astrocytes
(1,058.76±154.97 pg/ml; P<0.05 vs. OGD group; Fig. 4A). IL-1β was also significantly
upregulated following OGD induction in astrocytes (324.47±46.69
pg/ml) compared with the control group (28.97±14.82 pg/ml;
P<0.01), and this effect was significantly attenuated in
astrocytes pre-treated with acetylpuerarin (134.06±23.66 pg/ml;
P<0.01 vs. OGD group; Fig.
4B).
Acetylpuerarin suppresses the mRNA
expression of pro-inflammatory cytokines in astrocytes induced by
OGD
It is well established that astrocytes serve a role
in normal and abnormal processes associated with the CNS via the
release of cytokines (1,11). Therefore, the expression of
pro-inflammatory cytokines following OGD induction with or without
acetylpuerarin was investigated. RT-qPCR results revealed that OGD
induction significantly increased the expression of
pro-inflammatory cytokines, including TNF-α (12.77±1.18; P<0.01)
and IL-1β (25.11±2.37; P<0.01); however, pre-treatment with
acetylpuerarin significantly attenuated the OGD-induced
upregulation of TNF-α (7.18±0.59; P<0.01) and IL-1β (13.21±2.15;
P<0.01) mRNA expression (Fig. 4C and
D).
Discussion
Astrocytes are multifunctional glial cells that
serve important roles in neurogenesis and neuron repair in the CNS.
During ischemic injury of the brain, astrocytes may protect and
repair damaged neurons (12,13). Furthermore, the microenvironment of
astrocytes may be modified by numerous pro-inflammatory and
anti-inflammatory cytokines secreted by astrocytes (14). Glial cells regulate inflammatory
processes associated with the production of immunomodulatory
molecules, phagocytosis of cellular debris and recruitment of
immune cells from the peripheral blood (1). Although glial cell activation is
essential for the maintenance of neuronal function under stress or
pain conditions, an excessive response induces cell damage and
hinders regeneration in the injured CNS (15). HIF-1 is a dimeric transcriptional
complex that serves a key role in maintaining oxygen and energy
homoeostasis, and in immune responses (16). Immunomodulatory cytokines, including
IL-1 and TNF-α, are able to stimulate HIF-1-dependent gene
expression, even in normoxic cells (17). In addition, the activation of HIF-1
has been reported to be associated with the PI3K and MAPK signaling
pathways (17). HIF-1 increases the
transcription of several proteins associated with inflammation; for
example, endothelial and inducible NOS and COX-2 upregulate
pro-inflammatory cytokine expression, which propagates
neuroinflammation.
Our previous study demonstrated that acetylpuerarin
inhibits lipopolysaccharide (LPS)-induced arachidonic acid
(AA)-metabolizing enzymes and AA metabolites in astrocytes via
downregulating secretory phospholipase A2 (sPLA2), as well as
phosphorylating extracellular signal-regulated kinase (ERK)1/2,
cytosolic phospholipase A2α (cPLA2α) and NF-κB. Furthermore, our
previous study revealed that acetylpuerarin inhibits the
LPS-induced activation of NF-κB and ERK1/2, as well as the
expression of important regulatory enzymes in primary rat
astrocytes, including sPLA2, cPLA2α, COX-2 and
5-lipoxygenase (8). These reports
indicate that acetylpuerarin possesses anti-inflammatory
properties; however, the underlying mechanism remains unclear. As
such, the effect of acetylpuerarin on HIF-1α and associated
signaling pathways was investigated in the present study. The
results revealed that OGD significantly induces astrocyte damage
and morphological changes, while treatment with acetylpuerarin
attenuates these changes. Furthermore, the results demonstrated
that acetylpuerarin suppresses OGD-induced HIF-1α and NF-κB
expression in astrocytes. In addition, HIF-1 is associated with
MAPK signaling and increased NOS and COX-2 expression, which in
turn stimulates the downstream inflammatory response.
In conclusion, the results of the present study
indicate that acetylpuerarin attenuates the inflammatory response
and the secretion of pro-inflammatory cytokines in OGD-induced
astrocytes. Furthermore, it was demonstrated that acetylpuerarin
suppresses HIF-1α expression and NF-κB activation. However, whether
acetylpuerarin protects astrocytes from ischemic injury and
neuroinflammation via the HIF-1α and NF-κB signaling pathways in
vivo remains unclear. Future studies should investigate the
underlying regulatory mechanism of acetylpuerarin associated with
neuroinflammation in astrocytes in vivo, utilizing animal
models to investigate neurological diseases.
Acknowledgements
Not applicable.
Funding
This study was supported by grants from Shandong
Provincial Natural Science Foundation, China (grant no.
BS2015YY015), the National Nature Science Foundation of China
(grant nos. 81503061 and 81500557) and the Foundation for Excellent
Young and Middle-Aged Scientists of Shandong Province (grant no.
BS2014YY018).
Availability of data and materials
The analyzed data sets generated during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
YX designed the present study, performed cell
culture and wrote the manuscript; PD performed cell intervention
and immunocytochemistry; XZ performed MMT and LDH assays and acted
as the advisor for other experiments; SB performed statistical
analysis of data and wrote the manuscript; GJ peformed western
blotting, RT-qPCR and ELISA; and HL supervised the present study
and performed statistical analysis.
Ethics approval and consent to
participate
The current study was approved by the Scientific
Research Ethics Committee of Qilu Hospital of Shandong University
(Jinan, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Lau LT and Yu AC: Astrocytes produce and
release interleukin-1, interleukin-6, tumor necrosis factor alpha
and interferon-gamma following traumatic and metabolic injury. J
Neurotrauma. 18:351–359. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Dong Y and Benveniste EN: Immune function
of astrocytes. GLIA. 36:180–190. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ivan M, Kondo K, Yang H, Kim W, Valiando
J, Ohh M, Salic A, Asara JM, Lane WS and Kaelin WG Jr: HIFalpha
targeted for VHL-mediated destruction by proline hydroxylation:
implications for O2 sensing. Science. 292:464–468. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hellwig-Burgel T, Stiehl DP, Wagner AE,
Metzen E and Jelkmann W: Review: hypoxia-inducible factor-1
(HIF-1): A novel transcription factor in immune reactions. J
Interferon Cytokine Res. 25:297–310. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wu B, Liu M, Liu H, Li W, Tan S, Zhang S
and Fang Y: Meta-analysis of traditional Chinese patent medicine
for ischemic stroke. Stroke. 38:1973–1979. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Aronica E, Ravizza T, Zurolo E and Vezzani
A: Astrocyte immune responses in epilepsy. Glia. 60:1258–1268.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Xiang Y, Wei X, Chen L, Liu H, Liu X, Wang
T and Zhang X: Anti-inflammatory effect of acetylpuerarin on
eicosanoid signaling pathway in primary rat astrocytes. J Mol
Neurosci. 52:577–585. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Begum G, Kintner D, Liu Y, Cramer SW and
Sun D: DHA inhibits ER Ca2+ release and ER stress in astrocytes
following in vitro ischemia. J Neurochem. 120:622–630. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kintner DB, Luo J, Gerdts J, Ballard AJ,
Shull GE and Sun D: Role of Na+-K+-Cl- cotransport and Na+/Ca2+
exchange in mitochondrial dysfunction in astrocytes following in
vitro ischemia. Am J Physiol Cell Physiol. 292:C1113–C1122. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lee KS, Kim SR, Park SJ, Park HS, Min KH,
Jin SM, Lee MK, Kim UH and Lee YC: Peroxisome proliferator
activated receptor-gamma modulates reactive oxygen species
generation and activation of nuclear factor-kappaB and
hypoxia-inducible factor 1alpha in allergic airway disease of mice.
J Allergy Clin Immunol. 118:120–127. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ridet JL, Malhotra SK, Privat A and Gage
FH: Reactive astrocytes: Cellular and molecular cues to biological
function. Trends Neurosci. 20:570–577. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Song H, Stevens CF and Gage FH: Astroglia
induce neurogenesis from adult neural stem cells. Nature.
417:39–44. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Emsley JG, Arlotta P and Macklis J.D:
Star-cross'd neurons: Astroglial effects on neural repair in the
adult mammalian CNS. Trends Neurosci. 27:238–240. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Benarroch EE: Neuron-astrocyte
interactions: Partnership for normal function and disease in the
central nervous system. Mayo Clin Proc. 80:1326–1338. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hellwig-Bürgel T, Stiehl DP, Wagner AE,
Metzen E and Jelkmann W: Review: hypoxia-inducible factor-1
(HIF-1): A novel transcription factor in immune reactions. J
Interferon Cytokine Res. 25:297–310. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hwang KY, Oh YT, Yoon H, Lee J, Kim H,
Choe W and Kang I: Baicalein suppresses hypoxia-induced HIF-1alpha
protein accumulation and activation through inhibition of reactive
oxygen species and PI 3-kinase/Akt pathway in BV2 murine microglial
cells. Neurosci Lett. 444:264–269. 2008. View Article : Google Scholar : PubMed/NCBI
|