Introduction
Eosinophilic cystitis (EC) is a type of cystitis
with unknown etiology, which was first reported by Brown et
al (1) in 1960 (2). The disease rarely occurs in children
and, to the best of our knowledge, only 59 cases have been
previously reported. As for children, adults are rarely affected by
EC. Incidence in males is larger compared with females and onset
develops at a mean age of 6.0±0.5 years (3,4). EC has
been reported to be local manifestations of systemic
‘hypereosinophilic’ syndromes and allergic diseases, and is
associated with bladder trauma, infections and certain medications,
including iodine or anesthetic ointments (5,6). The
clinical manifestations of EC include hematuria, irritative voiding
symptoms and bladder wall thickening, which is often misdiagnosed
as tumors (7). The pathological
manifestation of EC includes eosinophilic infiltration into layers
of the bladder wall (4). EC may be
caused by immune system regulation disorders, which induce antigen
stimulation and lead to immunoglobulin E (IgE)-mediated eosinophil
activation and degranulation of mast cells, triggering the release
of inflammatory mediators and injuring the bladder wall (8). Adult patients with EC may present with
a variety of urinary symptoms, including frequent urination, urgent
urination, odynuria, hematuria, dysuria and suprapubic pain;
children with EC often have similar symptoms (9,10).
Pathologic biopsy findings are therefore paramount for differential
diagnosis (11). Treatments for EC
include observation, identification of allergens, antibiotics,
antihistamines and steroids (12).
For intractable hematuria, transurethral resection of bladder
lesions and partial cystectomy may also be used (8). The current study presents two cases of
EC, which were diagnosed and treated at the Children's Hospital of
Hunan (Changsha, China) between January 2016 and March 2017.
Clinical manifestations, pathological features, corresponding
treatments and follow-ups are reported.
Case report
Case 1
Patient 1 was a 6-year-old male who first presented
with intermittent gross hematuria, frequent urination, urgent
urination, odynuria and abdominal pain in January 2016 at the
Children's Hospital of Hunan (Changsha, China). The frequency of
urination reached 30 times a day, however the increased rate of
urination was primarily at night. Urination was difficult, although
the patient was able to form a stream. The patient had no history
of hemorrhage or allergy and no lumbago or fever on admission
(Table I). The patient was diagnosed
as having a urinary infection and given one week of
anti-inflammatory treatment (Cefmetazole, 100 mg/kg/d) two days
prior to the aggravation of dysuresia symptoms. Ultrasonography
revealed a thickened bladder wall. Following anti-inflammatory
treatment (Cefmetazole, 100 mg/kg/d) for further 3 days, the
symptoms disappeared. The patient had normal blood pressure on
admission and routine blood tests revealed eosinophil ratios of
24.6, 33.0 and 29.4% (normal range, 0.5–5%) (13) on day 1, at the end of week 1 and at
the end of week 2, respectively. In addition, the red blood cell
count and hemoglobin were observed using peripheral blood smears
(data not shown); all results were normal. During hospitalization,
urine samples were collected by urinary bladder catheterization and
sent to the hospital laboratory. Urine cultures were prepared on
blood agar medium and MacConkey agar (PlastLabor Microbiologica,
Rio de Janeiro, Brazil). A Vitek (bioMérieux, Marcy-l'Étoile,
France) was used to identify and test the susceptibility of
strains. A urinary tract infection was established at ≥50,000
colony forming units/ml of a single microbial growth. These urine
culture tests yielded negative results and a routine urine test and
microscopy revealed 20–25 red blood cells in each high-power field
of view (data not shown). The specific gravity of urine was 1.025
and a urine nitrite test was negative (data not shown). Renal
functions were normal and the concentration of complement C3 was
122 mg/dl. A tuberculin test had negative results and urine
polymerase chain reaction (PCR) revealed no adenovirus or
Mycobacterium tuberculosis (data not shown). Levels of
Dermatophagoides pteronyssinus (D1) and Dermatophagoides
culinae (D2) allergens were grade 2 (14). Ultrasonography indicated obvious
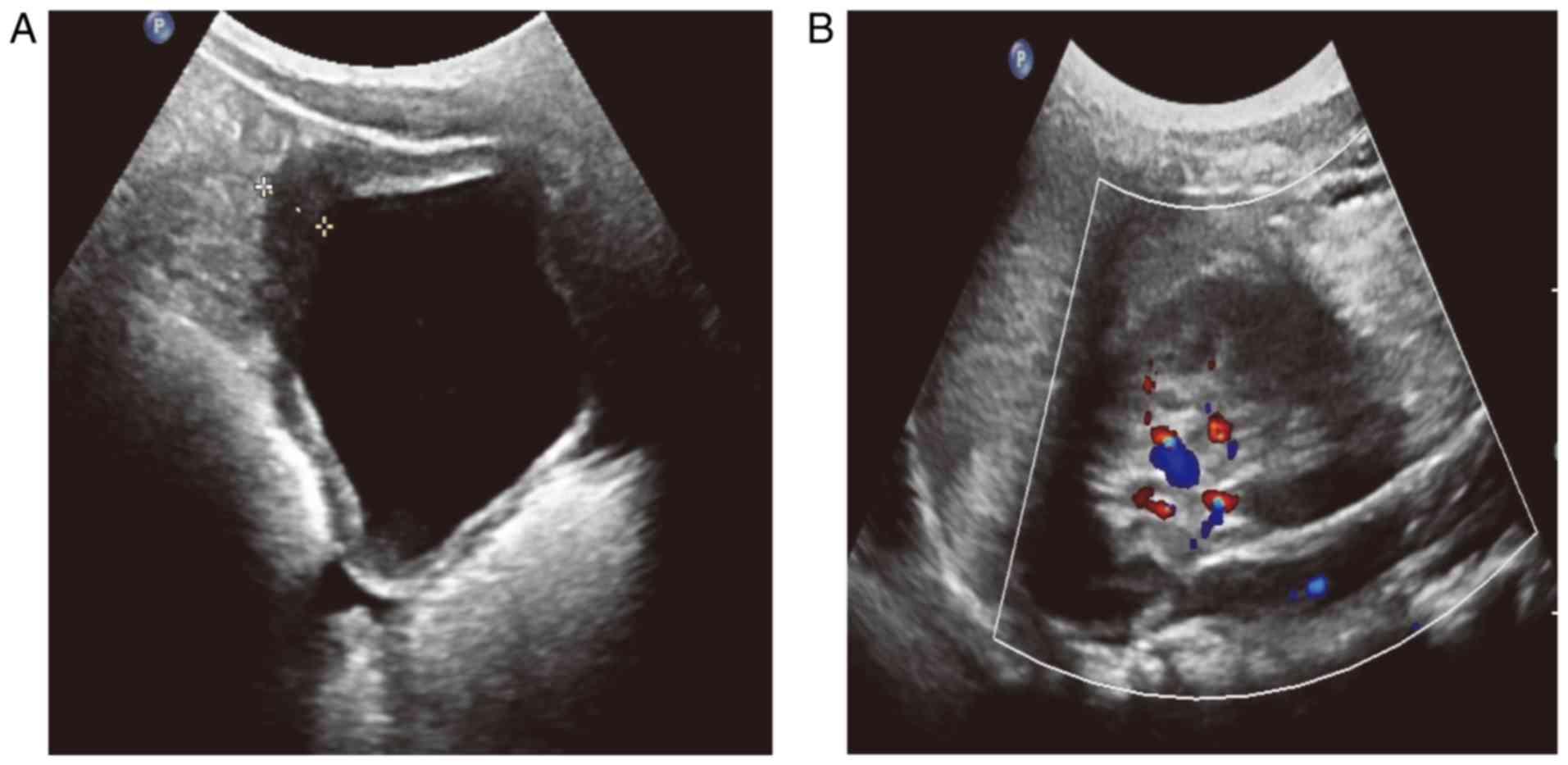
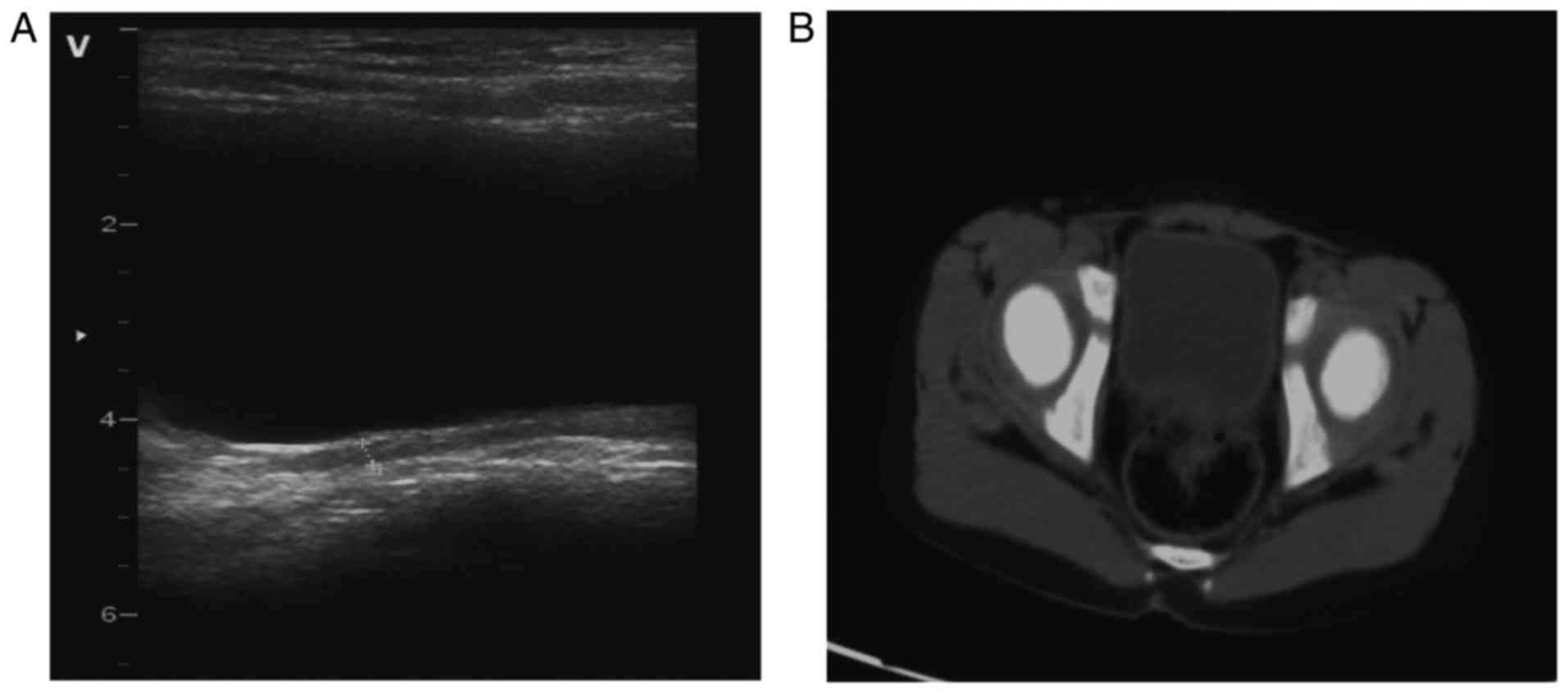
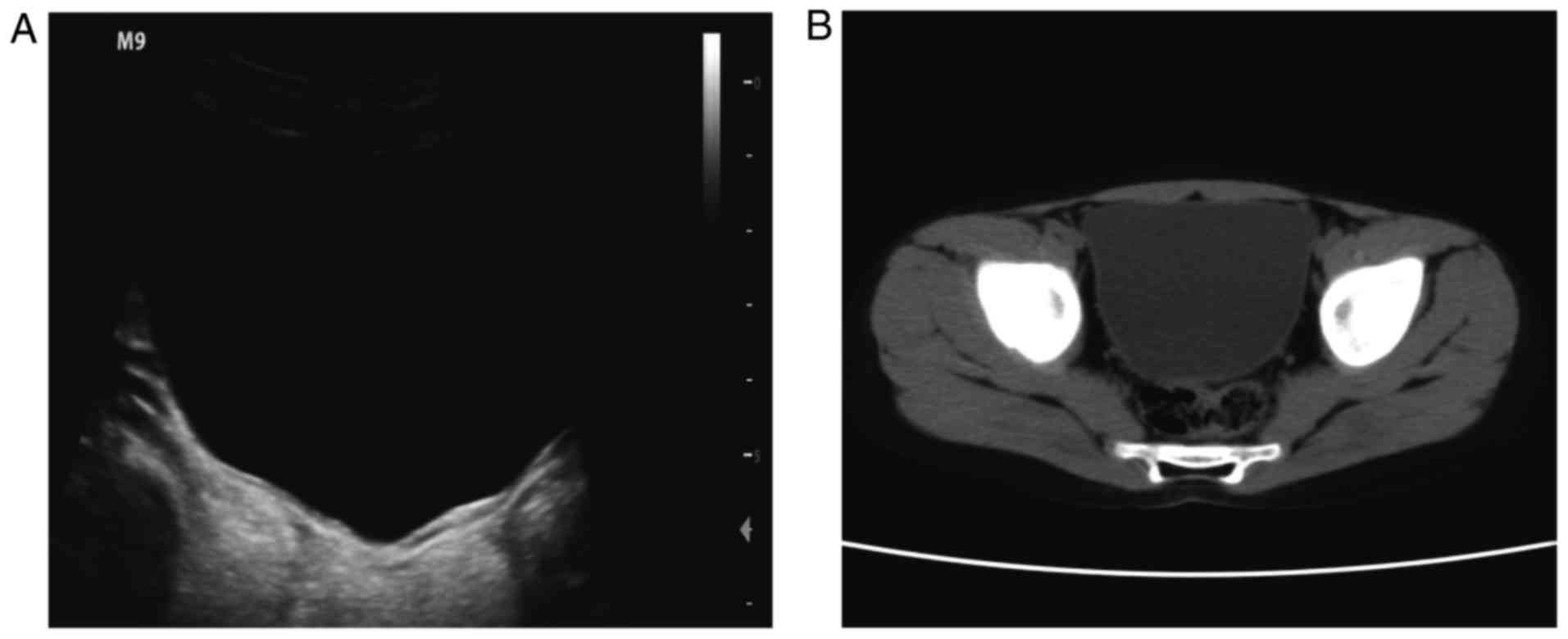
thickening and abundant blood flow in the bladder wall (Fig. 1A and B). Computed tomography revealed
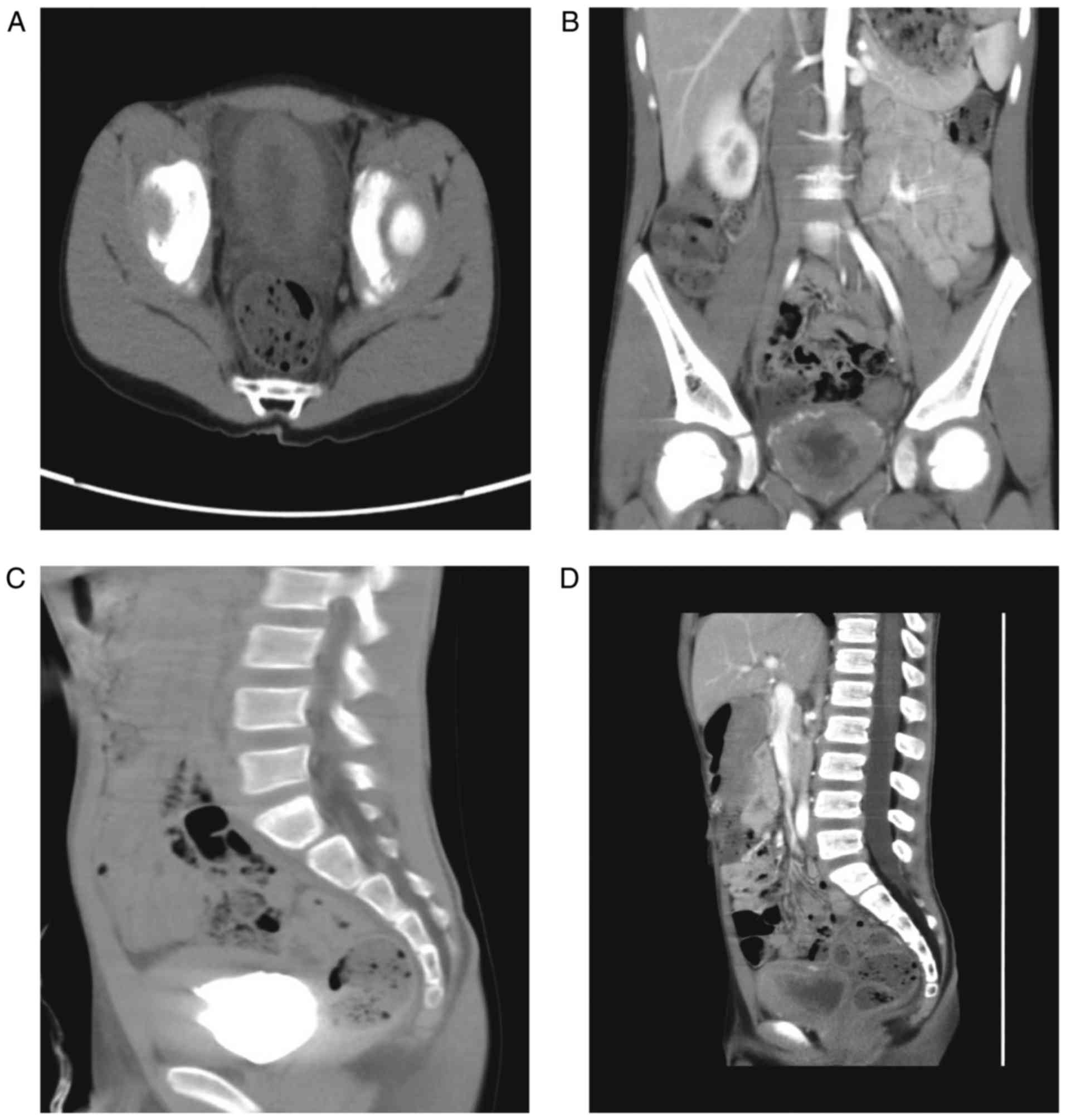
local mucosal lesions with thickening of the right and posterior
wall of the bladder (Fig. 2A-D).
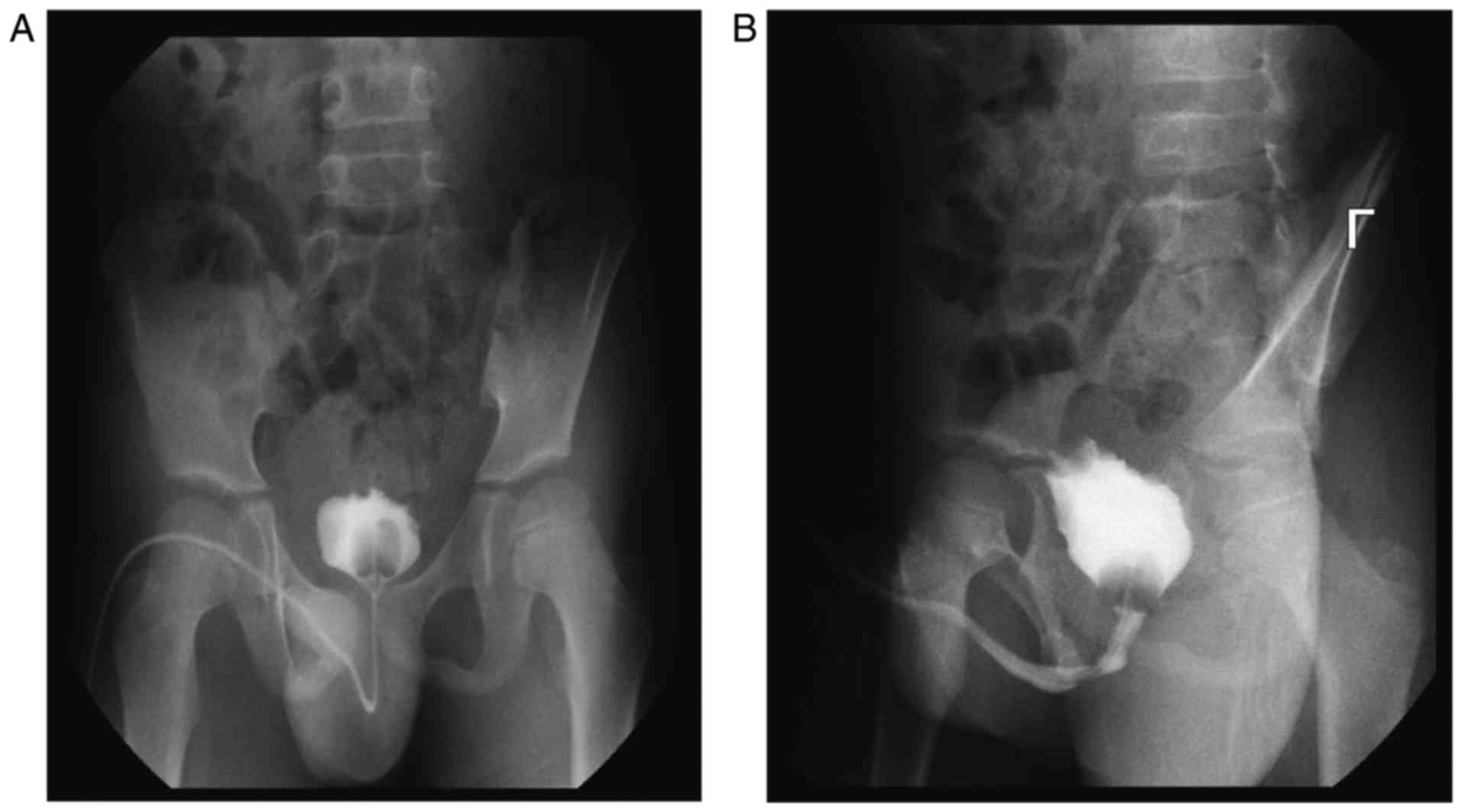
Retrograde angiography of the bladder determined that the bladder
volume decreased and the bladder wall was irregular (Fig. 3A and B). Cystoscopy also revealed
that the bladder volume became smaller and the mucosa at the
bladder floor and neck became red (data not shown). Based on the
above examinations, rhabdomyosarcoma of the bladder was initially
considered as a diagnosis. Biopsy of the lesions through the
urethra was performed and specimen were fixed at room temperature
in 10% formalin for 12–24 h, embedded in paraffin at 62°C for 3 h,
cut into 4-µm-thick sections and stained with a hematoxylin
(Shanghai Regal Biology Technology Co., Ltd., Shanghai, China) and
eosin (Sinopharm Chemical Reagent Co., Ltd., Shanghai, China)
according to the manufacturer's protocol. Specimens were examined
histopathologically using a light microscope (magnification, ×400;
DM 2000 LED; Leica Microsystems, Inc., Buffalo Grove, IL, USA) to
assess the types of lesions (15).
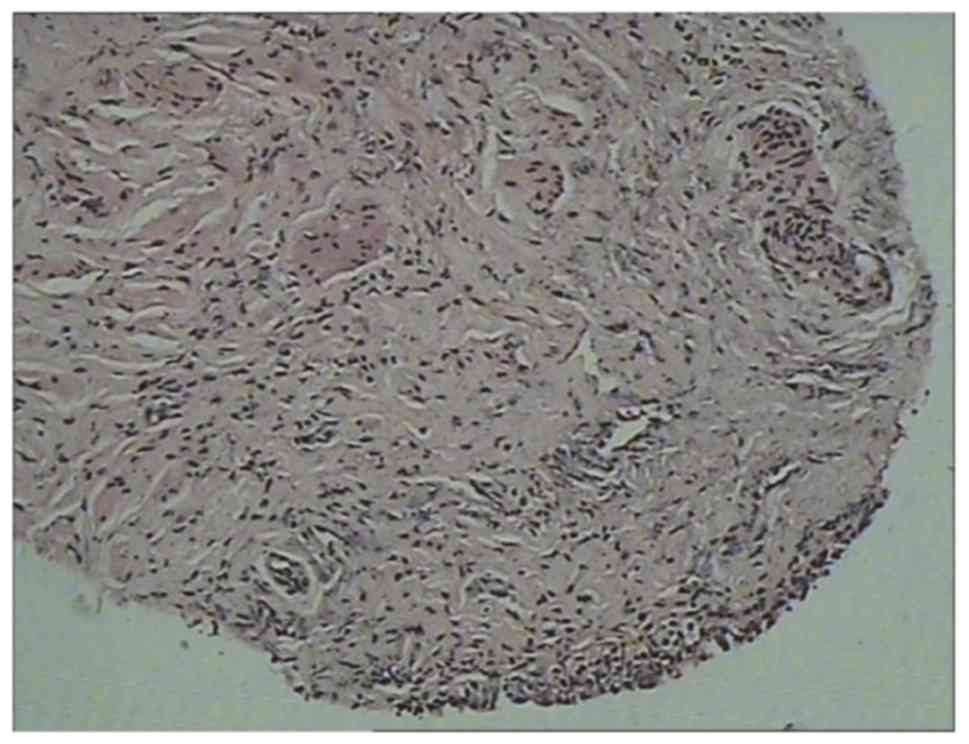
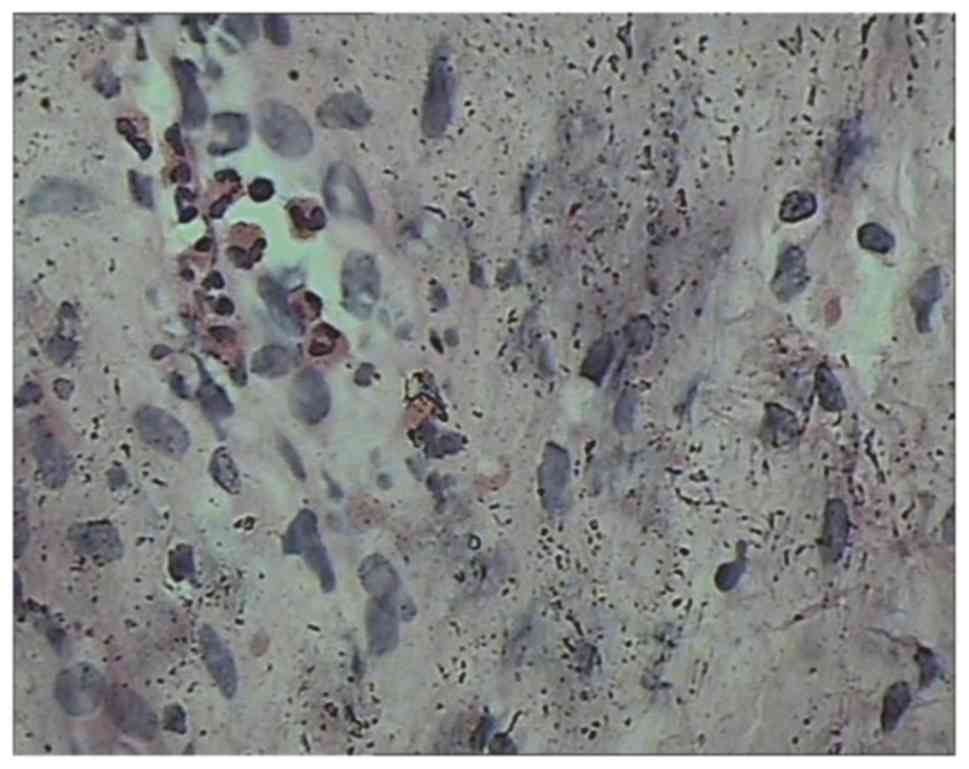
Lesions revealed congestion and edema of the bladder mucosa
(Fig. 4) and infiltration of the
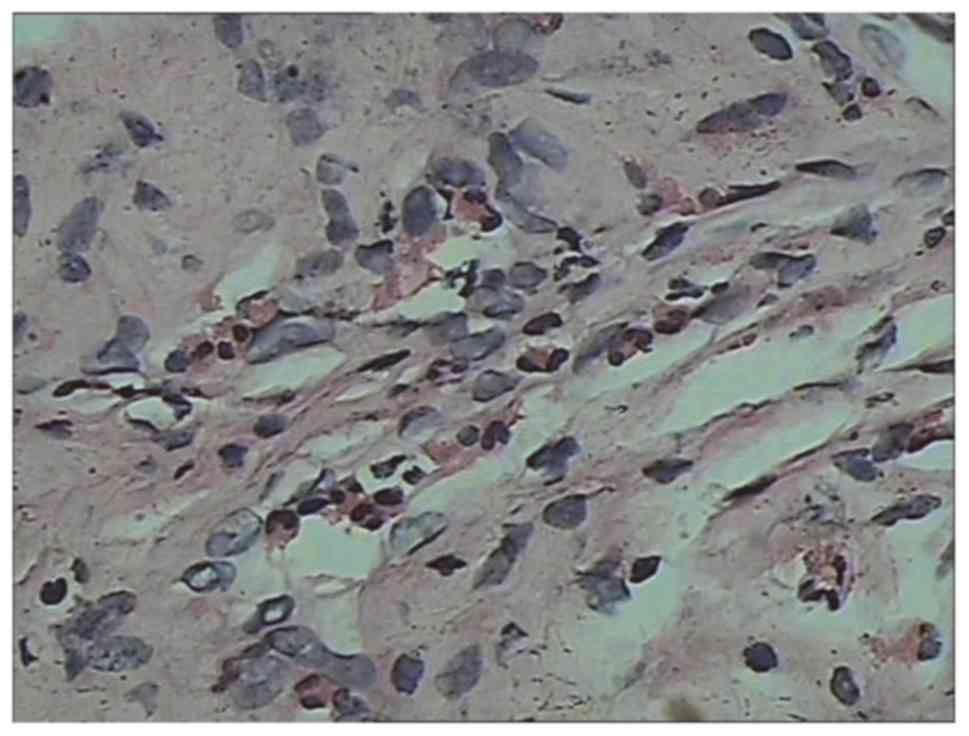
blood vessels and eosinophils into the muscular layer (Fig. 5) accompanied by focal muscle necrosis
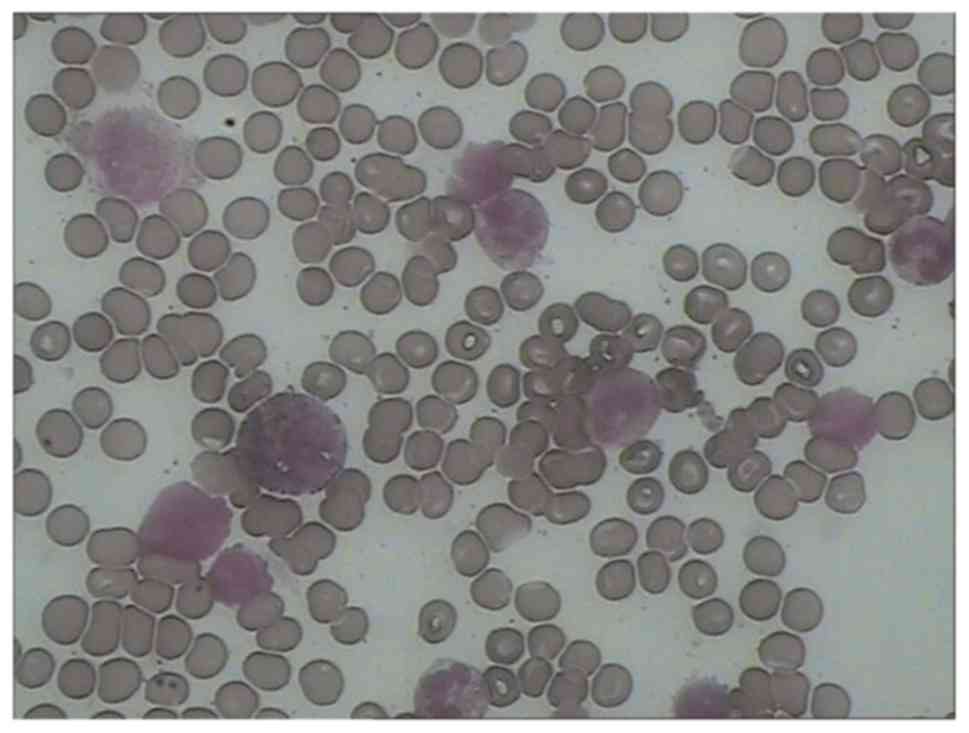
(Fig. 6). A bone marrow biopsy
demonstrated eosinophilia (Fig. 7;
Table II). Following diagnosis, the
patient was given anti-inflammatory (Cefmetazole, 100 mg/kg/d) and
cetirizine hydrochloride (1 ml/day) treatments for a week, followed
by 6 weeks of prednisone dose-reduction therapy (16). One month later, re-examination
revealed that the symptoms had disappeared and imaging results were
normal (Fig. 8A and B). The blood
eosinophil content was 1.19%. Following continued intake of
low-dose prednisone (5 mg/day) for 2 months, the level of blood
eosinophils was normal. Imaging examination also revealed
completely normal results (Fig. 9A and
B; Table III).
 | Table I.Clinical features and manifestations
of the disease following admission to hospital. |
Table I.
Clinical features and manifestations
of the disease following admission to hospital.
|
|
|
| Symptoms |
|
|
|
|---|
|
|
|
|
|
|
|
|
|---|
| Case | Sex | Age (years) | 1 | 2 | 3 | 4 | 5 | Onset time | Allergy history | First diagnosis of
EC |
|---|
| I | M | 6 | + | – | + | – | + | Acute | Yes | Yes |
| II | M | 7 | – | + | + | + | + | Acute | No | Yes |
 | Table II.Imaging examination, laboratory
examination, cystoscopy and pathological examination. |
Table II.
Imaging examination, laboratory
examination, cystoscopy and pathological examination.
|
| Eosinophilic
granulocytes in blood (%) |
| Ultrasonography | Computed
tomography |
|
|
|
|---|
|
|
|
|
|
|
|
|
|
|---|
| Case | 1 | 2 | 3 | 4 | 5 | Routine urine
test | 1 | 4 | 5 | 1 | 4 | 5 | Retrograde
angiography | Cystoscopy | Pathological
examination |
|---|
| I | 24.60 | 33.00 | 29.40 | 1.19 | 2.34 | – | a, b | a, b | – | a, b | a, b | – | a, b | c | d, e |
| II | 7.90 | 5.60 | 0.09 | 1.87 | 2.65 | – | a, b | a, b | – | a, b | a, b | – | a, b | c | d, e |
 | Table III.Treatment, follow-up and prognosis of
patients. |
Table III.
Treatment, follow-up and prognosis of
patients.
| Case | Treatments | Duration of
treatment (weeks) | Follow-up | Prognosis |
|---|
| I | Anti-inflammation,
cetirizine hydrochloride and prednisone | 12 | Monthly | Recovered |
| II | Anti-inflammation
and cetirizine hydrochloride | 12 | Monthly | Recovered |
Case 2
Patient 2 was a 7-year-old male who presented with
acute dysuria, suprapubic pain, frequent urination, urgent
urination, urgent incontinence and odynuria. The patient had no
history of allergies and had normal body development (Table I). On admission in March 2017, the
patient had a normal blood pressure and the eosinophil ratios were
7.9, 5.6 and 0.09% on day 1, at the end of week 1 and at the end of
week 2, respectively. Normal red blood cells and normal hemoglobin
were observed in the peripheral blood smears (data not shown). A
urine culture test yielded negative results, while a routine urine
test and microscopy revealed no abnormalities (data not shown). The
specific gravity of the urine was 1.030 and a nitrite test in the
urine yielded negative results (data not shown). The patient's
renal functions were normal and the concentration of complement C3
was 109 mg/dl. PPD experiments yielded negative results and urine
PCR revealed no adenovirus or Mycobacterium tuberculosis
(data not shown). No clear allergens were observed in the allergen
screening (data not shown). Computed tomography and retrograde
angiography of the bladder revealed local mucosal lesions with
thickening of each side and the posterior wall of the bladder (data
not shown). Cystoscopy revealed that the bladder volume was reduced
and the mucosa at the bladder floor and neck was red (data not
shown). The symptoms of EC included acute dysuria, suprapubic pain,
frequent urination, urgent urination, urge incontinence and
odynuria. Biopsy of the lesions through the urethra revealed
infiltration of blood vessels and eosinophils into the muscular
layer, accompanied by focal muscle necrosis (data not shown). EC
was diagnosed, however there was no eosinophilic proliferation in
the bone marrow biopsy (Table II).
Anti-inflammatory (Cefmetazole, 100 mg/kg/day) and cetirizine
hydrochloride (1 ml/day) treatments were administered for a week.
Two months later, the symptoms had disappeared (data not shown).
Imaging examinations revealed normal results and the blood
eosinophils were normal (Table
III). The data which are not presented for case 2 were
comparable to the images presented for case 1.
Discussion
EC is an uncommon primary inflammatory disorder of
the bladder with uncertain etiology. The incidence of EC in male
adults is increased compared with female adults. Similarly, the
incidence of EC in male children is increased compared with
females; the average age of onset in children is 6 years old
(17,18). The exact cause of EC remains unclear,
however certain studies have suggested that anaphylaxis may be a
trigger (5–7). Allergens may include food, dust mites,
pollen, condom antigens, iodine and anesthetic creams. Asthma and
celiac disease are also associated with EC (8). In the present study, the patient in
case 1 was sensitive to Dermatophagoides pteronyssinus and
Dermatophagoides culinae, while the patient in case 2 had no
specific allergies. The pathogenesis of EC involves IgE-mediated
eosinophil activation, with subsequent mast cell degranulation and
muscle damage (8). Patients with EC
often exhibit a series of urinary symptoms, including frequent
urination, hematuria, suprapubic pain, dysuria and daytime and
nocturnal enuresis; children with EC may have a clear suprapubic
mass (19). The clinical
manifestations in the cases presented in the present study were
consistent with EC; however, the clinical manifestations of EC are
often varied and may be easily confused with nonspecific cystitis
(2). Children with suprapubic masses
should also be differentiated from those with malignant bladder
tumors. EC is a rare condition that can mimic invasive bladder
cancer symptoms. EC diagnosis may be considered if a bladder tumor
is associated with eosinophilia (20). The eosinophil count in the blood was
significantly increased in each of the 2 cases and a bone marrow
biopsy revealed increased eosinophils. In a number of patients with
EC, peripheral blood eosinophilia occurs without reaching the level
of eosinophilia syndrome. Patient 2 only received treatment by
anti-inflammatory and cetirizine hydrochloride without steroid
therapy. During hospitalization, the eosinophils were found to be
decreased in a routine blood test and the patient's symptoms
gradually improved. It has previously been suggested that EC may
also present a self-healing trend (3). In the present study, patient 1
presented with similar symptoms 3 years earlier; an ultrasonography
revealed thickening of the bladder wall and following 3 days of
anti-inflammatory treatment, the symptoms disappeared. In addition,
patient 1 was allergen-positive. The authors hypothesize that the
onset of the disease in case 1 was associated with allergens and
that, with the elimination of inflammatory mediators, symptoms may
disappear (3). It has previously
been reported that urine cultures are positive in EC patients
(4). However, the urine cultures in
each of the cases presented herein were negative. Eosinophils are
rarely observed in urine sediments and are rapidly degraded or
rarely detached from the mucosa (21). Certain EC patients have hematuria
symptoms (22,23). If an imaging examination reveals
bladder wall thickening, which is similar to mass infiltration, a
tumor is suggested. Cystoscopy results usually suggest bladder
rhabdomyosarcoma (6). Intense
inflammatory changes, including congestion and edema of the bladder
wall, may result in intense inflammatory changes in the bladder
wall and associated with this, lesion may produce excrescences,
which resemble vesical rhabdomyosarcoma (24). As EC is very rare in children there
are no ideal guidelines for its treatment and follow-up and
treatment is typically based on experience (25). First-line treatments typically
involve the removal of any suspected allergens, followed by the use
of antihistamines and corticosteroids. It has been reported that
corticosteroids may accelerate the remission of symptoms and
stabilize lysosomal membranes due to their anti-inflammatory
effects (16). For refractory cases,
cyclosporine A is administered orally for 8 months (26). For children with peripheral blood
eosinophilia, montelukast sodium is used (27). Researchers have also tried
intravesical instillation of dimethyl sulfoxide twice a week
(27). EC in children is normally
benign and self-limiting, however it may still develop into bladder
fibrosis and secondary urinary tract obstruction. The diagnosis and
treatment of EC depends on clinical suspicions and
histopathological examination.
In conclusion, EC in children is similar to a tumor,
however it has its own characteristics. Although it is a rare
disease it should be considered when urinary tract symptoms and
bladder wall thickening are observed in children. Bladder biopsy
and histopathological evaluation are important for the diagnosis of
EC and allow for the selection of an appropriate treatment.
Acknowledgements
The authors would like to thank Dr Li Hong Tan for
his support and encouragement. The authors would also like to thank
Dr. Feng Ning and Professor Weijian Chen for their technical
support and pathological advice.
Funding
This work was supported by the Natural science
foundation of Hunan Province (grant no. 10JJ5042).
Availability of data and materials
The data analyzed during the current study are
available from the corresponding author on reasonable request.
Authors' contributions
JuH, FN and LT analyzed and interpreted data
regarding eosinophilic cystitis. JiH obtained imaging data and WC
performed histopathological experiments. JuH contributed to writing
the manuscript. YZ conceived the study and helped revise the
manuscript. All authors have read and approved the final
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
The patients provided written informed consent for
the publication of their data and associated images.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Brown EW: Eosinophilic granuloma of the
bladder. J Urol. 83:665–668. 1960. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Saadi A, Bouzouita A, Ayed H, Kerkeni W,
Cherif M, Ben Slama RM, Derouiche A and Chebil M: Pseudotumoral
Eosinophilic cystitis. Urol Case Rep. 3:65–67. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Park H: Eosinophilic cystitis with
recurrent urinary retention: Case report. Res Rep Urol. 9:51–53.
2017.PubMed/NCBI
|
|
4
|
Runge SB, Høyer S and Winding L:
Macroscopic hematuria and a bladder mass: Eosinophilic cystitis in
a 7-year-old boy. Case Rep Radiol. 2016:93462182016.PubMed/NCBI
|
|
5
|
Sparks S, Kaplan A, DeCambre M, Kaplan G
and Holmes N: Eosinophilic cystitis in the pediatric population: A
case series and review of the literature. J Pediatr Urol.
9:738–744. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Li G, Cai B, Song H and Yang Z: Clinical
and radiological character of eosinophilic cystitis. Int J Clin Exp
Med. 8:533–539. 2015.PubMed/NCBI
|
|
7
|
Ozdoğan EB, Arslansoyu Çamlar S, Bilen S,
Imamoğlu M, Tıraş S, Cansu A and Ozoran Y: An unusual cause of
terminal hematuria in a child: Eosinophilic cystitis. Can Urol
Assoc J. 8:E867–E871. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ladocsi LT, Sullivan B and Hanna MK:
Eosinophilic granulomatous cystitis in children. Urology.
46:732–735. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Rossanese M, Palumbo V, Sioletic S,
Crestani A, Giannarini G and Ficarra V: Surgical treatment of
Eosinophilic cystitis in adults: A report of two cases and a
literature review. Urol Int. Mar 19–2018.(Epub ahead of print).
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Shaocong Z, Yufeng L, Dao W, Bai L,
Shufang S and Linlin W: Eosinophilic cystitis in children: A report
of 7 cases and literature review. J Clin Pediatr. 35:304–306.
2017.
|
|
11
|
Zhou AG, Amin A, Yates JK, Diamond DA,
Tyminski MM, Badway JA, Ellsworth PI, Aidlen JT and Owens CL: Mass
forming Eosinophilic cystitis in pediatric patients. Urology.
101:139–141. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Mosholt KS, Dahl C and Azawi NH:
Eosinophilic cystitis: Three cases, and a review over 10 years. BMJ
Case Rep 2014. pii:bcr2014205708. 2014.
|
|
13
|
Saad AG: Normal quantity and distribution
of mast cells and eosinophils in the pediatric colon. Pediatr Dev
Pathol. 14:294–300. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Casanovas M, Fernández-Caldas E, Alamar R
and Basomba A: Comparative study of tolerance between unmodified
and high doses of chemically modified allergen vaccines of
Dermatophagoides pteronyssinus. Int Arch Allergy Immunol.
137:211–218. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Al Johi RS, Seifeldein GS, Moeen AM,
Aboulhagag NA, Moussa EM, Hameed DA and Imam HM: Diffusion weighted
magnetic resonance imaging in bladder cancer, is it time to replace
biopsy? Cent European J Urol. 71:31–37. 2018.PubMed/NCBI
|
|
16
|
Gerharz EW, Grueber M, Melekos MD,
Weingaertner K, Barth P and Riedmiller H: Tumor-forming
eosinophilic cystitis in children: Case report and review of
literature. Eur Urol. 25:138–141. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Thomas JC and Ross JH: Eosinophilic
cystitis in a child presenting with a bladder mass. J Urol.
171:1654–1655. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sujka SK, Fisher JE and Greenfield SP:
Eosinophilic cystitis in children. Urology. 40:262–264. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Thijssen A and Gerridzen RG: Eosinophilic
cystitis presenting as invasive bladder cancer: Comments on
pathogenesis and management. J Urol. 144:977–979. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Werbrouck C, Marrannes J, Verhamme L,
Steenkiste E, Laridon E and Van Holsbeeck B: Eosinophilic cystitis
mimicking bladder tumor. JBR-BTR. 97:3752014.PubMed/NCBI
|
|
21
|
Popescu OE, Landas SK and Haas GP: The
spectrum of eosinophilic cystitis in males case series and
literature review. Arch Pathol Lab Med. 133:289–294.
2009.PubMed/NCBI
|
|
22
|
Chia D: Eosinophilic cystitis and
haematuria: Case report of a rare disease and common presentation.
Int J Surg Case Rep. 24:43–45. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Venkatesh KS and Bhat S: Eosinophilic
cystitis: A rare cause of hematuria in children. Case Rep Nephrol.
2012:7102302012.PubMed/NCBI
|
|
24
|
Redman JF and Parham DM: Extensive
inflammatory eosinophilic bladder tumors in children: Experience
with three cases. South Med J. 95:1050–1052. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Castillo J Jr, Cartagena R and Montes M:
Eosinophilic cystitis: A therapeutic challenge. Urology.
32:535–537. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Aleem S, Kumar B, Fasano MB, Takacs E and
Azar AE: Successful use of cyclosporine as treatment for
eosinophilic cystitis: A case report. World Allergy Organ J.
9:222016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zaman SR, Vermeulen TL and Parry J:
Eosinophilic cystitis: Treatment with intravesical steroids and
oral antihistamines. BMJ Case Rep 2013. pii:bcr2013009327.
2013.
|