Introduction
Fat is an important component of a healthy human
diet and is essential for the synthesis of hormones, as well as the
delivery of essential vitamins throughout the body (1). However, excessive consumption of
dietary fat may increase the cholesterol levels in the human body,
enhancing the chance of heart disease, cancer and type 2 diabetes
(2).
It is well known that lipids may be oxidised in
vitro or in vivo by multiple oxidants, the products of
which are key factors in conditions including atherosclerosis,
neurological disorders, cancer and aging (3–5).
Although oxidation may trigger a chain of chemical reactions and
damage cells, certain antioxidants are able to terminate these
chain reactions, exerting beneficial effects with regard to health
maintenance and disease prevention (6–8).
Oxidation due to exposure to oxygen and sunlight makes unsaturated
fats turn rancid and become discoloured, and therefore,
antioxidants are widely used as food additives to protect against
degradation (9).
It has been reported that certain plants (e.g.,
berries) contain abundant and numerous bioactive compounds, natural
phytochemicals, which may be used as antioxidants to protect lipids
from undesired oxidative modification (3,6).
Chicoric acid (CA) is a caffeic acid derivative, which extensively
occurs in certain edible plants and vegetables, including chicory
(Cichorium intybus), lettuce and those of the genera
Echinacea and dandelion (Taraxacum). CA has been regarded as a
nutraceutical with potent antioxidant activities (10,11), and
previous studies have indicated that CA attenuated inflammation
through suppression of the phosphoinositide-3 kinase/Akt and
nuclear factor-κB signalling pathways to markedly inhibit a
high-fat diet-induced inflammatory response (12,13).
However, the potential of CA as a natural food antioxidant has
remained to be determined.
In the present study, the antioxidant effects and
inhibition of foodborne pathogens by CA was systematically
evaluated in vitro, and the attenuation of inflammation as
well as the inhibition of lipid droplet formation (which evaluated
the inhibition of adipogenic differentiation in pre-adipogenic
cells and the regulation of lipid metabolism by adipocytes) was
studied using RAW264.7 and 3T3-L1 cells, respectively. To the best
of our knowledge, the present study was the first to investigate
the potential use of CA as a natural food antioxidant.
Materials and methods
Antioxidant activity of CA
The clearance effect of CA on the
2,2-diphenyl-1-picrylhydrazyl (DPPH) radical was estimated as
follows: Vincristine (VC) or CA at 95, 190 or 380 mg/ml was mixed
with 100 mM Tris-HCl buffer (pH=7.5) and then added to 1 ml of 500
µM DPPH in ethanol (250 µM). The mixture was vigorously agitated at
room temperature in the dark and the absorbance of the resulting
solution was spectrophotometrically determined by measuring the
absorption at 517 nm (14,15).
To determine the ·OH radical scavenging activity, 1
ml of reaction mixture containing 2.8 mM deoxyribose, 20 mM
phosphate buffer (pH 7.4), CA or VC (95, 190 or 380 mg/ml), 100 mM
FeCl3, 104 mM EDTA, 1 mM H2O2 and
100 mM ascorbic acid was incubated at 37°C for 60 min.
Subsequently, 50 ml 2% (w/v) butylated hydroxytoluene, 1 ml 2.8%
(w/v) trichloroacetic acid and 1 ml 1% (w/v) 2-thiobarbituric acid
were added and the mixture was incubated at 80°C for 20 min. The
reaction was terminated by incubation in an ice-water bath for 5
min, 2 ml n-butanol was added to each tube and the degree of
deoxyribose degradation was spectrophotometrically determined by
measuring the absorption at 532 nm (16).
To assess the Fe2+ chelation ability, CA
or VC solution was mixed with 2 mM FeCl2 and 5 mM
ferrozine at a ratio of 10:1:2 in PBS. After incubation for 10 min
at room temperature, the absorbance was measured at 562 nm
(14).
The oxygen reduction ability of CA was determined as
follows: CA or VC solution was mixed with potassium ferricyanide
(the final concentration was 0.5%) in phosphate buffer (pH 6.6).
The mixture was incubated at 50°C for 20 min. Subsequently, an
equal volume of 1% trichloroacetic acid was added to the mixture,
which was centrifuged at 5,000 × g for 10 min. The upper phase was
mixed with distilled water and 0.1% FeCl3 at a ratio of
1:1:2, and the absorbance was measured at 700 nm (14).
Anti-oil oxidation effect of CA
CA was added to 30 g rapeseed oil (Shandong Luhua
Group Co., Ltd., Laiyang, China) to reach the final concentrations
of 0.02 and 0.05%. Pure rapeseed oil was used as a control group
and rapeseed oil with 0.02% tert-butylhydroquinone (TBHQ) was used
as a positive control. All samples were heated to 60°C over 30 min
and then maintained at this temperature. The peroxide value (POV)
of each system was measured after 1, 3 and 5 days. The POV was
tested using the analytical method of the sanitary standard of
edible lard and rapeseed oil, People's Republic of China
(GB/T5009.37-1996) (17).
Anti-microbial activity of CA
The agar diffusion assay was performed to examine
the anti-microbial activity of different concentrations of CA on
Lactobacillus plantarum (isolated from human feces; State
Key Laboratory of Food, Nanchang University, Nanchang, China),
L. rhamnosus (Culturelle; i-Health, Inc., Cromwell, CT,
USA), L. crispatus (isolated from vaginal secretion of
women; Institute of Translational Medicine, Nanchang University)
and Bifidobacterium longum isolated from a probiotic drug
(Bifico; Shanghai Xinyi Pharmaceutical Factory Co., Ltd., Shanghai,
China), as well as Salmonella enteritidis, Shigella typhimurium,
Escherichia coli and Listeria monocytogenes (isolated
from human feces; State Key Laboratory of Food, Nanchang
University). The isolation of the microorganisms was performed as
reported by previous studies by our group (15,18–22).
Cultures of the probiotics L. plantarum, L. rhamnosus, L.
crispatus, B. longum grown anaerobically for 24 h in De Man,
Rogosa and Sharpe medium (Oxoid Ltd; Thermo Fisher Scientific,
Inc., Waltham, MA, USA) were centrifuged at 8,000 × g for 10 min at
4°C to obtain supernatants. Overnight (12 h) cultures of pathogenic
microorganisms including S. enteritidis, S. typhimurium, E.
coli and L. monocytogenes were spread on the surface of
the Lysogeny Broth agar (Oxoid, Ltd.; Thermo Fisher Scientific,
Inc.) plates. Aliquots (200 µl) of the supernatant were loaded into
an Oxford cup (outer diameter, 7.8±0.1 mm; inner diameter, 6.0±0.1
mm; height, 10.0±0.1 mm), which was placed on the surface of the
agar. The size of the clear zone formed around the Oxford cup was
measured.
Oil red O staining for intracellular
triglycerides
3T3-L1 cells (Pre-adipocytes derived from mouse 3T3
cells that is used in biological research on adipose tissue;
ATCC-CL-173; American Type Culture Collection, Manassas, VA, USA)
were maintained in Dulbecco's modified Eagle's medium (DMEM)
supplemented with 10% fetal bovine serum (FBS; both Gibco; Thermo
Fisher Scientific, Inc.) and antibiotics (100 U/ml penicillin and
100 µg/ml streptomycin) at 37°C in a humidified atmosphere
containing 5% CO2, and intracellular lipid accumulation
in adipocytes was assessed using Oil Red O staining. In brief, In
brief, 3T3-L1 cells were cultured in a 6-well plate at a density of
1×105 cells/well and CA was incubated with 3T3-L1 cells
at final concentrations of 12.5, 25 or 50 µM for 24 h at 37°C in a
5% CO2 incubator. The cells were gently washed with
ice-cold PBS (pH 7.4) twice and fixed with 10% formalin at room
temperature for at least 30 min. Subsequently, the wells were
washed with 60% isopropyl alcohol for 5 min and then exhaustively
with PBS. The wells were allowed to dry completely prior to the
addition of filtered Oil Red O solution, followed by incubation for
30 min at room temperature. The stained lipid droplets in 3T3-L1
pre-adipocytes were exhaustively rinsed three times with PBS.
Stained oil droplets were extracted with 100% isopropanol for 10
min to quantify intracellular lipids. The dye extract was then
immediately removed by gentle pipetting and its absorbance was
measured spectrophotometrically at 520 nm. The amount of Oil Red
O-stained material (OROSM, %) was compared to that in control wells
containing cell culture medium without CA and calculated according
to the following formula: OROSMe% = absorbance of tested sample
extract/absorbance without tested sample extract ×100 (23).
Anti-inflammatory effects of CA
As primary macrophages isolated from humans are
difficult to culture due to their high sensitivity to culture
conditions, which makes them prone to losing their biological
characteristics (24), the RAW264.7
murine macrophage cell line (passage 8–20; ATCCTIB-71; American
Type Culture Collection) was used in the present study. RAW264.7
cells were maintained in DMEM supplemented with 10% FBS and
antibiotics (100 U/ml penicillin and 100 µg/ml streptomycin) at
37°C in a 5% CO2 incubator.
RAW 264.7 cells were seeded into 24-well plates
(5×104 cells per well), allowed to attach for 24 h and
then washed with PBS. Cells were treated with PBS (Control group),
lipopolysaccharide (LPS; 1 µg/ml each), LPS + 5 µM CA, LPS + 10 µM
CA, LPS + 20 µM CA or LPS + 40 µM CA for 24 h, and the supernatants
were harvested. The amounts of interleukin (IL)-1β and tumor
necrosis factor (TNF)-α secreted into the cell supernatants were
determined using ELISA kits for IL-1β (cat. no. 88-7013) and TNF-α
(cat. no. 88-7324; both Thermo Fisher Scientific, Inc., Waltham,
MA, USA).
Statistical analysis
Values are expressed as mean ± standard deviation.
Data were analyzed with SPSS software (version 30.0; IBM Corp.,
Armonk, NY, USA) using one-way analysis of variance followed by the
least-significant difference post-hoc test. P<0.05 was
considered to indicate a statistically significant difference.
Results
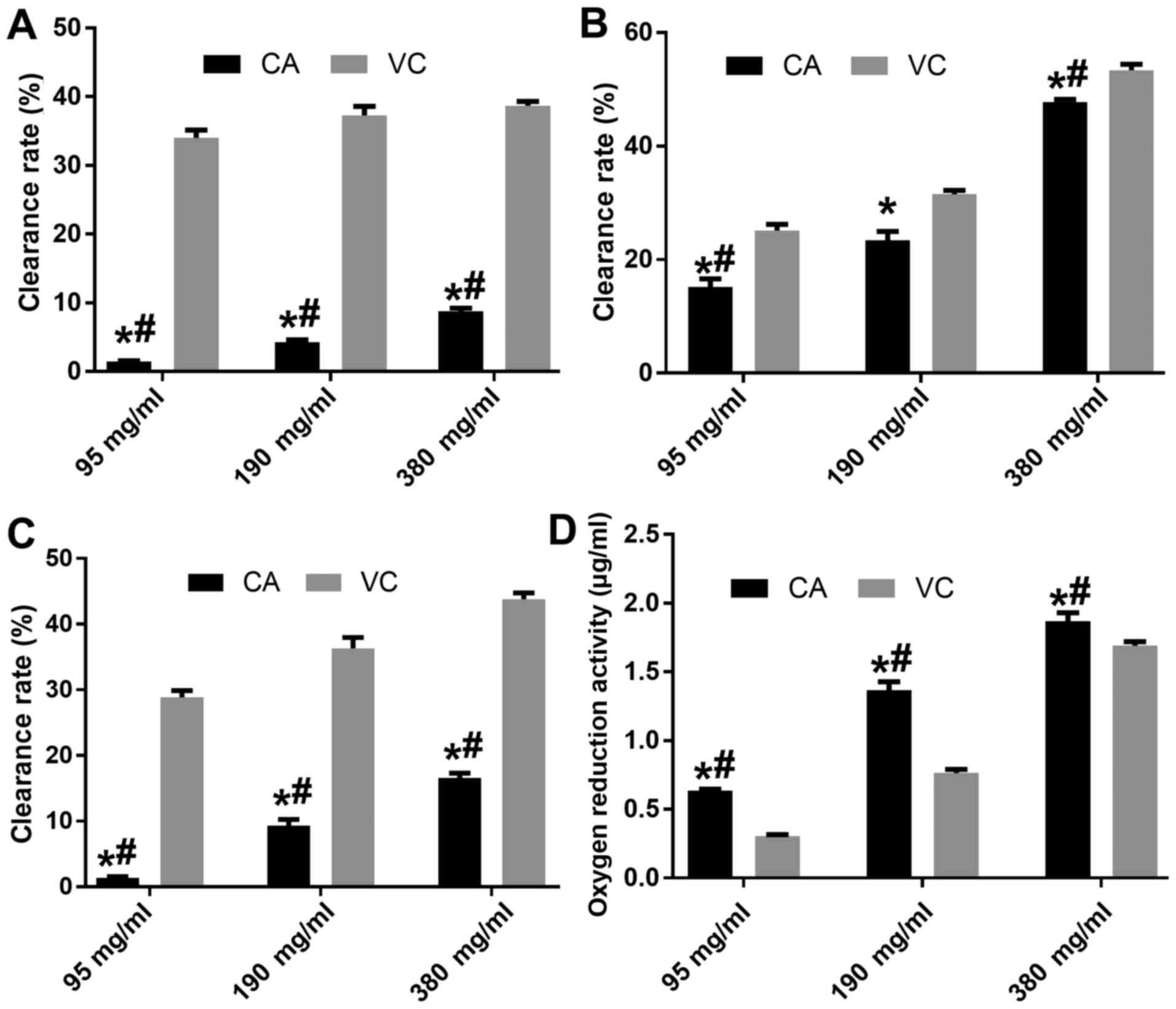
Anti-oxidative activity of CA
The anti-oxidative activity of different doses of CA
was evaluated with VC used at the same doses as a positive control
(Fig. 1). The results indicated that
CA had a dose-dependent anti-oxidative activity, and that the
clearance effect of CA on ·OH, DPPH and Fe2+ was weaker
than that of VA at the same concentration (P<0.05), while 380
mg/ml CA possessed a better clearance effect of DPPH compared with
95 mg/ml VC (Fig. 1A-C).
Furthermore, CA had a dose-dependent oxygen-reduction activity,
which was higher compared with that of VC at the same concentration
(P<0.05; Fig. 1D).
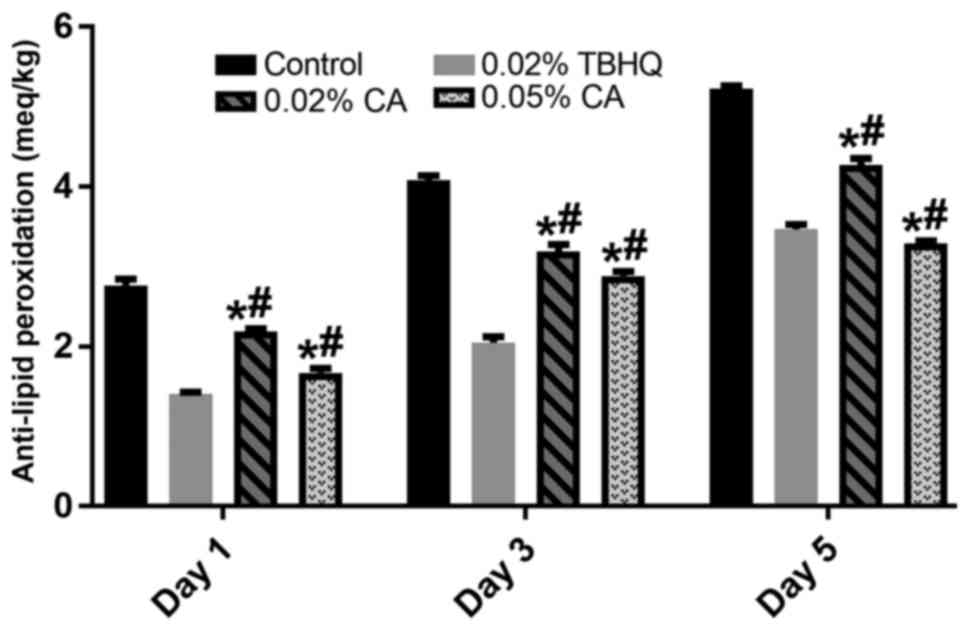
Detection of the presence of peroxide provides
initial evidence of rancidity in unsaturated dietary fat, with POV
being the most widely used method. As displayed in Fig. 2, 0.02 and 0.05% CA significantly
inhibited the oxidation of rapeseed oil on days 1, 3 and 5 compared
with that in the control group (P<0.05). Compared with the 0.02%
TBHQ group, the anti-oxidation effect of 0.02 and 0.05% CA was
lower on days 1 and 3, while CA at 0.05% possessed a better
inhibitory effect than that of TBHQ from the 5th day.
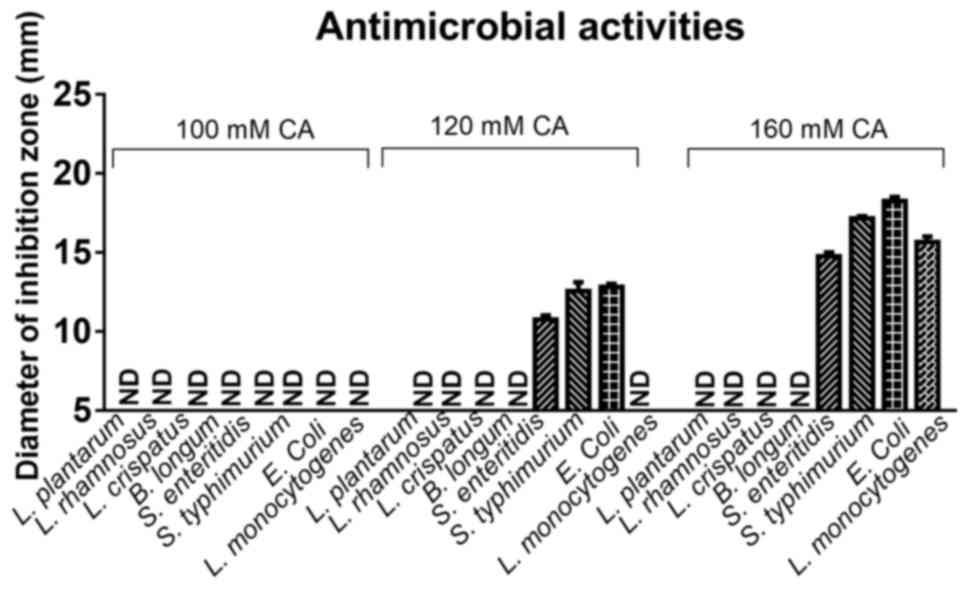
Anti-bacterial activity of CA
To test the influence of different concentrations of
CA on the human intestinal microbiota, CA at 100, 120 and 160 mM
was used to evaluate its effect on the growth of various beneficial
microbes and pathogens of the microbiota. These concentrated were
selected from previous studies and preliminary results (10,13). As
presented in Fig. 3, 100, 120 and
160 mM exerted no effect on the growth of the beneficial microbes
L. plantarum, L. rhamnosus, L. crispatus, B. longum, while
120 and 160 mM CA significantly inhibited the growth of the
pathogens S. enteritidis, S. typhimurium, E. coli and L.
monocytogenes.
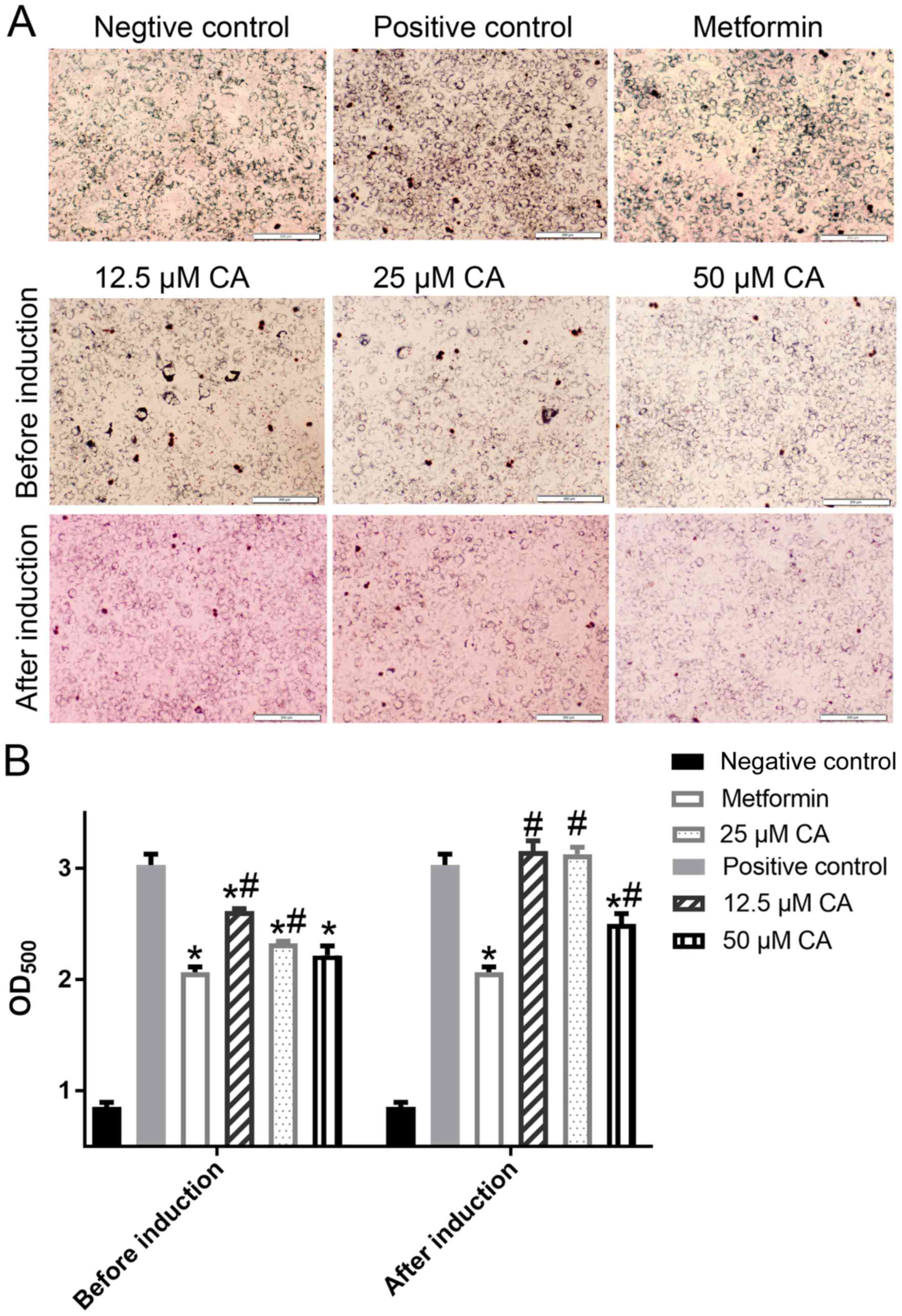
Anti-obesity activity of CA
Adipose tissue has an important role in maintaining
lipid homeostasis and energy balance by storing triglycerides or
liberating free fatty acids in response to changes in energy
demands. As presented in Fig. 4,
when CA was added prior to the induction of 3T3-L1, 12.5, 25 and 50
µM CA significantly inhibited the formation of fat droplets, and
the inhibitory effects of 50 µM CA possessed a similar effect to
that of metformin. However, when CA was added after induction, no
obvious inhibition was observed in the 12.5 and 25 µM CA groups,
with only CA at 50 µM achieving a significant inhibition of fat
droplet formation in 3T3-L1 cells.
Anti-inflammatory activity of CA via
suppressing pro-inflammatory cytokines
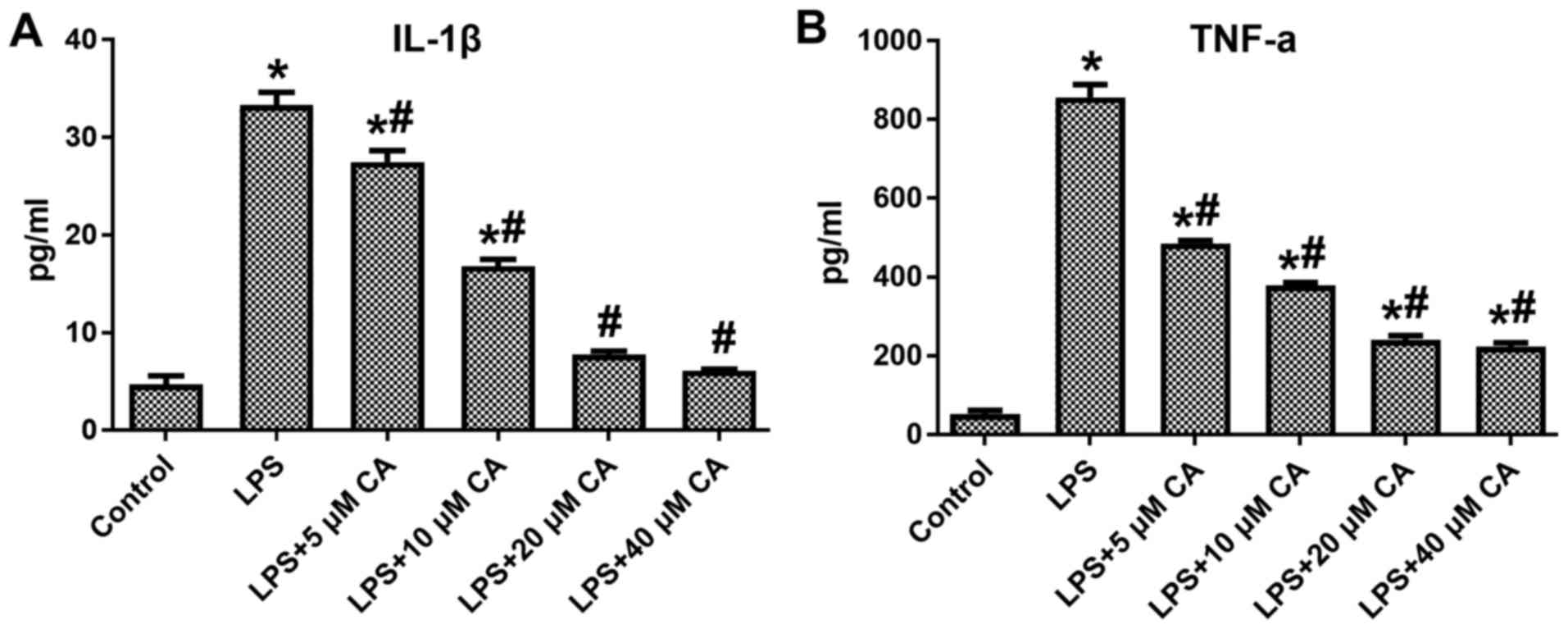
During inflammation, excess levels of cytokines
(IL-1β and TNF-α) damage cells and tissues, and may also activate
macrophages and cause inflammation-associated diseases. To assess
the anti-inflammatory effects of CA on the production of IL-1β and
TNF-α, RAW264.7 cells were treated with different concentrations of
CA (5, 10, 20 or 40 µM). The results indicated that at all
concentrations, CA significantly inhibited the LPS-induced
production of IL-1β and TNF-α by RAW264.7 cells, and a
dose-dependent effect of CA was observed (Fig. 5).
Discussion
Lipid oxidation is the oxidative degradation of
lipids, which most frequently occurs in polyunsaturated fatty acids
that contain multiple double bonds. Antioxidants, such as vitamin
C, are reducing agents acting as antioxidants and may reduce
oxidizing substances, such as hydrogen peroxide; however, they also
reduce metal ions that generate free radicals through the Fenton
reaction (25). Plants and animals
have developed complex and overlapping systems of oxidants and
antioxidants to balance the oxidative state of components of their
biomolecular system. The antioxidant activity of natural products
is an area of current research.
Oxidative stress may be considered as either a cause
or consequence of certain diseases; antioxidants are not only used
as food additives to help protect against food deterioration, but
also serve as antioxidant dietary supplements. Certain
antioxidants, including β-carotene, vitamin A, vitamin E and
selenium, either individually or in combination, have been
determined to be associated with survival (25,26), and
may lower the risk of cancer (27)
and cardiovascular diseases (28).
In the present study, the phytochemical CA, which possesses potent
antioxidant and anti-inflammatory effects (10,11), was
assessed for its potential as a food antioxidant and to improve the
health of humans.
First, the anti-oxidative activity of CA was
evaluated. At all of the tested concentrations, CA exerted a marked
anti-oxidative effect in terms of clearing ·OH, DPPH and
Fe2+, and displaying an oxygen-reducing activity, all of
which was in a dose-dependent manner. Although according to most
studies, 95 mg/ml VC is sufficient for use as a positive control,
190 and 380 mg/ml VC were also used in the present study to compare
their anti-oxidative activity with that of the same doses of CA
(4,14). For ·OH and Fe2+ clearance,
the results indicated that the anti-oxidative activity of CA was
significantly lower than that of VC at the same concentrations
(P<0.05), and that the clearance rate of ·OH and Fe2+
in the 380 mg/ml group was only 8.75 and 16.55%, respectively,
which was significantly lower than in the 95 mg/ml VC group
(P<0.05). For DPPH, although the clearance of CA was
significantly lower than that in the VC group at the same doses,
380 mg/ml CA achieved a DPPH clearance of 47.8%, which was
significantly higher than that in the 95 mg/ml VC group
(P<0.05). Furthermore, the 95, 190 and 380 mg/ml CA groups
possessed a significantly higher oxygen-reduction activity than VC
at the same dose, and 380 mg/ml CA possessed a high
oxygen-reduction activity of 1.87 µg/ml (P<0.05). Therefore, due
to its high anti-oxidative activity, CA may have potential use a
natural food antioxidant and may be used as an antioxidant to
protect human health.
Autoxidation is a free radical reaction involving
oxygen and leading to the deterioration of fats and oils, causing
off-flavours and off-odours. The POV is useful to assess the extent
to which spoilage has advanced. In the present study, 0.02 and
0.05% CA significantly reduced the POV compared with that in the
control group at days 1, 3 and 5 (P<0.05). Although the POV
values for 0.02 and 0.05% CA at days 1 and 3 were significantly
lower than those in the TBHQ group (P<0.05), 0.05% CA achieved a
significantly higher POV on day 5 than that in the 0.02% TBHQ group
(P<0.05), indicating that the CA has a potent anti-oxidation
effect on the oil and that it has a better long-term protection
ability than TBHQ.
Numerous studies have indicated that intestinal
microbes have an important role in human health (20,29–32).
Therefore, after confirmation of the antioxidant effect of CA, the
present study further investigated the potential effect of CA on
common beneficial microbes and pathogens in the human microbiota.
No anti-microbial activity of CA on L. plantarum, L. rhamnosus,
L. crispatus and B. longum was observed at
concentrations of 100, 120 and 160 mM, while the concentration of
120 mM obviously inhibited the growth of pathogens, including S.
enteritidis, S. typhimurium and E. coli. In addition,
160 mM CA had a potent inhibitory effect on all of the tested
pathogens, namely S. enteritidis [inhibition zone diameter
(IZD), 15 mm], S. typhimurium (IZD, 17 mm), E. coli
(IZD, 18 mm) and L. monocytogenes (IZD, 16 mm). The absence
of inhibitory effects of CA at a final concentration of ~120 mM on
beneficial microbes of the healthy intestinal flora and its potent
inhibitory effects on pathogens of the microbiota indicated that
supplementation with CA may support human intestinal health.
The present study then assessed whether CA may be
able to offset the harmful effects caused by excessive oil intake.
For this, the effects of CA to inhibit intracellular lipid
accumulation and inflammation were assessed using 3T3-L1 and
RAW264.7 cells, respectively. In a preliminary experiment, the
effects of different concentrations of CA on cell viability were
assessed, revealing that CA at concentrations of <50 µM had no
marked effect on the growth of 3T3-L1 cells and RAW264.7 cells
(data not shown). When CA was added to the oil prior to adipogenic
induction, 12.5, 25 and 50 µM CA significantly inhibited fat
droplet formation compared with that in the positive control group
(P<0.05), with 50 µM CA having a similar effect to that of
metformin. However, when CA was added to the oil after induction,
only 50 µM CA significantly inhibited the fat droplet formation
compared with that in the positive control group (P<0.05), and
its inhibitory effects were significantly lower than those in the
metformin group. Oil red O staining indicated that although a high
dose of CA obviously reduced the presence of fat droplets even
after they had been formed, and was therefore able to break down
the fat droplets, only low doses achieved a significant inhibition
if CA was added prior to adipogenic induction to inhibit droplet
formation. The desired effect of CA to inhibit the formation of fat
droplets also confirmed that CA treatment may reduce the likelihood
of obesity in healthy individuals. Therefore, when CA is used as a
natural food antioxidant and mixed with oil to enter the human
intestine, it may potentially be able to effectively inhibit the
harmful effects of excessive oil intake.
As is already known, excess levels of IL-1β and
TNF-α produced by activated eosinophils, macrophages, mononuclear
phagocytes and neutrophils cause damage to cells and tissues, which
eventually causes inflammation-associated diseases; macrophages
have an important role in regulating several immunopathological
conditions and inducing the overexpression of pro-inflammatory
mediators of the inflammatory process (15). Therefore, RAW264.7 cells were used in
the present study. In the present study, CA at 5, 10, 20 and 40 µM
significantly reduced the production of IL-1β and TNF-α compared
with that in the LPS group (P<0.05), with their effect being
dose-dependent. In addition, 20 µM CA was sufficient to restore
IL-1β to near-normal levels. As CA exerted potent anti-inflammatory
effects by inhibiting pro-inflammatory cytokines, it is indicated
that it may efficiently prevent diseases caused by chronic
inflammation.
To date, only few studies have been performed to
systematically evaluate the role of CA as an antioxidant oil
additive, as well as its potential benefits on human health
involving immunity, obesity and the intestinal microbiota. The
potent anti-oxidative activity (prolongation of the shelf life of
oil), anti-bacterial activity (inhibition of the proliferation of
pathogenic microbes in the host's intestines), anti-inflammatory
effects and inhibition of intracellular triglyceride accumulation
by CA suggest that it may promote intestinal and overall health. CA
has potential use as a functional natural food antioxidant to
prevent the oxidation of oil, protect human intestinal health,
delay senescence and prevent chronic diseases.
Acknowledgements
Not applicable.
Funding
The present study was supported by grants from the
Open Foundation of Hubei Key Laboratory of Lipid Chemistry and
Nutrition (grant no. 201602), the National Natural Science
Foundation of China (grant nos. 31560264, 81503364), and grants
from Jiangxi Province (grant nos. 20171BCB23028 and 20175526).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
TC and MF designed the present study; XZ, FH and XX
performed the experiments; MF and TC analyzed all data and wrote
the manuscript. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of the Second Affiliated Hospital of Nanchang University
(Nanchang, China), and the sampling of human specimens was
performed after obtaining consent from the patients or
volunteers.
Patient consent for publication
Patients or their guardians provided written
informed consents for publication.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Willett WC: Is dietary fat a major
determinant of body fat? Am J Clin Nutr. 67 Suppl 3:556S–562S.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Balabanova B and Gulaboski R: Human health
risks from heavy metals via consumption of contaminated food.
International Symposium at Faculty of Medical Sciences. http://eprints.ugd.edu.mk/14904/November
24–2015
|
|
3
|
Morita M, Naito Y, Yoshikawa T and Niki E:
Antioxidant capacity of blueberry extracts: Peroxyl radical
scavenging and inhibition of plasma lipid oxidation induced by
multiple oxidants. J Berry Res. 7:1–9. 2017. View Article : Google Scholar
|
|
4
|
Niki E: Oxidative stress and antioxidants:
Distress or eustress? Arch Biochem Biophys. 595:19–24. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Niki E: Biomarkers of lipid peroxidation
in clinical material. Biochim Biophys Acta. 1840:809–817. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Prior RL, Sintara M and Chang T:
Multi-radical (ORACMR5) antioxidant capacity of selected berries
and effects of food processing. J Berry Res. 6:159–173. 2016.
View Article : Google Scholar
|
|
7
|
Ivanova-Petropulos V, Hermosín-Gutiérrez
I, Boros B, et al: Phenolic compounds and antioxidant activity of
macedonian red wines. J Food Compos Anal. 41:1–14. 2015. View Article : Google Scholar
|
|
8
|
Forbeshernandez TY, Gasparrini M, Afrin S,
Bompadre S, Mezzetti B, Quiles JL, Giampieri F and Battino M: The
healthy effects of strawberry polyphenols: Which strategy behind
antioxidant capacity? Crit Rev Food Sci Nutr. 1 Suppl 56:S46–S59.
2016. View Article : Google Scholar
|
|
9
|
Kader AA, Zagory D and Kerbel EL: Modified
atmosphere packaging of fruits and vegetables. Crit Rev Food Sci.
28:1–30. 1989. View Article : Google Scholar
|
|
10
|
Xiao H, Wang J, Yuan L, Xiao C, Wang Y and
Liu X: Chicoric acid induces apoptosis in 3T3-L1 preadipocytes
through ROS-mediated PI3K/Akt and MAPK signaling pathways. J Agric
Food Chem. 61:1509–1520. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Liu Q, Chen Y, Shen C, Xiao Y, Wang Y, Liu
Z and Liu X: Chicoric acid supplementation prevents systemic
inflammation-induced memory impairment and amyloidogenesis via
inhibition of NF-κB. FASEB J. 31:1494–1507. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Park CM, Jin KS, Lee YW and Song YS:
Luteolin and chicoric acid synergistically inhibited inflammatory
responses via inactivation of PI3K-Akt pathway and impairment of
NF-κB translocation in LPS stimulated RAW 264.7 cells. Eur J
Pharmacol. 660:454–459. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Xiao H, Xie G, Wang J, et al: Chicoric
acid prevents obesity by attenuating hepatic steatosis,
inflammation and oxidative stress in high-fat diet-fed mice. Food
Res Int. 54:345–353. 2013. View Article : Google Scholar
|
|
14
|
Lai LS, Chou ST and Chao WW: Studies on
the antioxidative activities of hsian-tsao (mesona procumbens
hemsl) leaf gum. J Agric Food Chem. 49:963–968. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Jiang M, Deng K, Jiang C, Fu M, Guo C,
Wang X, Wang X, Meng F, Yang S, Deng K, et al: Evaluation of the
antioxidative, antibacterial, and anti-inflammatory effects of the
aloe fermentation supernatant containing lactobacillus plantarum
HM218749.1. Mediators Inflamm. 2016:29456502016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Dorman HJD, Peltoketo A, Hiltunen R and
Tikkanen MJ: Characterisation of the antioxidant properties of
de-odourised aqueous extracts from selected lamiaceae herbs. Food
Chem. 83:255–262. 2003. View Article : Google Scholar
|
|
17
|
Cheng-Lun L, Derong T and Le L: Research
on the extracting and anti-oxidation dynamic characteristics of
ginger oleoresin. Int J Food Sci Tech. 43:517–525. 2008. View Article : Google Scholar
|
|
18
|
Chen T, Wu Q, Li S, Xiong S, Jiang S, Tan
Q, Zhang Z, Zhu D and Wei H: Microbiological quality and
characteristics of probiotic products in china. J Sci Food Agric.
94:131–138. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Meng F, Chen T, Wang X, Wang X, Wei H,
Tian P, Wang H, Zhao X, Shen L and Xin H: Evaluation of the
accuracy and sensitivity of high-throughput sequencing technology
using known microbiota. Mol Med Rep. 17:408–413. 2018.PubMed/NCBI
|
|
20
|
Chen T, Wu Q, Zhou H, et al: Assessment of
commercial probiotic products in china for labelling accuracy and
probiotic characterisation of selected isolates. Int J Dairy
Technol. 70:119–126. 2017. View Article : Google Scholar
|
|
21
|
Wang X, Wu Q, Deng K, Wei Q, Hu P, He J,
Liu H, Zheng Y, Wei H, Shah NP and Chen T: A novel method for
screening of potential probiotics for high adhesion capability. J
Dairy Sci. 98:4310–4317. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Deng K, Chen T, Wu Q, Xin H, Wei Q, Hu P,
Wang X, Wang X, Wei H and Shah NP: In vitro and in vivo examination
of anticolonization of pathogens by lactobacillus paracasei
FJ861111.1. J Dairy Sci. 98:6759–6766. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lim SM, Goh YM, Kuan WB and Loh SP: Effect
of germinated brown rice extracts on pancreatic lipase,
adipogenesis and lipolysis in 3T3-L1 adipocytes. Lipids Health Dis.
13:1692014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wójcik M, Wessely-Szponder J, Cichoż-Lach
H, Celiński K and Bobowiec R: In vitro proinflammatory polarization
of macrophages isolated from hepatocarcinogenic stage in humans and
rats. In Vivo. 30:853–862. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Carr A and Frei B: Does vitamin C act as a
pro-oxidant under physiological conditions? FASEB J. 13:1007–1024.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Abner EL, Schmitt FA, Mendiondo MS, Marcum
JL and Kryscio RJ: Vitamin E and all-cause mortality: A
meta-analysis. Curr Aging Sci. 4:158–170. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Caraballoso M, Sacristan M, Serra C and
Bonfill X: Drugs for preventing lung cancer in healthy people.
Cochrane Database Syst Rev. 10:CD0021412003.
|
|
28
|
Rees K, Hartley L, Day C, Clarke A and
Stranges S: Selenium supplementation for the primary prevention of
cardiovascular disease. Cochrane Database Syst Rev.
1012:CD0096712012.
|
|
29
|
Zhao X, Chen T, Meng F, Wang H, Tian P,
Tang X, Wang X, Wang X, Xin H and Wei H: Therapeutic effect of herb
residue fermentation supernatant on spleenâ-‘deficient mice. Mol
Med Rep. 17:2764–2770. 2018.PubMed/NCBI
|
|
30
|
Zhang F, Zhang M, Wang Y, Li C and Chen T:
Comparison of the common bacteria in human and mouse tumours using
high-throughput sequencing. Mol Med Rep. 17:6717–6722.
2018.PubMed/NCBI
|
|
31
|
Chen T, Yan S, Wang X, Wang X, Meng F,
Yang S, Yang J and Xin H: High-throughput sequencing analyses of
oral microbial diversity in healthy people and patients with dental
caries and periodontal disease. Mol Med Rep. 16:127–132. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Chen H, Luo T, Chen T and Wang G: Seminal
bacterial composition in patients with obstructive and
non-obstructive azoospermia. Exp Ther Med. 15:2884–2890.
2018.PubMed/NCBI
|