Introduction
Coronary heart disease (CHD), is considered to be
one of the diseases that have the greatest threat to human health
in recent decades. According to surveys, the total CHD patients in
developed countries are over 750,000. There are more than 450,000
patients with secondary CHD (1).
Other studies have shown (2) that in
2004 there were approximately 400,000 people who died of
cardiovascular disease globally. The incidence of CHD in China is
relatively low, but with the development of the aging population,
the incidence rate is also rising year by year (3). This not only threatens the patient's
prognosis and lifespan, but also has an impact on the social and
economic benefits such as labor loss and brings a heavy burden on
society and the family. CHD is due to changes in coronary function,
leading to stenosis or occlusion of the lumen, causing myocardial
ischemia and hypoxia in patients (4). The pathogenesis of CHD is not yet
clear. At present, most scholars believe that CHD is caused by a
combination of multiple genes and is associated with environmental
and genetic factors (5).
The Notch signaling pathway serves as a
highly-conserved cell-to-cell communication pathway. Notch was
first discovered when a mutant allele caused an incision in the
wing of the fly (6). Studies have
shown that (7) Notch-1 signaling
pathway plays a key role in the physiology, pathology, occurrence
and development of cardiovascular system. In recent years, with
in-depth study, it was found that Notch signaling pathway plays a
regulatory role in the course of CHD (8). Nuclear factor-κB (NF-κB) is a protein
complex that regulates transcription of DNA, and NF-κB is expressed
in all animal cells (9). In recent
years, studies have shown that (10)
NF-κB-p65 signaling pathway also plays a regulatory role in the CHD
process. However, Notch-1 signaling is associated with NF-κB-p65
signaling pathway at multiple levels. For example, NF-κB-p65
signaling pathway is involved in the development of rheumatoid
arthritis. The activation of Notch-1 signaling pathway is closely
related to the occurrence and development of tumors (11,12). We
speculate that Notch-1 and NF-κB-p65 may be involved in the
pathogenesis of CHD.
Through this study, the changes of Notch-1 and NF-κB
pathways in the pathogenesis of CHD were explored, in order to
provide a theoretical basis for clinical prevention and control of
CHD.
Materials and methods
Animal sources
A total of 96 wistar rats were used in this study,
including 48 male and 48 female rats. The weight range was 160–210
g, the average weight was 185±25 g, age 8–12 weeks, and the average
age 10.51±1.25 weeks, provided by Daping Medical Laboratory Animal
Center, Third Military Medical University, license no. SCXK (Yu)
2012-0005.
Experimental materials
Tissue protein lysate, BCA protein quantification
kit (Biotime Biotechnology Institute, Shanghai, China), TUNEL
apoptosis detection kit (Sigma-Aldrich; Merck KGaA, Darmstadt,
Germany), rabbit anti-mouse Notch-1 polyclonal antibody and
NF-κB-p65 polyclonal antibody (cat. nos. 4147 and 4764; both from
Cell Signaling Technology, Inc., Danvers, MA, USA), horseradish
peroxidase labeled goat anti-rabbit IgG (cat. no. 31460; Zymed;
Thermo Fisher Scientific, Inc., Waltham, MA, USA), and GAPDH
internal reference was provided by Cell Signaling Technology, Inc.
Isoproterenol hydrochloride was purchased from Shanghai Shifeng
Biotechnology Co., Ltd. (Shanghai, China). The study was approved
by the Ethics Committee of Weifang People's Hospital (Weifang,
China).
Animal model construction
A total of 96 rats were randomly divided into a
control and an observation group according to random method. The
rats of the control group were 25 males and 23 females, and the age
was 10.05±1.62 weeks. There were 23 males and 25 females in the
observation group, aged 10.32±1.32 weeks, there was no
statistically significant difference in sex and age between the two
groups, and there was comparability. In the week before modeling,
the rats of the two groups were fed with common feed, and had free
access to drinking water with a 12 h light/dark cycle to adapt to
the environment. After one week, the rats in the observation group
were fed with high-fat diet for 6 weeks by the laboratory (propyl
thioxymidine 0.2%, sodium taurocholate 0.5%, cholesterol 2%, lard
10%, and basal diet 87.3%). After 6 weeks, the rats were injected
with isoproterenol hydrochloride (5 mg/kg) continuously for 3 days,
once daily. After 3 days of injection, 10% chloral hydrate (0.3
ml/100 g) was used for intraperitoneal anesthesia and fixed. The ST
segment changes were observed using an electrocardiograph and the
ST segment elevation ≥0.1 mv in the rat electrocardiogram suggested
that the modeling was successful. The control group was fed with
conventional feed for 6 weeks.
Tissue collection
Rats were sacrificed by cervical dislocation on the
1st, 3rd, 7th, and 14th days after successful modeling. Each time,
12 rats were deprived of their hearts. The infarcted and
non-infarcted areas were separated and some of them were subjected
to follow-up experiments. The rest of the tissue was stored at 80°C
until use.
Detection of Notch-1 and NF-κB
proteins in rat cardiomyocytes by western blot analysis
The collected tissues were lysed using the cell
lysate RIPA, and the total protein was lysed. The protein
concentration was measured using a BCA protein quantitation kit,
electrophoresis separation was performed using a 12% SDS-PAGE gel,
and electrotransfer was performed at a constant voltage. The
membrane was sealed in 5% skim milk in TBS buffer and protected
from light at room temperature for 1 h. Primary antibody (dilution,
1,000 both for rabbit anti-mouse Notch-1 polyclonal antibody and
NF-κB-p65 polyclonal antibody) was plated overnight at 4°C. Washing
with PBS followed by labeling with horseradish peroxidase goat
anti-rabbit IgG secondary antibody, incubated at 4°C on a rocking
shaker for one hour, shaking, and visualization. GAPDH was used as
an internal control in this study. Gray scale of the protein bands
was measured using the Quantity One 1-D analysis software (Bio-Rad
Laboratories, Inc., Hercules, CA, USA). Product relative expression
= grayscale of target protein / grayscale of internal control
bands.
Apoptosis
Apoptosis of rat cardiomyocytes was detected using
the TUNEL Cell Apoptosis Detection kit. The experimental method was
performed in strict accordance with the manufacturer's
instructions. After staining, observations were performed with an
optical microscope (Olympus, Tokyo, Japan). After nuclear
myocardial cell nuclear staining, the normal myocardial cell
nuclear staining was blue, and the apoptotic cardiomyocyte nuclear
staining was yellow or brown-yellow. Each field was randomly taken
from 5 fields to observe and count the apoptotic cells in the
visual field. Apoptosis rate = total apoptotic number / total cell
number ×100%.
Statistical analysis
In this study, we used SPSS20.0 software (IBM Corp.,
Armonk, NY, USA) to perform statistical analysis on all collected
data, GraphPad Prism 7 software (GraphPad Software, Inc., La Jolla,
CA, USA) was used to create images. Enumeration data are expressed
as rate (%), examined using Chi-square test, and measurement data
used for the analysis were expressed as mean ± standard deviation
(SD). The t-test was used for analysis between the two groups.
Multiple groups were compared using repeated measures analysis of
variance (ANOVA) and the post hoc test used was Fisher's test.
Pearson's analysis was used to analyze protein expression
correlations. P<0.05 was considered to indicate a statistically
significant difference.
Results
Rat modeling
We established the two groups of rat models in 6
weeks. The results showed that the rats in the observation group
successfully established the CHD model in 6 weeks. During the
modeling period, the two groups of model rats did not die.
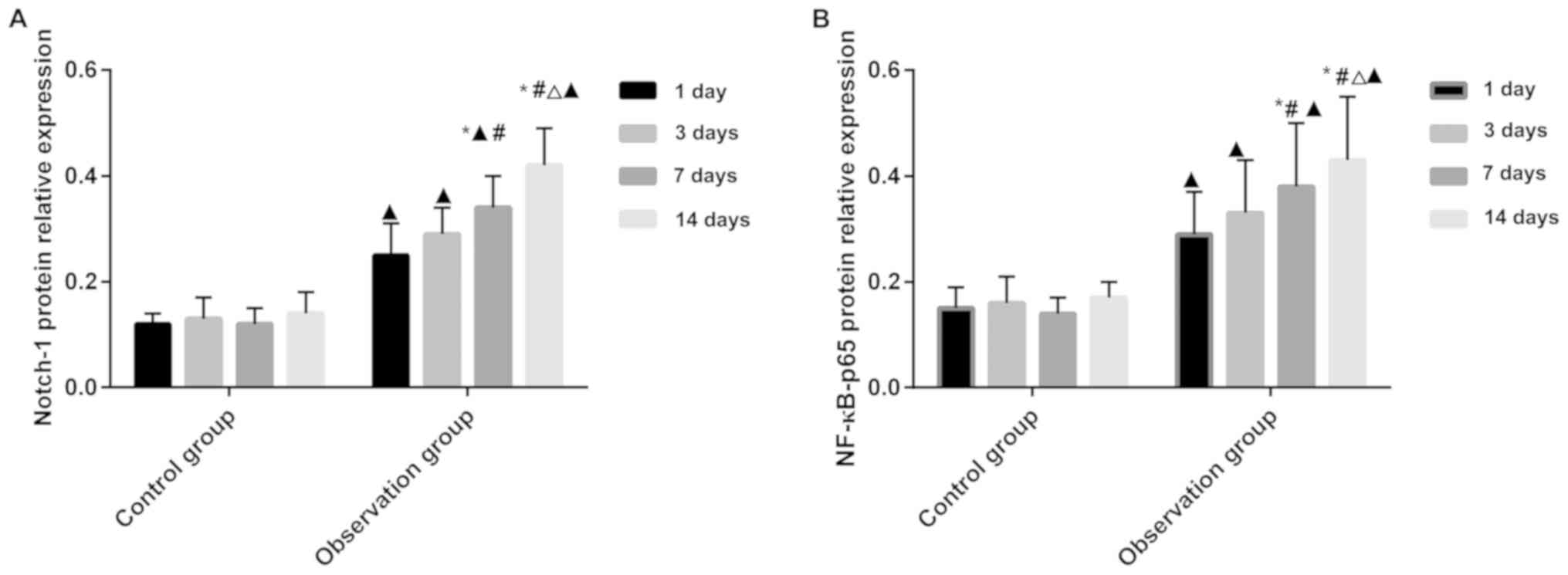
The expression of Notch-1 and
NF-κB-p65 protein in rat cardiomyocytes
After successfully establishing the rat model, rats
were sacrificed on the 1st, 3rd, 7th, and 14th day. The rat
cardiomyocytes were used to detect the expression of Notch-1 and
NF-κB-p65 protein. The expression of Notch-1 and NF-κB-p65 protein
was significantly higher in the observation than in the control
group (P<0.05). Repeated measurement analysis of variance
revealed that the Notch-1 and NF-κB-p65 proteins were expressed in
the control group and there was no statistical difference
(P>0.05), but the expression of Notch-1 and NF-κB-p65 protein in
the observation group was statistically different (P<0.05).
There was a statistically significant difference between in the
expression of Notch-1 and NF-κB-p65 protein in the observation
group between 7 days, 14 days and 1 day, 3 days of modeling
(P<0.05), and the expression of Notch-1 protein in the
observation group at day 7 was different from that at the 14 day
(P<0.05) (Fig. 1; Tables I and II).
 | Table I.The expression of Notch-1 protein in
two groups of rats. |
Table I.
The expression of Notch-1 protein in
two groups of rats.
| Items | 1 day (n=12) | 3 day (n=12) | 7 day (n=12) | 14 day (n=12) | F-value | P-value |
|---|
| Control | 0.12±0.02 | 0.13±0.04 | 0.12±0.03 | 0.14±0.04 | 0.978 | 0.412 |
| Observation | 0.25±0.06 | 0.29±0.05 |
0.34±0.06a–c |
0.42±0.07a,b | 17.644 | 0.001 |
| t value | 7.120 | 8.656 | 11.361 | 12.031 |
|
|
| P-value | 0.001 | 0.001 | 0.001 | 0.001 |
|
|
 | Table II.The expression of NF-κB-p65 protein in
two groups of rats. |
Table II.
The expression of NF-κB-p65 protein in
two groups of rats.
| Items | 1 day (n=12) | 3 day (n=12) | 7 day (n=12) | 14 day (n=12) | F-value | P-value |
|---|
| Control | 0.15±0.04 | 0.16±0.05 | 0.15±0.03 | 0.17±0.03 | 0.746 | 0.531 |
| Observation | 0.29±0.08 | 0.33±0.10 |
0.38±0.12a,b |
0.43±0.12a,b | 3.920 | 0.015 |
| t value | 5.422 | 5.267 | 6.411 | 7.281 |
|
|
| P-value | 0.001 | 0.001 | 0.001 | 0.001 |
|
|
Relationship between expression of
Notch-1, NF-κB-p65 protein and sex and age of rats
We observed the expression of Notch-1 and NF-κB-p65
protein in the observation group at the 14th day after successful
modeling. There was no statistical difference between the
expression of Notch-1 and NF-κB-p65 protein and the sex and age of
the rats (P>0.05) (Table
III).
 | Table III.Relationship between expression of
Notch-1, NF-κB-p65 protein and sex, age in rats (n, %). |
Table III.
Relationship between expression of
Notch-1, NF-κB-p65 protein and sex, age in rats (n, %).
|
| Notch-1 protein
expression |
| NF-κB-p65 protein
expression |
|
|---|
|
|
|
|
|
|
|---|
| Groups | Low expression
(n=6) | High expression
(n=6) | P-value | Low expression
(n=6) | High expression
(n=6) | P-value |
|---|
| Sex |
| Male
(n=6) | 3 (50) | 3 (50) | >0.05 | 3 (60) | 3 (42.86) | >0.05 |
| Female
(n=6) | 3 (50) | 3 (50) |
| 2 (40) | 4 (57.14) |
|
| Age |
| >10
weeks (n=5) | 3 (50) | 2 (40) | >0.05 | 2 (40) | 3 (42.86) | >0.05 |
| ≤10
weeks (n=7) | 3 (50) | 4 (60) |
| 3 (60) | 4 (57.14) |
|
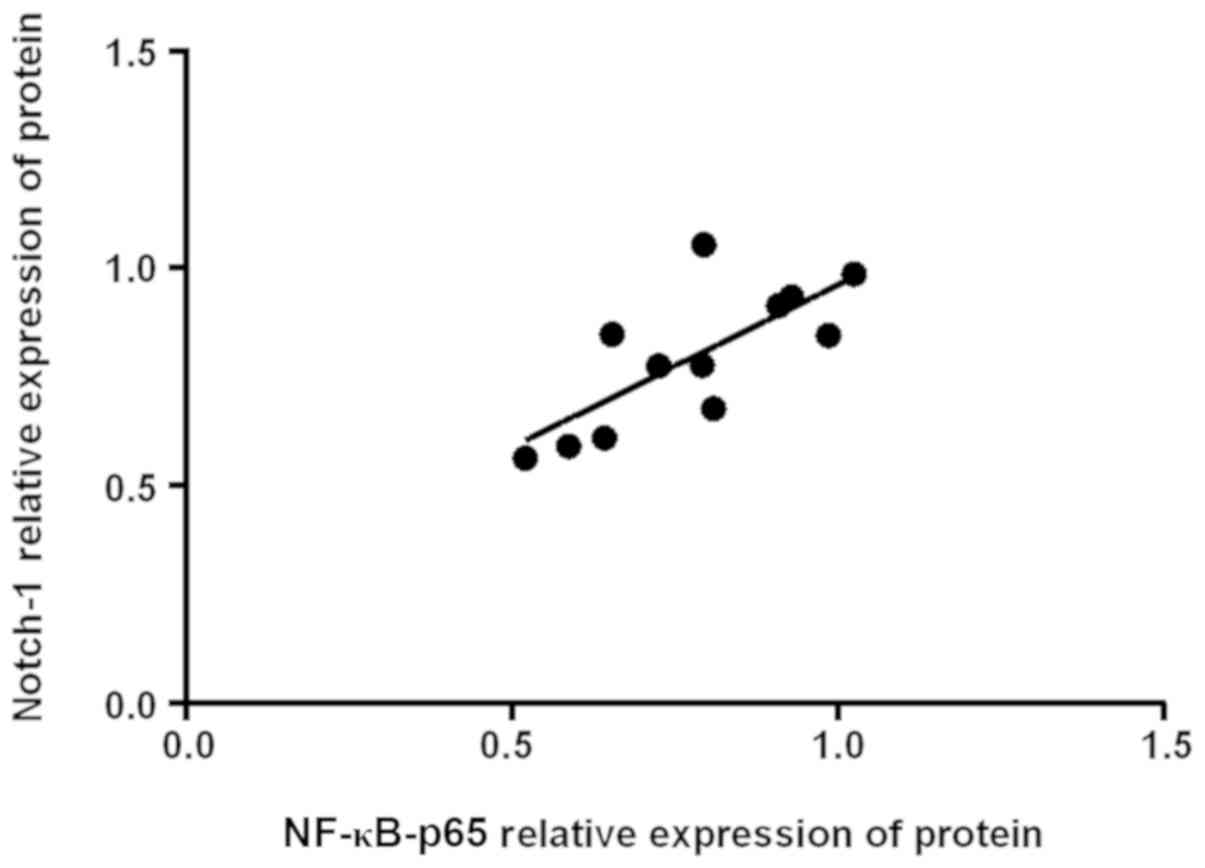
Correlation analysis between Notch-1
and NF-κB-p65 protein expression
Pearson's correlation analysis of Notch-1 and NF-κB
protein in rats in 14 days showed that Notch-1 was positively
correlated with NF-κB-p65 protein expression (r=0.745, P=0.05)
(Fig. 2).
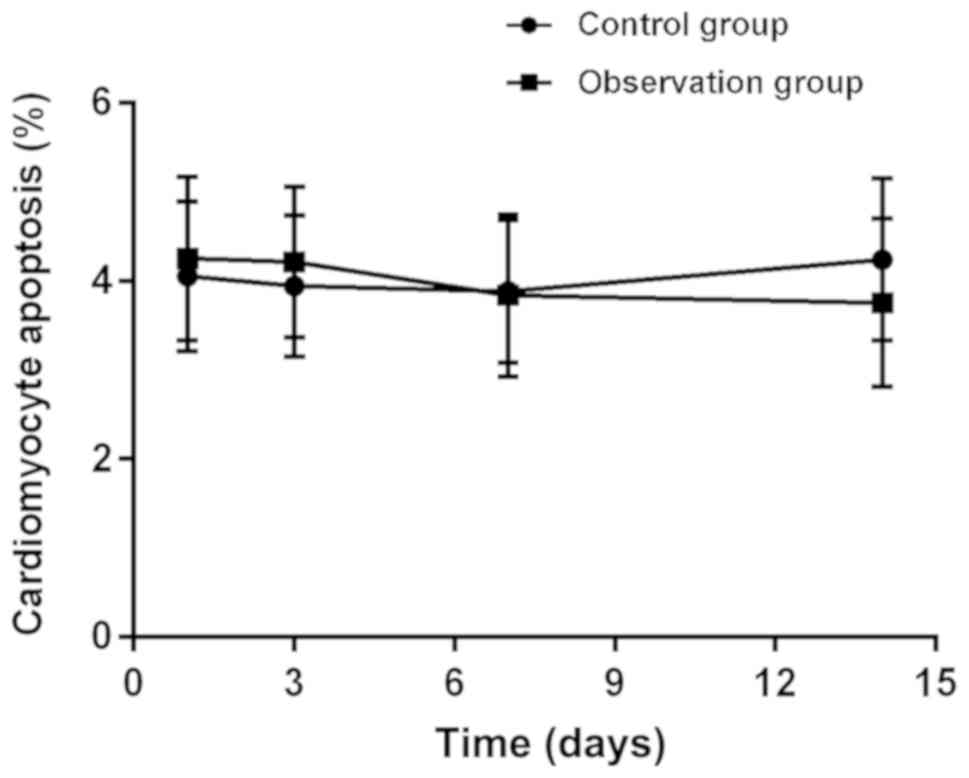
Apoptosis
The TUNEL assay was used to detect apoptosis of the
two groups of rats according to the time period. The results showed
that after the rats were successfully modeled, there was no
statistical difference between the observation group and the
control group (P<0.05), repeated measurement analysis of
variance found that there was no statistical difference in
myocardial cell apoptosis between the control and the observation
group (P>0.05) (Fig. 3; Table IV).
 | Table IV.Cardiomyocyte apoptosis expression in
rats. |
Table IV.
Cardiomyocyte apoptosis expression in
rats.
| Items | 1 day (n=12) | 3 day (n=12) | 7 day (n=12) | 14 day (n=12) | F-value | P-value |
|---|
| Control | 4.05±0.84 | 3.94±0.79 | 3.98±0.80 | 4.24±0.91 | 0.304 | 0.823 |
| Observation | 4.25±0.92 | 4.21±0.95 | 3.84±0.81 | 3.75±0.85 | 0.993 | 0.405 |
| t value | 0.556 | 0.806 | 0.426 | 1.363 |
|
|
| P-value | 0.584 | 0.429 | 0.674 | 0.186 |
|
|
Discussion
The total number of cardiovascular patients in China
is as high as 290 million, and the death rate is >30%. The
number of deaths due to cardiovascular and cerebrovascular diseases
is second only to malignant tumors. There are also data showing
that the age of onset is tending to be younger (13). CHD is the most common disease in
cardiovascular and cerebrovascular diseases. CHD is caused by
atherosclerosis of the coronary arteries causing obstruction or
stenosis of the coronary arteries, which leads to myocardial
ischemia and hypoxia. It is common in clinical, if the treatment is
not timely, it may lead to death or disability of the patient or
cause serious effects on the quality of life (14).
On the occurrence and development process of CHD,
there are various opinions from various angles, including
thrombosis, cloning of smooth muscle cells, lipid infiltration, and
endothelial injury response (15).
At present, most scholars still believe that the endothelium injury
response is the main cause of CHD, and the theory of endothelial
injury response is that the coronary endothelium or endometrial
injury causes inflammation forming atherosclerosis (16).
Notch/NF-κB signaling pathway has been fully studied
as a classical signaling pathway. Compared with non-classical
pathways, the crosstalk between Notch/NF-κB signaling pathways is
relatively simple (17), and studies
have shown (18) that Notch
signaling pathway is linked to various signals, but their crosstalk
relationship is more complex and requires further studies. In the
past decade, a large number of studies have shown that (19) Notch and NF-κB are interrelated in the
life cycle and the cancer process. Notch signaling pathway is
composed of Notch receptor and Notch ligand and CSL
(CBF1/RBP-J/SU(H)/Lag-1). It mainly exists in mammals in four forms
(Notch1-4) (20). Studies have shown
that (21) Notch-1 regulates the
development and function of inflammatory cells, and acts on T cells
in the thymus to mediate its activation, proliferation, and
secretion of cytokines. NF-κB signaling is an important signaling
pathway for multicellular animals to determine cell fate. As an
important transcription factor, NF-κB is widely present in
eukaryotic cells. After activation by multiple stimuli, NF-κB
transcription factors directly regulate target genes, among which
NF-ĸB plays an important role in immune and inflammatory responses.
NF-ĸB is also a very important inflammatory factor, and may be
responsible for regulating a large number of inflammatory factors
(IL-1, IL-2, TNF-α) and death-related factors (Bcl-2, Bax, Fas)
(22), and NF-ĸB-p65 is one of the
important subgroups of the NF-ĸB pathway, and its expression
changes can well reflect the conditions of the pathway (23).
In this study, we successfully established a rat
model of CHD and detected the expression of Notch-1 and NF-κB-p65
protein in rat cardiomyocytes. The results showed that the Notch-1
and NF-κB-p65 protein expression in the observation group 1 day
after successful modeling was significantly higher than that of the
control group, and the expression was significantly higher at the
subsequent time. However, the expression of Notch-1 and NF-κB-p65
protein was found to be different on day 7 after the modeling
success compared to day 1 and day 3. This may suggest that the
Notch and NF-κB pathways were activated and involved in the
development of CHD one week after rat modelling. In the study of
Jin et al (24), the
expression of Notch-1 receptor protein and its soluble
intracellular fragment NICD1 protein in the rat model of myocardial
infarction was significantly increased, and the downstream target
gene Hes1 was found to increase. In addition, the NF-κB pathway in
the myocardial infarction group was activated in the first week,
which was significantly higher than that in the unmodeled control
group. Myocardial infarction is the basis of CHD, a variety of
incentives lead to coronary atherosclerotic plaque rupture, causing
platelet aggregation in the rupture and the formation of
thrombosis, which is the main cause of death in patients with CHD.
The above literature and the results of this study suggest that
Notch-1 and NF-κB-p65 signaling pathway may be involved in the
development of CHD. We also examined the correlation between the
two proteins and whether they differed in sex and age. We found
that the expression of Notch-1 and NF-κB-p65 protein was positively
correlated. In the study of Li et al (25), increasing the activity of Notch-1 can
upregulate the expression of NF-κB target genes, and we have better
proved the relationship between them by Pearson correlation test.
Moreover, we analyzed the expression of Notch-1 and NF-κB-p65
proteins with sex, and age, but we have few specimens, which cannot
explain its accuracy. Studies have shown that (26) the inhibition of Notch-1 signaling
pathway by pcDNA3.1-Myc-His plasmid and RNA interference can
inhibit the expression of Bcl-2 and Bax proteins and inhibit
cardiomyocyte apoptosis, and our study found that there was no
difference in apoptosis of murine cardiomyocytes between the two
groups, which may be due to the fact that we did not modulate them
resulting in insignificant differences. Through the above we
initially demonstrated the relationship between Notch-1 and NF-κB
signaling pathway in the development of CHD, but failed to further
detect its target genes, and we have fewer samples in this study,
which cannot explain the accuracy of the results. We hope to
increase our testing projects in future research, conduct more
in-depth research, and obtain more accurate results to support our
experimental conclusions.
In conclusion, the expression levels of Notch-1 and
NF-κB proteins were increased in CHD rat cardiomyocytes, and
Notch-1 was positively correlated with NF-κB protein expression,
they are involved in the development of CHD.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
JZ and SZ were responsible for animal model
construction. JZ, SZ and TW were mainly devoted to western blot
analysis and TUNEL assay. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
Weifang People's Hospital (Weifang, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Dawber TR, Moore FE and Mann GV: II.
Coronary heart disease in the Framingham study. Int J Epidemiol.
44:1767–1780. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lloyd-Jones D, Adams R, Carnethon M, De
Simone G, Ferguson TB, Flegal K, Ford E, Furie K, Go A, Greenlund
K, et al: American Heart Association Statistics Committee and
Stroke Statistics Subcommittee: Heart disease and stroke statistics
- 2009 update: A report from the American Heart Association
Statistics Committee and Stroke Statistics Subcommittee.
Circulation. 119:480–486. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Xie W, Li G, Zhao D, Xie X, Wei Z, Wang W,
Wang M, Li G, Liu W, Sun J, et al: Relationship between fine
particulate air pollution and ischaemic heart disease morbidity and
mortality. Heart. 101:257–263. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
McClelland RL, Jorgensen NW, Budoff M,
Blaha MJ, Post WS, Kronmal RA, Bild DE, Shea S, Liu K, Watson KE,
et al: 10-year coronary heart disease risk prediction using
coronary artery calcium and traditional risk factors: Derivation in
the MESA (Multi-Ethnic Study of Atherosclerosis) with validation in
the HNR (Heinz Nixdorf Recall) study and the DHS (Dallas Heart
Study). J Am Coll Cardiol. 66:1643–1653. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Patel TP, Rawal K, Bagchi AK, Akolkar G,
Bernardes N, Dias DS, Gupta S and Singal PK: Insulin resistance: An
additional risk factor in the pathogenesis of cardiovascular
disease in type 2 diabetes. Heart Fail Rev. 21:11–23. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhou QZ, Zhang G, Long HB, Lei F, Ye F,
Jia XF, Zhou YL, Kang JP and Feng DX: Effect of spinal cord
extracts after spinal cord injury on proliferation of rat embryonic
neural stem cells and Notch signal pathway in vitro. Asian Pac J
Trop Med. 7:562–567. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kozmann G, Jókuthy A, Virányi V and
Vassányi I: Methodological studies related to cardiovascular risk
assessment. Stud Health Technol Inform. 90:635–638. 2002.PubMed/NCBI
|
|
8
|
Jin Y, Liu K, Peng J, Wang C, Kang L,
Chang N and Sun H: Rhizoma Dioscoreae Nipponicae polysaccharides
protect HUVECs from H2O2-induced injury by
regulating PPARγ factor and the NADPH oxidase/ROS-NF-κB signal
pathway. Toxicol Lett. 232:149–158. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hamid T, Guo SZ, Kingery JR, Xiang X, Dawn
B and Prabhu SD: Cardiomyocyte NF-kappaB p65 promotes adverse
remodelling, apoptosis, and endoplasmic reticulum stress in heart
failure. Cardiovasc Res. 89:129–138. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Mnafgui K, Hajji R, Derbali F, Gammoudi A,
Khabbabi G, Ellefi H, Allouche N, Kadri A and Gharsallah N:
Anti-inflammatory, antithrombotic and cardiac remodeling preventive
effects of eugenol in isoproterenol-induced myocardial infarction
in wistar rat. Cardiovasc Toxicol. 16:336–344. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yin G, Wang Y, Cen XM, Yang M, Liang Y and
Xie QB: Lipid peroxidation-mediated inflammation promotes cell
apoptosis through activation of NF-κB pathway in rheumatoid
arthritis synovial cells. Mediators Inflamm. 2015:4603102015.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Fender AW, Nutter JM, Fitzgerald TL,
Bertrand FE and Sigounas G: Notch-1 promotes stemness and
epithelial to mesenchymal transition in colorectal cancer. J Cell
Biochem. 116:2517–2527. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Satija A, Bhupathiraju SN, Spiegelman D,
Chiuve SE, Manson JE, Willett W, Rexrode KM, Rimm EB and Hu FB:
Healthful and unhealthful plant-based diets and the risk of
coronary heart disease in U.S. adults. J Am Coll Cardiol.
70:411–422. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
De Bruyne B, Fearon WF, Pijls NH, Barbato
E, Tonino P, Piroth Z, Jagic N, Mobius-Winckler S, Rioufol G, Witt
N, et al: FAME 2 Trial Investigators: Fractional flow
reserve-guided PCI for stable coronary artery disease. N Engl J
Med. 371:1208–1217. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hu SS, Kong LZ, Gao RL, Zhu ML, Wang W,
Wang YJ, Wu ZS, Chen WW and Liu MB: Editorial Board: Outline of the
report on cardiovascular disease in China, 2010. Biomed Environ
Sci. 25:251–256. 2012.PubMed/NCBI
|
|
16
|
Hansson GK, Libby P and Tabas I:
Inflammation and plaque vulnerability. J Intern Med. 278:483–493.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Franck G, Mawson T, Sausen G, Salinas M,
Masson GS, Cole A, Beltrami-Moreira M, Chatzizisis Y, Quillard T,
Tesmenitsky Y, et al: Flow perturbation mediates neutrophil
recruitment and potentiates endothelial injury via TLR2 in mice:
Implications for superficial erosion. Circ Res. 121:31–42. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Braune EB and Lendahl U: Notch - a
goldilocks signaling pathway in disease and cancer therapy. Discov
Med. 21:189–196. 2016.PubMed/NCBI
|
|
19
|
Gu JW, Rizzo P, Pannuti A, Golde T,
Osborne B and Miele L: Notch signals in the endothelium and cancer
‘stem-like’ cells: Opportunities for cancer therapy. Vasc Cell.
4:72012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ray A, Vasudevan S and Sengupta S:
6-Shogaol inhibits breast cancer cells and stem cell-like spheroids
by modulation of Notch signaling pathway and induction of
autophagic cell death. PLoS One. 10:e01376142015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wilson A, MacDonald HR and Radtke F: Notch
1-deficient common lymphoid precursors adopt a B cell fate in the
thymus. J Exp Med. 194:1003–1012. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Vlantis K, Wullaert A, Polykratis A,
Kondylis V, Dannappel M, Schwarzer R, Welz P, Corona T, Walczak H,
Weih F, et al: NEMO prevents RIP kinase 1-mediated epithelial cell
death and chronic intestinal inflammation by NF-κB-dependent and
-independent functions. Immunity. 44:553–567. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Twait E, Williard DE and Samuel I:
Dominant negative p38 mitogen-activated protein kinase expression
inhibits NF-kappaB activation in AR42J cells. Pancreatology.
10:119–128. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Jin JL, Deng ZT, Lyu RG, Liu XH and Wei
JR: Expression changes of Notch and nuclear factor-κB signaling
pathways in the rat heart with myocardial infarction. Zhonghua Xin
Xue Guan Bing Za Zhi. 45:507–512. 2017.(In Chinese). PubMed/NCBI
|
|
25
|
Li L, Zhao F, Lu J, Li T, Yang H, Wu C and
Liu Y: Notch-1 signaling promotes the malignant features of human
breast cancer through NF-κB activation. PLoS One. 9:e959122014.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yu B and Song B: Notch 1 signalling
inhibits cardiomyocyte apoptosis in ischaemic postconditioning.
Heart Lung Circ. 23:152–158. 2014. View Article : Google Scholar : PubMed/NCBI
|