Introduction
Homeostasis of tissues affected by periodontal
disease is achieved by the mechanical removal of bacterial deposits
on the surface of teeth by scaling and root planing (SRP) (1). However, this procedure cannot remove
residual bacteria in root locations that are inaccessible to
mechanical instrumentation; therefore, subgingival biofilm cannot
be completely eliminated (2–4). The insufficient reduction of bacteria
is associated with therapeutic failures, and the persistence of
bacterial species after mechanical debridement has been associated
with additional tissue destruction (4–6). Under
these conditions, the use of systemic antibiotics as an adjunct to
initial periodontal therapy may be helpful, especially because the
disruption of biofilm by mechanical instrumentation makes bacteria
more susceptible to antibiotics (7).
Until recently, the antibiotic combination of amoxicillin (AMX)
plus metronidazole (MTZ) administered for 7–8 days has been the
protocol most widely used to achieve clinical and microbiological
efficacy (8–18). However, despite the impressive amount
of research that has demonstrated the beneficial effects of the AMX
+ MTZ combination as an adjuvant to SRP, there is still no
consensus in the literature concerning the optimal duration and
antibiotic dosing. Various adjunctive regimens of AMX + MTZ have
been proposed. In the literature, the durations of systemic
treatment with MTZ, AMX or their combination range from 7–8 days
(6,8,10,13–16,19)
to 10–14 days (20–25). When both the duration of
administration and the doses are considered, the variation in study
design is even greater. The only exception appears to be the dose
of AMX, which in the vast majority of studies is 500 mg per day,
three times daily (TID) (12,17,18,20–26).
On the other hand, the recommended ideal dose of MTZ has been cited
as 200, 250, 400 or 500 mg TID (8,13,19–24).
Current authoritative textbooks of Periodontology based on reputed
papers (27) still recommend using
the 250 mg TID dose regimen of both AMX and MTZ to treat
periodontal diseases (28). However,
regardless of the dose and duration, it is important that
antibiotics be taken at their minimum bactericidal concentration
(29) to limit the risk for side
effects and the development of microbial antibiotic resistance
(4).
Antibiotics have paved the way for unprecedented
medical and societal developments, and are now widely used by all
health systems (31). In recent
decades, the global emergence of antimicrobial resistance has
become a pre-eminent concern among medical and public health
professionals, as well as a widespread problem. The causative
factors for this resistance remain uncontrolled, and national
strategies to address the problem are lacking (32). Recently, substantial changes have
been proposed to the adjuvant systemic antibiotic administration
protocol used to treat patients with chronic periodontitis. In
studies evaluating exclusively the change in clinical parameters in
patients with chronic periodontitis, the proposed changes have
reduced the duration of antibiotic intake from 7 to 3 days, and
increase the dose from 3 × 250–375 mg/day to 3 × 500 mg/day
(4,30). These modifications are controversial
due to the possibility that periopathogenic bacteria might develop
antibiotic resistance. Currently, the antibiotic resistance of
periopathogens exposed to adjunctive systemic antibiotics after SRP
has been insufficiently addressed in the literature. Most
investigators who have addressed this issue identified naturally
resistant bacteria prior to periodontal therapy (33–37). In
the USA, patients with CP frequently harbor subgingival periodontal
pathogens that display in vitro resistance to therapeutic
concentrations of antibiotics that are commonly used in clinical
periodontal practice (33).
Moreover, the different species of periodontal pathogens often have
different susceptibilities to particular antibiotics (38). Few authors have investigated the
resistance of periopathogenic bacteria to antibiotics both before
and after periodontal treatment. However, the existing studies show
that despite the initial antibiotic resistance of some species,
most periopathogens are sensitive to antibiotics during their
administration, and systemic antibiotics reduce the number of
resistant strains at the end of non-surgical periodontal therapy,
when compared with the initial situation (34,36,37).
In periodontal disease, pathogens such as
Porphyromonas gingivalis, Treponema denticola, and
Tannerella forsythia interact with the host organism and
produce a systemic inflammation, which affects the tissue balance
through the action of a large number of cytokines and chemokines
released by normal connective tissue residents such as mast cells,
fibroblasts or by acquired connective tissue participants such as
activated macrophages (39).
Recently, a Gram-positive, non-spore-forming,
nonmotile and strictly anaerobic rod (Slakia exigua) has
been isolated and identified in periodontal and periapical
infections, as well (40). The
induction of pro-inflammatory cytokines, chemokines, and an
enhanced immune response, through mast cell degranulation, with
subsequent histamine and pro-inflammatory cytokines release can
amplify the inflammatory process and result in an increase in the
number and activity of polymorphonuclear cells (PMNs) (41,42).
PMNs produce reactive oxygen species (free radicals) via the
respiratory burst mechanism as part of a defense response to
infection (43). The deliberate
generation of free radicals occurs during phagocytosis as part of
the bactericidal reaction (44). The
production of reactive oxygen species is a component of the bone
resorption process that occurs during periodontal disease (41,43), and
contributes to its aggravation. However, the source of reactive
oxygen species and their role in the pathogenesis of periodontitis
remain unclear (45). Studies in
human subjects (46–49) have revealed that periodontal disease
is associated with systemic oxidative stress that induces some
minor localized inflammation. Currently, the 3-day systemic
antibiotic adjunctive regimen used in non-surgical periodontal
therapy has been compared with a 7-day regimen in only two studies
conducted by the same investigators who only evaluated changes in
clinical periodontal parameters (4,30).
In this study, we compared various periodontal
clinical parameters, microbiological changes, and systemic
oxidative stress in patients with chronic periodontitis who
received adjuvant systemic administration of AMX and MTZ following
non-surgical periodontal therapy. These comparisons were made
between groups of patients who received short-term (3 days) or
long-term (7 days) antibiotic treatment regimens. Additionally, we
assessed changes in the resistance of subgingival pathogens to the
prescribed antibiotics before and after their use in periodontal
treatment.
Patients and methods
Between October 2017 and August 2018, this
prospective, placebo-controlled, triple-blinded, randomized
clinical trial enrolled 46 subjects who were outpatients at the
Clinic of Periodontology of the Faculty of Dental Medicine of the
‘Victor Babeş’ University of Medicine and Pharmacy in Timișoara,
Romania. The study protocol was approved by the Research Ethics
Committee of the ‘Victor Babeş’ University of Medicine and Pharmacy
(approval no. 06/07.05.2018). The study is registered in the ISRCTN
Registry of Clinical Trials (ISRCTN12816166), and follows the
guidelines described in the CONSORT 2010 statement on clinical
trials. The study was conducted over a period of 14 months (October
2017-December 2018) in accordance with principles outlined in the
Declaration of Helsinki on experimentation involving human
subjects. All subjects were informed about the nature and purpose
of the study, and each subject signed an informed consent document
giving permission for the dental procedures and sampling of
biological material.
Patient population
The study population consisted of males and females
(mean age, 46.24±12.81 years; range, 27–80 years) who had clinical
and radiographic signs of generalized chronic periodontitis (CP),
as described by Armitage in 1999 (50). The study eligibility criteria
included the presence of at least 10 natural teeth that were
distributed in all four quadrants. Among the teeth, at least six
had to exhibit one site with a pocket depth (PD) ≥5 mm at baseline.
Subjects who had received periodontal therapy or taken an
antibiotic during the previous six months were excluded from the
study. Other exclusion criteria included: any systemic disorder
that might affect the progression and treatment of periodontal
disease (eg. diabetes type 1 or 2), taking a medication that might
interact with amoxicillin or metronidazole (eg. coumarin
derivatives, alcohol derivatives, 5-fluorouracil/disulfiram
derivatives, oral solutions with amprenavir, lopinavir/ritonavir,
methotrexate or tetracyclines, oral contraceptives, mebendazole,
busulfan, timidazole, vitamin K antagonists, barbiturates and
lithium), pregnancy and lactation. To be classified as a smoker,
the subject had to report smoking >10 cigarettes per day
(Tonetti et al 1995) (51).
Clinical measurements
All patients underwent a clinical and radiographic
baseline (before therapy) examination that assessed the following
parameters: Periodontal pocket depth (PPD), clinical attachment
level (CAL), full mouth bleeding score (FMBS), and full-mouth
plaque score (FMPS). The evaluation results were recorded in a
periodontal chart (http://www.periodontalchart-online.com/uk/) that was
subsequently saved in a pdf format, printed, and attached to the
patient's observation file. Three months later, measurements of PPD
and the CAL were made at six sites per tooth (mesio-buccal, buccal,
disto-buccal, disto-lingual, lingual, and mesio-lingual) at all
teeth, excluding third molars, to the nearest millimeter with a
periodontal probe (PCPUNC 15; Hu-Friedy), and using the
cement-enamel junction (CEJ) as a reference point for CAL. When the
CEJ was missing, measurements were made by using the most apically
located margin of the restoration as a reference point. Tooth
mobility was assessed based on the Miller classification system
(1985) (52), and furcation
involvement (FI) was assessed by using a Nabers probe #2N hdl #7,
markings: 3-6-9-12 mm, (Hu-Friedy®), with the results
classified according to Hamp et al 1975 (53).
After completing the measurements, all pockets with
PPD ≥4 mm were scaled and root planed under local anesthesia with
Gracey curettes (Hu-Friedy®) and ultrasonic instruments
(Piezon® 250; Electro Medical Systems SA) by the same
clinician (SB) who followed the protocol used for One-Stage
Full-Mouth Disinfection-OSFMD (54).
As home care, the patients were advised to rinse their mouth twice
daily for 2 min with a 0.2% chlorhexidine digluconate solution
(Dentaton; Ghimas S.p.A.) for 14 days.
Randomization, blinding, and treatment
allocation
At the end of the non-surgical therapy session, one
investigator (the randomizer, SIS) used a number generator
(www.random.org) to assign each patient to one of
three treatment groups. Each position on the randomization list was
associated with a medication package number that corresponded to a
pre-packed medication bag that contained instructions for
medication intake. One bag was handed to each patient. The patients
in group A (control group, n=14) received non-surgical periodontal
treatment plus treatment with placebos for 7 days. Patients in
group B (n=16) received non-surgical periodontal treatment combined
with the systemic administration of AMX and MTZ (SRP + AMX + MTZ;
500 mg, TID) for 3 days, followed by treatment with placebos for 4
days. Patients in group C (test group, n=16) received non-surgical
periodontal treatment combined with SRP + AMX + MTZ (500 mg TID)
for 7 days.
Each medication bag contained four identical vials,
and each vial contained tasteless and identical types of capsules.
Each vial was numbered, and the patient was instructed to take one
capsule from each vial every 8 h as follows: from vials no. 1 and 2
during the first 3 days after SRP, and from vials no. 3 and 4
during the following 4 days. The placebo group (group A) had only
placebo capsules in all the vials. Group B had AMX and MTZ in vials
no. 1 and 2, and placebo in vials no. 3 and 4. Group C had AMX and
MTZ in all four vials. The medication bags were prepared by the
pharmacy of the University of Medicine and Pharmacy ‘Victor Babeş’.
A followup appointment was scheduled for 2 weeks after each patient
had begun taking their medication. During that appointment, each
patient was asked whether they had experienced any adverse reaction
or breached the study's inclusion criteria. The SB, the SIS and the
patients were blinded against the antibiotic regimens.
Microbiological sampling
During the initial evaluation, samples of
subgingival plaque were collected from the deepest periodontal
pockets in each quadrant and used to identify the existing
bacterial strains and their resistance to systemic antimicrobial
agents prior to treatment. This protocol was repeated at the
three-month re-evaluation to assess post-treatment bacterial
suppression and identify strain resistance after long- or
short-term antibiotic intake periods. Samples were collected by
using eight sterile paper points (ProTaper Next® Paper
Points X2; Dentsply Sirona) that were inserted into the gingival
sulcus after having removed any supragingival plaque with sterile
cotton gauzes, isolated the site with cotton rolls, and taken
measures to avoid contamination with saliva. After the targeted
tooth surface was dried with a gentle air spray, the paper points
were left in situ for 30 sec until they were completely
soaked.
Eight paper points were inserted in each patient,
and four of those paper points were inserted into sterile sealed
Eppendorf tubes and sent for polymerase chain reaction (PCR)
testing that was performed with a commercial
Micro-Ident® Kit (Hain Lifescience GmbH). The samples
were tested for the following bacterial strains: Aggregatibacter
actinomycetemcomitans (Aa), Porphyromonas
gingivalis (Pg), Prevotella intermedia
(Pi), Tanererella forsythia (Tf), and
Treponema denticola (Td). The PCR testing was
conducted at the laboratories of the Department of Biochemistry of
the ‘Victor Babeş’ University of Medicine and Pharmacy. Following
15 min of vortex mixing at room temperature, the cones were removed
and the eluates clarified by centrifugation for 5 min at 3,000 × g
at 23°C. The samples were stored for 1 day at −20°C, and then at
−80°C until a microbiological analysis was performed no more than
30 days later.
Genetic identification of
periodontopathogenic bacterial species and assessment of
antimicrobial resistance
DNA was extracted by using a QIAamp DNA Micro Kit
(Qiagen GmbH) according to the manufacturer's protocol. The
absolute yield and quality of the extracted DNA were assessed by
using a NanoDrop ND-1000 spectrophotometer (Thermo Fisher
Scientific Inc.). Semiquantitative assessments of bacteria were
performed using a commercial testkit-system (micro-IDent plus; Hain
Lifescience GmbH). Amplification was performed in a thermocycler
(Thermo Fisher Scientific Inc.) and using HotStar Taq polymerase
(Qiagen GmbH). Results were recorded and classified into the
following categories: 0, nondetectable; 1, 104
(103 for Aa); 2, 104−105
(103−104 for Aa); 3,
105−106 (104−105 for
Aa); and 4, >107 (106 for
Aa).
The other four paper points were placed in vials
that contained thioglycolate and resazurin (bioMérieux®)
and sent to the laboratories of the Department of Microbiology
within 1 h after sampling. The inoculated thioglycolate tubes were
agitated for 30 sec, and the homogenized samples were seeded into
Columbia-agar plates and Schaedler-agar plates
(bioMérieux®) (0.2 ml per plate). For Aa, TSBV
(Triptic Soy-Serum-Bacitracin-Vancomycin-Agar) (55) plates were used. After seeding, the
plates were incubated under anaerobic conditions at 37°C (GENbag
anaero; bioMérieux) for 48 h, after which, they were re-incubated
and evaluated for 7 days. The different strains of bacteria were
identified by using ViteK2 ANC Kits (bioMérieux®) (as
recommended in similar identification protocols) (56,57), and
Rapid ID 32A Kits (bioMérieux®). Bacterial resistance to
antibiotics was evaluated by comparing the identified bacterial
strains, at baseline and at three months. The minimum inhibitory
concentrations (MICs) for AMX and MTZ were determined by using the
Epsilometer technique (E-test®; AB Biodisk), on
Brucella Blood Agar plates (bioMérieux®). The MIC
values were expressed in units of µg/ml and were determined by
evaluating a drug concentration range of 0.02 to 256 µg/ml, and are
expressed in units of µg/ml. A control strain (Bacteroides
fragilis, ATCC 25285, Thermo Fisher Scientific) was also tested
for its identification and sensitivity to the antibiotics.
Oxidative stress assessment
Blood samples (1.3 ml) were collected from the
antecubital vein between 8:00 a.m. and 10:00 a.m., and transported
to the laboratories of the Department of Pathophysiology of the
‘Victor Babeş’ University of Medicine and Pharmacy within one hour
after the venipuncture. The samples were centrifuged for 10 min at
20°C at 2,057 × g. The supernatant was collected in an Eppendorf
tube and stored at −80°C until analysis. The derivatives reactive
oxygen metabolites (d-ROM) test and biologic antioxidant potential
(BAP) test were used to analyze reactive oxygen metabolites and
biological antioxidant potential, respectively, by use of
photometric methods (Diacron International®).
Supportive periodontal treatment
(SPT)
SPT was performed by the same clinician (SB) in all
patients on a three-month follow-up basis, The SPT consisted of
supra- and subgingival debridement by use of ultrasonic mechanical
instrumentation and prophylactic powder AIR FLOW®
CLASSIC powder (EMS) at all sites, in order to enhance biofilm
removal. During the follow-up appointment, various clinical
parameters (PPD, CAL, FMPS, FMBS, FI, and tooth mobility),
biochemical parameters (d-ROM and BAP), and microbiological changes
(Aa, Pg, Pi, Tf, Td and bacterial resistance) were recorded.
If necessary, individual oral hygiene was reinforced.
Intra-examiner reproducibility
In order to evaluate the intra-examiner
reproducibility of clinical data, five subjects who were not
involved with the study but satisfied the enrollment criteria were
evaluated on two occasions, 24 h apart. The intraclass correlation
coefficient (ICC) was used to assess the agreement of findings,
based on the PD measurements. The ICC was calculated using the R
package irr (59). An intra-examiner
agreement of 0.989 (95% CI, 0.916–0.999) was found.
Statistical analysis
All statistical analyses were performed using R
software, v. 3.4.0 (58).
Comparisons of clinical and microbiological parameters were
performed within each group (between the baseline and 3-month
re-evaluation), and also between groups. Changes between
appointments (Δ) were calculated as the difference between the
initial and final value of each parameter. The primary outcome was
the intergroup difference in reduction of PPD after intervention,
while CAL, FMPS, FMBS, number of sites with a PPD ≥6 mm (considered
deep periodontal pockets), and the microbiological parameters were
regarded as secondary outcomes. In order to achieve 80% power for
detecting a significant mean difference of 1 mm in the reduction of
PPD between groups (assuming a common standard deviation of 0.75 mm
and given significance level α=0.05), at least 14 patients needed
to be included in each group. The Pitman asymptotic relative
efficiency correction was applied in the sample size computation to
account for the use of nonparametric comparison tests. Changes in
the frequency of detection of major periopathogens were analyzed to
evaluate the microbiological status of the patients. Inter-group
comparisons were made using Kruskal-Wallis tests with post-hoc
Mann-Whitney tests for quantitative and ordinal variables, and
Fisher exact tests for proportions. The Benjamini-Hochberg method
was used to adjust P-values in multiple comparisons. Additionally,
comparisons among groups regarding changes that occurred in various
parameters between appointments after adjusting for baseline values
and smoking were made by rank-based ANCOVA (60) as performed with the R package
npsm (61). The absence of
interactions between groups and covariates (i.e. homogeneity of
regression slopes) was tested before considering adjusted
inference. Intra-group comparisons of clinical and biochemical
parameters and detection scores of pathogen species (Aa, Pg, Pi,
Td, Tf) between the baseline and 3 month re-evaluation
time-points, were performed using Wilcoxon Signed Rank tests. In
all analyses, a P-value <0.05 was considered to be statistically
significant.
Results
Patient population
The patient demographic characteristics are
described in Table I. The study
enrolled 46 patients (23 females and 23 males) with a mean age of
46.24±12.81 years. Among the enrolled patients, 28.26% were
smokers. No patient withdrew from the study during its
investigational period (Fig. 1), and
no adverse events attributable to treatment were reported.
 | Table I.Patient demographic characteristics
at baseline and P-values. |
Table I.
Patient demographic characteristics
at baseline and P-values.
|
Characteristics | Group A | Group B | Group C | P-value |
|---|
| Number of patients
(no.) | 14 | 16 | 16 | – |
| Age (years) | 46.71±12.99 | 51.88±14.51 | 40.19±7.90 | 0.052a |
| (min-max) | (30–67) | (33–80) | (27–54) |
|
| Females (%) | 9 (64.29) | 5 (31.25) | 9 (56.25) | 0.21b |
| Smokers (%) | 4 (28.57) | 7 (43.75) | 2 (12.50) | 0.147b |
Changes in clinical parameters
The PPD, CAL, and FMBS values, the incidence of deep
pockets (PPD ≥6 mm) and the corresponding CAL, and the number of
sites with a PPD ≥6 mm, all showed significant changes between the
initial and final evaluation in all three treatment groups. We also
found significant decreases in FMPS in groups A and B, but not in
group C (Table II). The mean
baseline values for the clinical parameters in the different study
groups are shown in Table III.
 | Table II.Intra-group comparisons of clinical
parameters before and after treatment, and the P-values of
differences (Wilcoxon test). |
Table II.
Intra-group comparisons of clinical
parameters before and after treatment, and the P-values of
differences (Wilcoxon test).
| Parameters | Study group | Baseline | 3-months
re-evaluation | P-value |
|---|
| PPD (mm) | A | 3.11±0.96 | 2.70±0.53 | 0.007a |
|
| B | 3.71±0.77 | 3.14±0.82 | 0.002a |
|
| C | 3.75±0.86 | 3.04±0.71 |
<0.001a |
| CAL (mm) | A | 3.47±1.19 | 3.08±0.73 | 0.017a |
|
| B | 4.38±1.19 | 3.83±1.16 | 0.001a |
|
| C | 4.56±1.89 | 3.83±1.58 |
<0.001a |
| FMPS (%) | A | 28.14±29.47 | 11.50±12.91 | 0.004a |
|
| B | 49.62±34.29 | 25.25±22.26 | 0.006a |
|
| C | 30.38±27.06 | 21.56±20.77 | 0.083 |
| FMBS (%) | A | 32.86±20.26 | 16.79±10.53 | 0.002a |
|
| B | 39.12±26.64 | 19.81±11.20 | 0.010a |
|
| C | 38.00±21.58 | 15.75±9.55 |
<0.001a |
| Number of sites
with PPD ≥6 mm | A | 12.36±17.59 | 4.21±5.32 | 0.004 |
|
| B | 18.69±24.43 | 7.56±11.27 |
<0.001a |
|
| C | 20.25±14.14 | 4.31±5.57 |
<0.001a |
| CAL of sites with
PPD ≥6 mm | A | 6.44±0.75 | 4.97±1.04 | 0.002a |
|
| B | 6.63±0.88 | 4.55±1.34 | 0.001a |
|
| C | 7.19±1.88 | 4.95±1.59 |
<0.001a |
 | Table III.Clinical characteristics of patients
at baseline and the p-values for comparisons between treatment
groups (Kruskal-Wallis test). |
Table III.
Clinical characteristics of patients
at baseline and the p-values for comparisons between treatment
groups (Kruskal-Wallis test).
|
Characteristics | Group A | Group B | Group C | P-value |
|---|
| PPD (mm) | 3.11±0.96 | 3.71±0.77 | 3.75±0.86 | 0.027a |
| CAL (mm) | 3.47±1.19 | 4.38±1.19 | 4.56±1.89 | 0.033a |
| FMPS (%) | 28.14±29.48 | 49.62±34.29 | 30.38±27.06 | 0.139 |
| FMBS (%) | 32.86±20.26 | 39.12±26.64 | 38.00±21.58 | 0.749 |
| Mean number of
sites with PPD 6 mm | 12.36±17.59 | 18.69±24.43 | 20.25±14.14 | 0.095 |
| PPD of sites with
PPD ≥6 mm | 6.26±0.39 | 6.56±0.85 | 6.55±0.43 | 0.170 |
| CAL of sites with
PPD ≥6 mm | 6.44±0.75 | 6.63±0.88 | 7.19±1.88 | 0.203 |
The baseline values for PPD and CAL showed
significant differences between groups (Table III). Post-hoc Mann-Whitney tests
revealed that the PPD values in group A were lower than those in
groups B and C (P=0.041 in both cases), whereas the baseline PPD
values in groups B and C were similar (P=1). Moreover, the values
for CAL in group A were lower than those in groups B and C (P=0.041
in both cases), and the latter two groups showed no significant
difference in CAL values (P=0.664). Table IV shows a comparison of changes that
occurred in the different groups between the baseline and 3 months
re-evaluation time-points. While more pronounced changes in PPD,
CAL and FMBS, and greater decreases in PPD and CAL in initial deep
sites were associated with a longer course of antibiotic intake,
those differences were not statistically significant. There were,
however, significant differences between groups with regard to the
decrease in the number of sites with a PPD ≥6 mm. Post-hoc tests
showed these differences were due to a greater decrease in group C
than in group A (P=0.023); however, the differences between groups
A and B, and between groups B and C, were not statistically
significant (P>0.05 in both cases). The absence of interactions
between groups and covariates was tested in all rank-based analyses
of covariance, and was confirmed in all cases, except for the
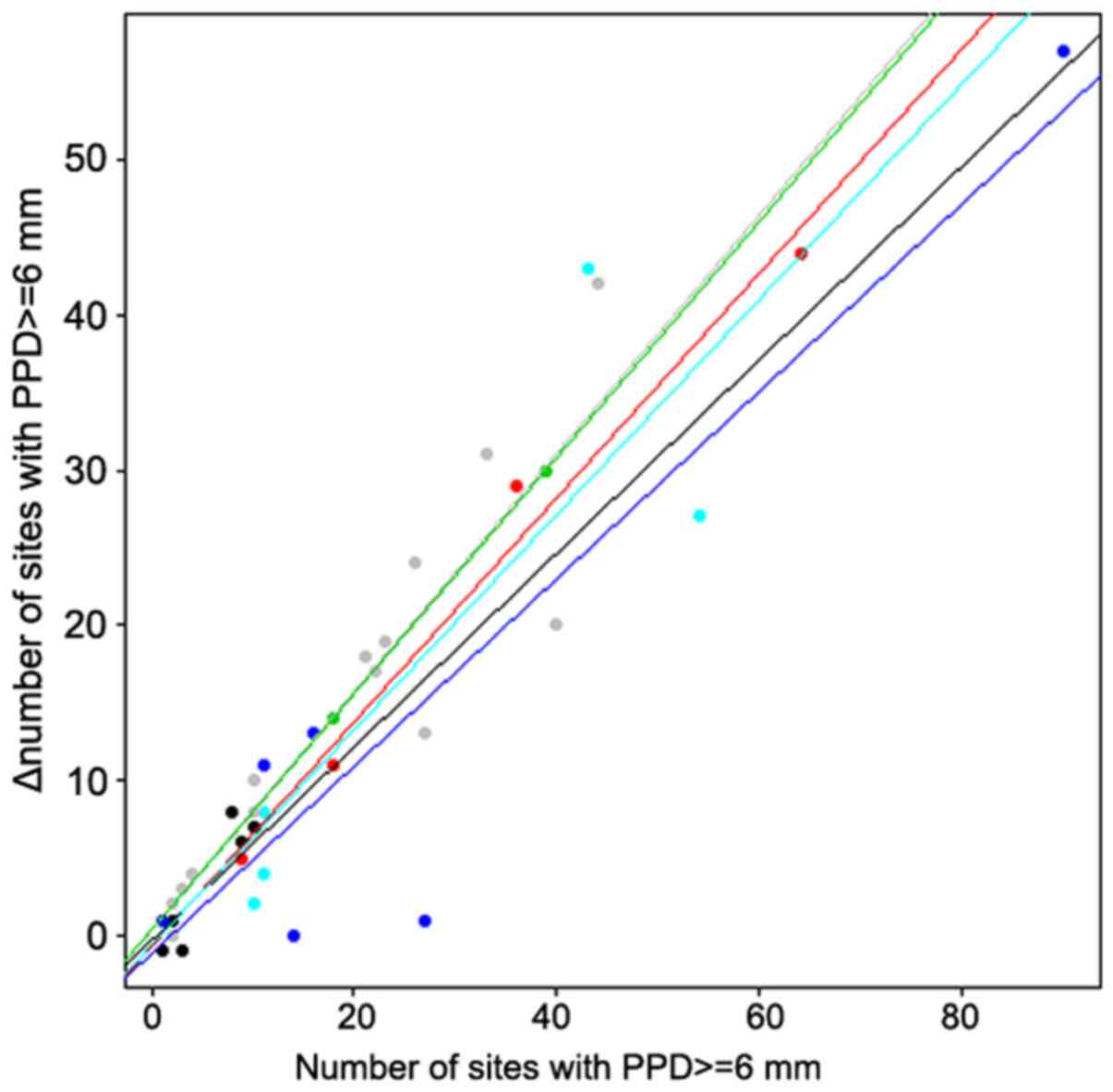
change in the number of sites with a PPD ≥6 mm (P=0.028).
Therefore, there was a significant interaction between groups and
covariates in that case, which can be visualized in Fig. 2. In groups A and B, when using a
fixed baseline value for the number of sites with a PPD ≥6 mm, the
decrease in the number of sites with a PPD ≥6 mm after treatment
was greater for smokers than for nonsmokers. Furthermore, that
decrease was more pronounced in group C than in the first two
groups, which showed no significant difference between smokers and
nonsmokers.
 | Table IV.Changes in clinical characteristics
between baseline and after three months, and the P-values for
comparisons between groups (Kruskal-Wallis test). |
Table IV.
Changes in clinical characteristics
between baseline and after three months, and the P-values for
comparisons between groups (Kruskal-Wallis test).
|
Characteristics | Group A | Group B | Group C | p1 | p2 |
|---|
| ΔPPD (mm) | 0.41±0.63 | 0.57±0.54 | 0.71±0.49 | 0.117 | 0.753 |
| ΔCAL (mm) | 0.39±0.63 | 0.55±0.47 | 0.73±0.67 | 0.135 | 0.282 |
| ΔFMPS (%) | 16.64±19.11 | 24.38±28.97 | 8.81±26.18 | 0.490 | 0.322 |
| ΔFMBS (%) | 16.07±15.28 | 19.31±24.21 | 22.25±17.18 | 0.537 | 0.769 |
| Δ mean number of
sites with PPD 6 mm | 8.14±12.87 | 11.12±16.84 | 15.94±11.74 | 0.053 | 0.050a |
| ΔPPD of sites with
PPD ≥6 mm | 1.58±0.96 | 1.78±0.98 | 2.07±0.79 | 0.321 | 0.518 |
| ΔCAL of sites with
PPD ≥6 mm | 1.46±0.91 | 1.64±1.05 | 2.24±1.10 | 0.164 | 0.313 |
Changes in microbiological
parameters
The detection scores for Aa, Pg, Pi, Tf and
Td at baseline were not significantly different among the
different treatment groups (P>0.05 for all species). In general,
the detection scores for those pathogens remained stationary or
decreased over time, with only a few exceptions, namely in the case
of Aa (1 patient in group A and 1 patient in group B) and
Pi (1 patient in group A and 1 patient in group B). The
decreases in detection scores for Pg, Tf and Td in
all three groups, and for Pi in groups B and C were
statistically significant. Significant differences among groups
were found regarding changes that occurred in the detection
frequency scores for Aa and Tf between baseline and
the 3-month re-evaluation (Table
VI). For Aa, Mann-Whitney tests showed differences
between groups A and C (P=0.048) and between groups B and C
(P=0.048), but not between groups A and B; whereas for Tf,
groups A and B were found to be different from group C (P<0.001
in both cases), but not from each other (P=0.920) (Table V).
 | Table VI.Antimicrobial susceptibility of the
periodontal isolates at baseline. |
Table VI.
Antimicrobial susceptibility of the
periodontal isolates at baseline.
|
|
| % |
|---|
|
|
|
|
|---|
| Microorganism and
antibiotics | Range of MIC
(mg/l) | S | I | R |
|---|
| Parvimonas
micra (N=12) |
|
AMX | ≤0.047–0.125 | 100 | 0 | 0 |
|
MTZ | ≤038–64 | 58.3 | 0 | 41.6 |
| Prevotella
intermedia (N=12) |
|
AMX | ≤0.064–0.125 | 100 | 0 | 0 |
|
MTZ | ≤0.5–3 | 100 | 0 | 0 |
| Actinomycens
naeslundii (N=9) |
|
AMX | ≤0.023–0.125 | 100 | 0 | 0 |
|
MTZ | ≤0.5–64 | 55.6 | 0 | 44.4 |
| Peptoniphilus
assayarolyticus (N=5) |
|
AMX | ≤0.125–0.47 | 100 | 0 | 0 |
|
MTZ | ≤1–4 | 100 | 0 | 0 |
| Porphyromonas
gingivalis (N=4) |
|
AMX | ≤0.125–0.23 | 100 | 0 | 0 |
|
MTZ | ≤0.5–1.5 | 100 | 0 | 0 |
|
Propionibacterium propionicus
(N=3) |
|
AMX | ≤0.125–0.19 | 100 | 0 | 0 |
|
MTZ | ≤1–32 | 66.6 | 0 | 33.3 |
| Gemella
morbillorum (N=2) |
|
AMX | ≤0.064–0.19 | 100 | 0 | 0 |
|
MTZ | ≤1–32 | 50 | 0 | 50 |
| Veillonella
spp. (N=2) |
|
AMX | ≤0.064–0.125 | 100 | 0 | 0 |
|
MTZ | ≤2–3 | 100 | 0 | 0 |
| Fusobacterium
nucleatum (N=2) |
|
AMX | ≤0.125–0.5 | 100 | 0 | 0 |
|
MTZ | ≤1.5–3 | 100 | 0 | 0 |
| Prevotella
bivia (N=2) |
|
AMX | ≤0.125–0.19 | 100 | 0 | 0 |
|
MTZ | 1 | 100 | 0 | 0 |
| Anaerococcus
prevotii (N=2) |
|
AMX | ≤0.064–0.125 | 100 | 0 | 0 |
|
MTZ | ≤0.5–64 | 50 | 0 | 0 |
| Other
(N=15)a |
|
AMX | ≤0.047–0.5 | 100 | 0 | 0 |
|
MTZ | ≤0.5–32 | 93.3 | 0 | 6.6 |
 | Table V.Detection scores for the species
Aa, Pg, Pi, Tf, Td at baseline and after 3 months in the
three groups. |
Table V.
Detection scores for the species
Aa, Pg, Pi, Tf, Td at baseline and after 3 months in the
three groups.
|
|
| Group A | Group B | Group C |
|
|---|
|
|
|
|
|
|
|
|---|
| Species | Detection
score | Baseline n (%) | 3 months n (%) | Baseline n (%) | 3 months n (%) | Baseline n (%) | 3 months n (%) |
P-valuec
Kruskal-Wallis test |
|---|
| Aa | 0 | 14 (100) | 13 (92.86) | 15 (93.75) | 15 (93.75) | 12 (75) | 15 (93.75) | 0.017a |
|
| 1 | – | – | 1 (6.25) | – | – | 1 (6.25) |
|
|
| 2 | – | – | – | 1 (6.25) | 1 (6.25) | – |
|
|
| 3 | – | 1 (7.14) | – | – | 3
(18.75) | – |
|
|
| 4 | – | – | – | – | – | – |
|
|
|
P-valueb | 1 | 1 | 0.095 |
|
|
| Wilcoxon tests for
intra-group comparison |
|
|
|
|
|
|
|
| Pg | 0 | – | 3
(21.43) | – | 4
(25) | 2 (12.5) | 13 (81.25) | 0.155 |
|
| 1 | 12 (85.71) | 11 (78.57) | 12 (75) | 12 (75) | 13 (81.25) | 3
(18.75) |
|
|
| 2 | 2
(14.29) | – | 4
(25) | – | 1 (6.25) | – |
|
|
| 3 | – | – | – | – | – | – |
|
|
| 4 | – | – | – | – | – | – |
|
|
|
P-valueb | 0.037a | 0.006a | 0.002a |
|
|
| Wilcoxon tests for
intra-group comparison |
|
|
|
|
|
|
|
| Pi | 0 | 6 (42.86) | 10 (71.43) | 5
(31.25) | 11 (68.75) | 4 (25) | 11 (68.75) | 0.443 |
|
| 1 | 8 (57.14) | 4
(28.57) | 11 (68.75) | 5
(31.25) | 10 (62.5) | 5
(31.25) |
|
|
| 2 | – | – | – | – | 2
(12.5) | – |
|
|
| 3 | – | – | – | – | – | – |
|
|
| 4 | – | – | – | – | – | – |
|
|
|
P-valueb | 0.129 | 0.041a | 0.003a |
|
|
| Wilcoxon tests for
intra-group comparison |
|
|
|
|
|
|
|
| Tf | 0 | – | 1 (7.14) | – | 1 (6.25) | 1 (6.25) | 10 (62.50) |
<0.001a |
|
| 1 | 4
(28.57) | 7 (50) | 5
(31.25) | 7
(43.75) | 5
(31.25) | 6
(37.50) |
|
|
| 2 | 9
(64.29) | 5 (35.72) | 9
(56.25) | 8 (50) | 5
(31.25) | – |
|
|
| 3 | 1 (7.14) | 1 (7.14) | 2 (12.5) | – | 5
(31.25) | – |
|
|
| 4 | – | – | – | – | – | – |
|
|
|
P-valueb | 0.037a | 0.048a |
<0.001a |
|
|
| Wilcoxon tests for
intra-group comparison |
|
|
|
|
|
|
|
| Td | 0 | – | 7 (50) | – | 8 (50) | 2
(12.5) | 14 (87.5) | 0.344 |
|
| 1 | 14 (100) | 7 (50) | 15 (93.75) | 8 (50) | 14 (87.5) | 2
(12.5) |
|
|
| 2 | – | – | 1 (6.25) | – | – | – |
|
|
| 3 | – | – | – | – | – | – |
|
|
| 4 | – | – | – | – | – | – |
|
|
|
P-valueb | 0.011a | 0.003a |
<0.001a |
|
|
| Wilcoxon tests for
intra-group comparison |
|
|
|
|
|
|
|
Evaluation of bacterial
resistance
A total of 69 bacterial isolates were identified in
the bacterial cultures (average of 1.5 isolates per patient), and
the isolates showed the following distribution pattern:
Parvimonas micra 17.39%, Prevotella intermedia
17.39%, Actinomyces naeslundii 13.04%, Peptoniphilus
assacharolyticus 7.24%, Porphyromonas gingivalis 5.79%,
Propionibacterium propionicus 4.34%, Gemella morbillorum,
Veillonella spp., Fusobacterium nucleatum, Prevotella bivia,
Anaerococcus prevotii, Actinomyces israeli 2.89% each, and
Bacteroides fragilis, Clostridium perfringens, Streptococcus
parasanguinis, Streptococcus agalactiae, Peptostreptococcus
anaerobius, Bacteroides ureolyticus, Actinomyces viscosus,
Clostridium clostridioforme, Eubacterium limosum, Pseudomonas
stutzeri, Peptostreptococcus anaerobius, Streptococcus oralis,
Streptococcus mitis, Prevotella disiens, Bacteroides spp. -
below 2% each. The antimicrobial susceptibility (MIC range),
percentage of susceptible, and the intermediate and resistant
strains of the different species identified at baseline are
presented in Table VI.
At the three-month reassessment, the total number of
isolates was considerably reduced; however, 27 species could still
be identified: Actinomyces naeslundii 22.22%,
Peptoniphilus assacharolyticus 14.81%, Propionibecterium
propionicus 11.11%, Actinomyces israelii, Veillonella
spp. and Prevotella intermedia 7.40% each. Parvimonas
micra, Porphyromonas gingivalis, Gemella morbillorum, Clostridium
perfringens, Prevotella bivia, Peptostreptococcus anaerobius,
Eubacterium limosum, and Anaerococcus prevotii were less
than 1.4% each. The antimicrobial susceptibility (MIC range),
percentage of ‘susceptible’, ‘intermediate’ and ‘resistant’ strains
of the different species identified at 3 months are presented in
Table VII.
 | Table VII.Antimicrobial susceptibility of the
periodontal isolates at the 3-month re-evaluation. |
Table VII.
Antimicrobial susceptibility of the
periodontal isolates at the 3-month re-evaluation.
|
|
| % |
|---|
|
|
|
|
|---|
| Microorganism and
antibiotics | Range of MIC
(mg/l) | S | I | R |
|---|
| Actinomyces
naeslundii (N=6) |
|
AMX | ≤0.064–0.5 | 100 | 0 | 0 |
|
MTZ | ≤0.5–1 | 100 | 0 | 0 |
| Peptoniphilus
assacharolyticus N=(4) |
|
AMX | 0.125 | 100 | 0 | 0 |
|
MTZ | ≤1–4 | 100 | 0 | 0 |
|
Propionibacterium propionicus
(N=3) |
|
AMX | ≤0.032–0.125 | 100 | 0 | 0 |
|
MTZ | ≤1.5–32 | 100 | 0 | 0 |
| Actinomyces
israelli (N=2) |
|
AMX | ≤0.125–4 | 50 | 0 | 50 |
|
MTZ | ≤1–4 | 100 | 0 | 0 |
| Veillonella
spp. (N=2) |
|
AMX | ≤0.032–0.12 | 100 | 0 | 0 |
|
MTZ | 1.5 | 100 | 0 | 0 |
| Prevotella
intermedia (N=2) |
|
AMX | 0.125 | 100 | 0 | 0 |
|
MTZ | ≤4–32 | 50 | 0 | 50 |
| Other
(N=8)a |
|
Amoxicillin | ≤0.032–6 | 87.5 | 0 | 12.5 |
|
Metronidazole | ≤2–64 | 87.5 |
| 12.5 |
There was no resistance to AMX prior to treatment;
however, after treatment, one strain of Actinomyces israelii
(group A) and one strain of Anareroccocus prevotii (group C)
were found to be resistant. The latter strain was identified in the
same patient in group C, and was classified as being antibiotic
sensitive prior to treatment, but later became resistant to both
AMX and MTZ.
Resistance to MTZ prior to treatment was identified
in a total of 13 strains (5 in group A, 5 in group B and 3 in group
C): 5 strains of Parvimonas micra (1 in group A, 2 in group
B, 2 in group C), 4 of Actinomyces naeslundii (3 in group A
and 1 in group B), 1 of Bacteriodes spp. (group A), 1 of
Gemella morbillorum (group B), 1 of Propionibacterium
propionicum (group B), and 1 of Anerococcus prevotii
(group C).
Resistance to MTZ after treatment was found in one
strain of Pi (group A) and one strain Anareroccocus
prevotii (group C).
Changes in oxidative stress
balance
There was a statistically significant decrease in
the mean d-ROM values in group C, when compared with the mean
baseline values (Table VIII).
 | Table VIII.Intra-group comparisons of the
oxidative stress values before and after treatment, and the
P-values of differences (Wilcoxon tests). |
Table VIII.
Intra-group comparisons of the
oxidative stress values before and after treatment, and the
P-values of differences (Wilcoxon tests).
| Oxidative stress
characteristics | Study group | Baseline | 3-months
re-evaluation | P-value |
|---|
| d-ROMs (Carratelli
units) | A |
499.50±114.24 |
498.40±126.84 | 0.903 |
|
| B |
523.60±127.75 |
495.70±109.99 | 0.130 |
|
| C |
550.40±139.73 |
429.60±127.09 |
<0.001a |
| BAP (µmol/l) | A |
1839±480.49 |
1879±527.45 | 0.903 |
|
| B |
1853±473.36 |
1814±482.59 | 0.597 |
|
| C |
1995±688.54 |
2072±509.07 | 0.117 |
There were also significant differences between
groups with respect to changes in d-ROM values (Table IX). The decreases in mean d-ROM
values in group C were significantly greater than those in groups A
(P=0.012) and B (P=0.025); however, there was no difference between
groups A and B (P=0.525).
 | Table IX.Changes in oxidative stress
characteristics between baseline and the 3-month re-evaluation. |
Table IX.
Changes in oxidative stress
characteristics between baseline and the 3-month re-evaluation.
| Oxidative stress
characteristics | Group A | Group B | Group C | p1 | p2 |
|---|
| Δd-ROM | 1.08±130.19 | 27.88±115.56 | 120.80±89.03 | 0.009a | 0.018 |
| ΔBAP | −40.64±847.33 | 39.17±699.87 | −76.54±931.86 | 0.394 | 0.231 |
Significant positive linear associations were found
only in group C, and those occurred between changes in d-ROM values
and the number of sites with a PPD ≥6 mm. Negative linear
associations were found between d-ROM changes and changes in
FMPS.
Discussion
To the best of our knowledge, this is the first
study to investigate the clinical and microbiological effects of
two different regimens of systemic antibiotic therapy given
adjunctive to non-surgical periodontal therapy in patients with
periodontitis. Moreover, this study also investigated changes that
occurred in systemic oxidative stress markers and the resistance of
main periopathogens. Unlike recent studies that only focused on
clinical changes in moderate and deep pockets (4,30), our
study also considered the mean full-mouth PPD as an endpoint.
As expected, the mean values for clinical parameters
significantly improved in the intra-group comparisons, with the
interesting exception of FMPS, for which the mean values
significantly decreased in groups A and B, but not in group C. No
explanation could be found for this outcome in the context of the
better clinical outcomes in group C at three months. The mean value
for intra-group full-mouth PPD reduction significantly decreased in
group B between baseline (3.71±0.77 mm) and 3 months (3.14±0.820
mm, P=0.02), and also in group C from baseline (3.75±0.86 mm) to 3
months (3.04±0.71 mm, P<0.001). These results showed that both
antibiotic regimens exhibited better clinical efficacy than that
obtained by using SRP alone, and are similar to results from other
studies that chose mean full-mouth PPD or CAL as the primary
outcome (13,20,24,25,62) to
evaluate the efficacy of various antibiotic regimens. Intra-group
comparisons showed that the mean CAL value significantly improved
in all three groups, and while inter-group comparisons showed more
pronounced improvements in group C, the differences were not
statistically significant. These results are in line to those
obtained by Carvalho et al (3), who used a treatment regimen of SRP +
MTZ (400 mg TID for 10 days) and by Matarazzo et al
(20), who treated patients with SRP
+ AMX (500 mg) + MTZ (400 mg) for 14 days.
An analysis of pockets ≥6 mm in depth, which are
known to harbour biofilm that is more difficult to reached with
mechanical instrumentation, was performed in our study in the
context of the antibiotic systemic medication given adjunctive to
SRP (12). The use of antibiotics
for 7 days significantly reduced the number of sites with a PD ≥6
mm, when compared with the placebo group (P=0.023). No
statistically significant difference was found between groups A and
B or between groups B and C.
Regarding the smoking status of the patients, in
both groups A and B, the decrease in the number of sites with PPD
≥6 mm was greater for smokers than for nonsmokers. This can be
partially explained by the fact that the smokers had more sites
with PPD ≥6 mm at baseline than did the non-smokers. The decrease
was most pronounced in group C, where there was no difference
between the smokers and nonsmokers; however, given the small sample
size and the small number of smokers in each group, this result
should be interpreted with caution.
When prescribing a systemic antibiotic regimen for
periodontal infection, the issues of antibiotic resistant species
being present prior to treatment and the possible creation of
antibiotic resistant species are of particular importance. When
prescribing antibiotics, practitioners have considered the chance
for side effects, the possibility of bacterial resistance, as well
as the chance of success when using the recommended dose of
antibiotics (63). However, several
studies demonstrated that systemically administered antibiotics
resulted in transitory selection of subgingival bacterial strains
resistant to tetracycline (64),
doxycycline (DOX) (65,66), AMX and MTZ (36). DOX-resistant baseline isolates were
reported in 12% of the total counts by Walker (67), 0.90% by Fiehn and Westergaard
(65), and 6% by Feres et al
(66).
No bacterial strains resistant to AMX were
identified at baseline; however, 13 strains were resistant to MTZ
at baseline. These resistant strains constituted 18% of the total
strains, and were present in all the treatment groups, even though
none of the patients had received antibiotics in the previous 6
months, and the patients who harbored the resistant isolates did
not recall having previously taken MTZ. In a similar study with 48
patients, bacterial species were identified in cultures, and the
five most common morphotypes were analyzed by PCR (37). Antibiotic susceptibility was
determined by the E-test®, which was also used in this
study. Among 261 identified isolates, the investigators found that
the S. viridans resistance rates for AMX, clindamycin, and
tetracycline were 0, 10 and 9–22%, respectively. As for
azithromycin, the resistance rates ranged from 18.2% for S.
sanguis to 47.7% for S. mitis. Prevotella
isolates were susceptible to AMX-clavulanic acid, but showed AMX
resistance rates of 17.1% for P. buccae and 26.3% for P.
denticola. Less than 6% of all Prevotella species were
resistant to MTZ, while 21.1% were resistant to clindamycin. In
this study, the highest resistance rates prior to therapy were
found for P. micra (7.24%) and A. naeslundii (5.79%).
Moreover, in the present study, the data confirmed that there was
no resistance to AMX prior to therapy. Similar results (no
resistance to AMX prior to therapy) were also found by Herrera
et al (68) and Ardila et
al (69); however, those
investigators found strains that were resistant to azithromycin
(68) and clindamycin (69).
The evolution of antibiotic resistance in this study
is similar to that reported in a study conducted with 20 patients
who were taking systemic DOX (100 mg/day) for 14 days (66). Despite an increase in resistance
detected during the treatment phase of that study (from 6±2 to
48±9%), those investigators found that the resistance decreased to
25±6% 2 weeks later, and to 9±2% at 90 days post-treatment. Similar
results were obtained by Fiehn and Westergaard (65), who found that the percentage of
organisms resistant to 10 µg/ml DOX increased from a baseline level
of 0.9 to 18.5% one week after completion of a 3-week treatment
regimen, and then subsequently returned to baseline levels at ~15
weeks. In a later study, resistant species were identified in
subgingival and saliva samples obtained from patients with chronic
periodontitis being treated with SRP and adjuvant antibiotic
therapy (AMX or MTZ) (36). That
study enrolled 20 patients who had undergone clinical and
microbiological assessments and were randomly assigned to two
groups: AMX 500 mg, TID × 14 days or MTZ 250 mg, TID × 14 days. It
was found that the resistant isolates detected during or after
therapy had also been detected prior to therapy. The most common
resistant species in the MTZ group were A. naeslundii, S.
constellatus, S. mitis, S. oralis, A. odontolyticus, and S.
sanguis, and in AMX treatment group were S. constellatus, P.
nigrescens, E. saburreum, A. naeslundii, S. oralis, P.
melaninogenica, and P. intermedia. While treatment with
systemic antibiotics gradually increased the percentage of
resistant subgingival species, a major component of the subgingival
plaque remained sensitive to antibiotics during their
administration. However, the antibiotic resistant isolates returned
to their baseline levels after 90 days. Other strains identified in
this study with antibiotic resistance prior to therapy were P.
micra, Bacteroides spp., Gemella morbillorum,
Propionibacterium propionicum and Anerococcus
prevotii.
In an in vitro study, Rams et al
(33) found that 30.3% of bacterial
strains were resistant to MTZ prior to therapy. In another study,
van Winkelhoff et al (34)
evaluated the antibiotic resistance of Aa in 118 patients
with localized, generalized, and refractory periodontitis. Similar
to this study, significant reductions in PPD and increases in CAL
were obtained in almost all patients. Four patients remained
positive for Aa after therapy. MTZ resistance was observed
in two out of four strains in those patients. In this study, we
similarly found a greater percentage of MTZ-resistant isolates
prior to therapy (18.84%) than after 3 months of therapy
(7.40%).
Data from the literature attest that periodontitis
patients are more susceptible to an imbalance in
oxidative-antioxidant processes when compared with healthy subjects
(48,49), and periodontal therapy can have a
beneficial effect on both clinical and biochemical parameters. High
levels of oxidative species are usually found in subjects with risk
factors such as cigarette smoking, alcohol abuse, an unbalanced
diet, or who have diseases associated with changes in oxidative
balance, such as cardiovascular diseases, metabolic diseases,
neurodegenerative diseases, autoimmune rheumatic diseases
(rheumatoid arthritis, systemic lupus erythematosus), skin diseases
like psoriasis, and periodontitis (70,71). A
reduction in BAP suggests a direct correlation with reduced
activity of the plasmatic antioxidant barrier. This is often seen
in elderly people (72) who are
undergoing hemodialysis (73) and in
patients with metabolic syndrome (74), but may not always correlate with an
inflammatory periodontal status (75,76). In
the present study, there were significant differences between
groups with respect to changes in d-ROM values [a significantly
greater decrease in d-ROMs values in group C when compared to the
changes in group A (P=0.012) and group B (P=0.025)]. These results
were similar to those obtained by Tamaki et al (71), who showed that non-surgical
periodontal treatment improved both periodontal clinical parameters
and plasma d-ROMs values at a 2-month reassessment. This suggests a
close relationship between periodontal conditions and systemic
oxidative status. Moreover, a trend of reduction in d-ROM levels as
measured at 1 month after therapy was observed in a study conducted
by D'Aiuto et al (77). In
this study, BAP levels were not significantly correlated with
periodontal parameters, and this was similar to results reported by
Tamaki et al (75) and
Machida et al (76).
One limitation of the present study is the small
number of patients we investigated. The fact that the three groups
were not homogeneous with respect to PPD and CAL at baseline could
also represent a potential limitation. However, in our analysis of
changes that occurred between appointments, this deficiency was
handled by adjusting for the baseline values of the parameters,
while testing for group effects in the analysis of covariance.
Furthermore, the fact that one strain of Anaeroccocus
prevotii was identified in the same patient as being antibiotic
sensitive prior to treatment, and then became resistant to both AMX
and MTZ after 7 days, suggests that certain strains can acquire
bacterial resistance during systemic antibiotic therapy.
From a clinical point of view, our microbiologic
and oxidative stress data suggest that a 7-day systemic antibiotic
regimen remains the regimen of choice as an adjuvant to SRP, both
in smokers and non-smokers. Further studies with larger groups of
patients and longer follow-up times, possibly based on new
fundamental integrative investigation methods (e.g. cellomics,
which integrates genomics and proteomics of diseased tissues)
(78) will offer a new insight in
understanding the complex interactions that the periodontal
pathogens undergo with the host, both in the health, disease and
during systemic antimicrobial treatments (79). A future direction could also focus on
the in vitro resistance of bacterial species interacting
with periodontal cells grown on materials biocompatible with the
oral environment (80). More
clinical studies are needed, as well, to fully evaluate the
evolution of parameters and variables associated with periodontal
inflammation, and their correlation over time during different
antibiotic treatment regimens.
In conclusion, within the limitations of this
study, it can be concluded that non-surgical periodontal therapy
given in combination with a 7-day course of antibiotics proved more
effective for improving clinical parameters when compared with a
3-day course of antibiotics. The detection of several pathogenic
bacteria (Aa and Td) showed greater improvement with
the 7-day antibiotic regimen, as well as the systemic oxidative
stress markers (decrease in d-ROMs). Bacterial resistance was found
in fewer strains after treatment than prior to treatment.
Acknowledgements
The authors thank Dr Octavia Vela and Dr Viorelia
Rădulescu for their assistance with patient management. We also
thank Ms. Claudia Zaharia for her kind assistance with statistical
processing of the data.
Funding
This study was supported by internal funds from
‘Victor Babeş’ University of Medicine and Pharmacy, Timişoara,
Romania: Doctoral Grant no. 13901/19.11.2014.
Availability of data and materials
The datasets used/analyzed during the current study
are available from the corresponding author on reasonable
request.
Authors contributions
SB, FB, MB and SIS participated in study design,
sample collection, and data acquisition. SB, MB and SIS drafted the
manuscript and critically revised it for intellectual content. DR,
AA, DM, CB, FH and ERB contributed to data acquisition. SB and FB
contributed equally to this study, and can therefore be regarded as
first authors. All authors read and approved the final version of
the manuscript.
Ethics approval and consent to
participate
The study protocol was approved by the Research
Ethics Committee of the ‘Victor Babeş’ University of Medicine and
Pharmacy (approval no. 06/07.05.2018). All subjects were informed
about the nature and purpose of the study, and each subject signed
an informed consent document giving permission for the dental
procedures and sampling of biological material.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Egelberg J: Periodontics the Scientific
Way, Synopses of Clinical Studies1. 3rd. Odonto Science; Malmo: pp.
121999
|
|
2
|
Cugini MA, Haffajee AD, Smith C, Kent RL
Jr and Socransky SS: The effect of scaling and root planing on the
clinical and microbiological parameters of periodontal diseases:
12-month results. J Clin Periodontol. 27:30–36. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Carvalho LH, D'Avila GB, Leão A, Haffajee
AD, Socransky SS and Feres M: Scaling and root planing, systemic
metronidazole and professional plaque removal in the treatment of
chronic periodontitis in a Brazilian population. I. Clinical
results. J Clin Periodontol. 31:1070–1076. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Cosgarea R, Juncar R, Heumann C, Tristiu
R, Lascu L, Arweiler N, Stavropoulos A and Sculean A: Non-surgical
periodontal treatment in conjunction with 3 or 7 days systemic
administration of amoxicillin and metronidazole in severe chronic
periodontitis patients. A placebo-controlled randomized clinical
study. J Clin Periodontol. 43:767–777. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Renvert S, Wikström M, Dahlén G, Slots J
and Egelberg J: Effect of root debridement on the elimination of
Actinobacillus actinomycetemcomitans and Bacteroides
gingivalis from periodontal pockets. J Clin Periodontol.
17:345–350. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Mombelli A, Gmür R, Gobbi C and Lang NP:
Actinobacillus actinomycetemcomitans in adult periodontitis.
I. Topographic distribution before and after treatment. J
Periodontol. 65:820–826. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Marsh PD: Dental plaque: Biological
significance of a biofilm and community life-style. J Clin
Periodontol. 32 (Suppl 6):7–15. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
van Winkelhoff AJ, Rodenburg JP, Goené RJ,
Abbas F, Winkel EG and de Graaff J: Metronidazole plus amoxycillin
in the treatment of Actinobacillus actinomycetemcomitans
associated periodontitis. J Clin Periodontol. 16:128–131. 1989.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Pavicić MJ, van Winkelhoff AJ, Douqué NH,
Steures RW and de Graaff J: Microbiological and clinical effects of
metronidazole and amoxicillin in Actinobacillus
actinomycetemcomitans-associated periodontitis. A 2-year
evaluation. J Clin Periodontol. 21:107–112. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Flemmig TF, Milián E, Karch H and Klaiber
B: Differential clinical treatment outcome after systemic
metronidazole and amoxicillin in patients harboring
Actinobacillus actinomycetemcomitans and/or Porphyromonas
gingivalis. J Clin Periodontol. 25:380–387. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Goutoudi P, Diza E and Arvanitidou M:
Effect of periodontal therapy on crevicular fluid interleukin-1beta
and interleukin-10 levels in chronic periodontitis. J Dent.
32:511–520. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Guerrero A, Griffiths GS, Nibali L, Suvan
J, Moles DR, Laurell L and Tonetti MS: Adjunctive benefits of
systemic amoxicillin and metronidazole in non-surgical treatment of
generalized aggressive periodontitis: A randomized
placebo-controlled clinical trial. J Clin Periodontol.
32:1096–1107. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ehmke B, Moter A, Beikler T, Milian E and
Flemmig TF: Adjunctive antimicrobial therapy of periodontitis:
Long-term effects on disease progression and oral colonization. J
Periodontol. 76:749–759. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Cionca N, Giannopoulou C, Ugolotti G and
Mombelli A: Amoxicillin and metronidazole as an adjunct to
full-mouth scaling and root planing of chronic periodontitis. J
Periodontol. 80:364–371. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Cionca N, Giannopoulou C, Ugolotti G and
Mombelli A: Microbiologic testing and outcomes of full-mouth
scaling and root planing with or without amoxicillin/metronidazole
in chronic periodontitis. J Periodontol. 81:15–23. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ribeiro EP, Bittencourt S, Zanin IC, Bovi
Ambrosano GM, Sallum EA, Nociti FH Jr, Gonçalves RB and Casati MZ:
Full-mouth ultrasonic debridement associated with amoxicillin and
metronidazole in the treatment of severe chronic periodontitis. J
Periodontol. 80:1254–1264. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yek EC, Cintan S, Topcuoglu N, Kulekci G,
Issever H and Kantarci A: Efficacy of amoxicillin and metronidazole
combination for the management of generalized aggressive
periodontitis. J Periodontol. 81:964–974. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Rodrigues AS, Lourenção DS, Lima Neto LG,
Pannuti CM, Hirata RD, Hirata MH, Lotufo RF and De Micheli G:
Clinical and microbiologic evaluation, by real-time polymerase
chain reaction, of non-surgical treatment of aggressive
periodontitis associated with amoxicillin and metronidazole. J
Periodontol. 83:744–752. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Rooney J, Wade WG, Sprague SV, Newcombe RG
and Addy M: Adjunctive effects to non-surgical periodontal therapy
of systemic metronidazole and amoxycillin alone and combined. A
placebo controlled study. J Clin Periodontol. 29:342–350. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Matarazzo F, Figueiredo LC, Cruz SE,
Faveri M and Feres M: Clinical and microbiological benefits of
systemic metronidazole and amoxicillin in the treatment of smokers
with chronic periodontitis: A randomized placebo-controlled study.
J Clin Periodontol. 35:885–896. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Mestnik MJ, Feres M, Figueiredo LC, Duarte
PM, Lira EA and Faveri M: Short-term benefits of the adjunctive use
of metronidazole plus amoxicillin in the microbial profile and in
the clinical parameters of subjects with generalized aggressive
periodontitis. J Clin Periodontol. 37:353–365. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Mestnik MJ, Feres M, Figueiredo LC, Soares
G, Teles RP, Fermiano D, Duarte PM and Faveri M: The effects of
adjunctive metronidazole plus amoxicillin in the treatment of
generalized aggressive periodontitis: A 1-year double-blinded,
placebo-controlled, randomized clinical trial. J Clin Periodontol.
39:955–961. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Heller D, Varela VM, Silva-Senem MX,
Torres MC, Feres-Filho EJ and Colombo AP: Impact of systemic
antimicrobials combined with anti-infective mechanical debridement
on the microbiota of generalized aggressive periodontitis: A
6-month RCT. J Clin Periodontol. 38:355–364. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Silva MP, Feres M, Sirotto TA, Soares GM,
Mendes JA, Faveri M and Figueiredo LC: Clinical and microbiological
benefits of metronidazole alone or with amoxicillin as adjuncts in
the treatment of chronic periodontitis: A randomized
placebo-controlled clinical trial. J Clin Periodontol. 38:828–837.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Feres M, Soares GM, Mendes JA, Silva MP,
Faveri M, Teles R, Socransky SS and Figueiredo LC: Metronidazole
alone or with amoxicillin as adjuncts to non-surgical treatment of
chronic periodontitis: A 1-year double-blinded, placebo-controlled,
randomized clinical trial. J Clin Periodontol. 39:1149–1158. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Griffiths GS, Ayob R, Guerrero A, Nibali
L, Suvan J, Moles DR and Tonetti MS: Amoxicillin and metronidazole
as an adjunctive treatment in generalized aggressive periodontitis
at initial therapy or re-treatment: A randomized controlled
clinical trial. J Clin Periodontol. 38:43–49. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Jorgensen MG and Slots J: Responsible use
of antimicrobials in periodontics. J Calif Dent Assoc. 28:185–193.
2000.PubMed/NCBI
|
|
28
|
Newman MG, Tkei HH, Klokkevold PR and
Carranza FA: Newman and Carranza's Clinical Periodontology.1. 13th.
Elsevier; Amsterdam: 2019
|
|
29
|
Vogelman B and Craig WA: Kinetics of
antimicrobial activity. J Pediatr. 108:835–840. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Cosgarea R, Heumann C, Juncar R, Tristiu
R, Lascu L, Salvi GE, Arweiler NB and Sculean A: One year results
of a randomized controlled clinical study evaluating the effects of
non-surgical periodontal therapy of chronic periodontitis in
conjunction with three or seven days systemic administration of
amoxicillin/metronidazole. PLoS One. 12:e01795922017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Laxminarayan R, Duse A, Wattal C, Zaidi
AK, Wertheim HF, Sumpradit N, Vlieghe E, Hara GL, Gould IM,
Goossens H, et al: Antibiotic resistance-the need for global
solutions. Lancet Infect Dis. 13:1057–1098. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Mah MW and Memish ZA: Antibiotic
resistance. An impending crisis. Saudi Med J. 21:1125–1129.
2000.PubMed/NCBI
|
|
33
|
Rams TE, Degener JE and van Winkelhoff AJ:
Antibiotic resistance in human chronic periodontitis microbiota. J
Periodontol. 85:160–169. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
van Winkelhoff AJ, Tijhof CJ and de Graaff
J: Microbiological and clinical results of metronidazole plus
amoxicillin therapy in Actinobacillus
actinomycetemcomitans-associated periodontitis. J Periodontol.
63:52–57. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
van Winkelhoff AJ, Herrera D, Oteo A and
Sanz M: Antimicrobial profiles of periodontal pathogens isolated
from periodontitis patients in The Netherlands and Spain. J Clin
Periodontol. 32:893–898. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Feres M, Haffajee AD, Allard K, Som S,
Goodson JM and Socransky SS: Antibiotic resistance of subgingival
species during and after antibiotic therapy. J Clin Periodontol.
29:724–735. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Maestre JR, Bascones A, Sánchez P,
Matesanz P, Aguilar L, Giménez MJ, Pérez-Balcabao I, Granizo JJ and
Prieto J: Odontogenic bacteria in periodontal disease and
resistance patterns to common antibiotics used as treatment and
prophylaxis in odontology in Spain. Rev Esp Quimioter. 20:61–67.
2007.PubMed/NCBI
|
|
38
|
Lakhssassi N, Elhajoui N, Lodter JP,
Pineill JL and Sixou M: Antimicrobial susceptibility variation of
50 anaerobic periopathogens in aggressive periodontitis: An
interindividual variability study. Oral Microbiol Immunol.
20:244–252. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Dehelean CA, Soica C, Pinzaru I, Coricovac
D, Danciu C, Pavel I, Borcan F, Spandidos DA, Tsatsakis AM and
Baderca F: Sex differences and pathology status correlated to the
toxicity of some common carcinogens in experimental skin carcinoma.
Food Chem Toxicol. 95:149–158. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Pricop R, Cristea VC, Gheorghe I, Tatu AL,
Mihaescu G and Chifiriuc MC: Matrix-assisted laser
desorption/ionization time-of-flight mas spectrometry (MALDI-TOF
MS) reveals the anaerobic Slakia exigua as unique etiology
of a dental abscess. Biointerface Res Appl Chem. 7:1995–1997.
2017.
|
|
41
|
Oktay S, Chukkapalli SS, Rivera-Kweh MF,
Velsko IM, Holliday LS and Kesavalu L: Periodontitis in rats
induces systemic oxidative stress that is controlled by
bone-targeted antiresorptives. J Periodontol. 86:137–145. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Căruntu C, Boda D, Musat S, Căruntu A and
Mandache E: Stress-induced mast cell activation in glabrous and
hairy skin. Mediators Inflamm. 2014:1059502014. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Dahiya P, Kamal R, Gupta R, Bhardwaj R,
Chaudhary K and Kaur S: Reactive oxygen species in periodontitis. J
Indian Soc Periodontol. 17:411–416. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Robinson JM: Reactive oxygen species in
phagocytic leukocytes. Histochem Cell Biol. 130:281–297. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Tóthová L and Celec P: Oxidative stress
and antioxidants in the diagnosis and therapy of periodontitis.
Front Physiol. 8:10552017. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Tsai CC, Chen HS, Chen SL, Ho YP, Ho KY,
Wu YM and Hung CC: Lipid peroxidation: A possible role in the
induction and progression of chronic periodontitis. J Periodontal
Res. 40:378–384. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Konopka T, Król K, Kopeć W and Gerber H:
Total antioxidant status and 8-hydroxy-2-deoxyguanosine levels in
gingival and peripheral blood of periodontitis patients. Arch
Immunol Ther Exp (Warsz). 55:417–422. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Akalin FA, Baltacioğlu E, Alver A and
Karabulut E: Lipid peroxidation levels and total oxidant status in
serum, saliva and gingival crevicular fluid in patients with
chronic periodontitis. J Clin Periodontol. 34:558–565. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Chapple IL, Brock GR, Milward MR, Ling N
and Matthews JB: Compromised GCF total antioxidant capacity in
periodontitis: Cause or effect? J Clin Periodontol. 34:103–110.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Armitage GC: Development of a
classification system for periodontal diseases and conditions. Ann
Periodontol. 4:1–6. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Tonetti MS, Pini-Prato G and Cortellini P:
Effect of cigarette smoking on periodontal healing following GTR in
infrabony defects. A preliminary retrospective study. J Clin
Periodontol. 22:229–234. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Miller PD Jr: A classification of marginal
tissue recession. Int J Periodontics Restorative Dent. 5:8–13.
1985.PubMed/NCBI
|
|
53
|
Hamp SE, Nyman S and Lindhe J: Periodontal
treatment of multirooted teeth. Results after 5 years. J Clin
Periodontol. 2:126–135. 1975. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Quirynen M, Bollen CML, Vandekerckhove BN,
Dekeyser C, Papaioannou W and Eyssen H: Full- vs. partial-mouth
disinfection in the treatment of periodontal infections: Short-term
clinical and microbiological observations. J Dent Res.
74:1459–1467. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Slots J: Selective medium for isolation of
Actinobacillus actinomycetemcomitans. J Clin Microbiol.
15:606–609. 1982.PubMed/NCBI
|
|
56
|
Tatu AL, Merezeanu N, Pântea O, Gheorghe
I, Popa M, Banu O, Cristea VC, Chifiriuc MC, Lazăr V and Marutescu
L: Resistance features of Pseudomonas aeruginosa strains
isolated from patients with infectious complications of
cardiovascular surgery. Biointerface Res Appl Chem. 7:2004–2008.
2017.
|
|
57
|
Gheorghe I, Tatu AL, Lupu I, Thamer O,
Cotar AI, Pircalabioru GG, Popa M, Cristea VC, Lazar V and
Chifiriuc MC: Molecular characterization of virulence and
resistance features in Staphylococcus aureus clinical strains
isolated from cutaneous lesions in patients with drug adverse
reactions. Rom Biotechnol Lett. 22:12321–12327. 2017.
|
|
58
|
R Core Team, . A language and Environment
for Statistical ComputingFoundation for Statistical Computing;
Vienna: pp. 2012017
|
|
59
|
Gamer M, Lemon J, Fellows I and Singh P:
irr: Various Coefficients of Interrater Reliability and Agreement.
R package version 0.84.1. 2019.
|
|
60
|
Hettmansperger TP and McKean JW: Robust
nonparametric statistical methods.1. 2nd. Chapman & Hall; Boca
Raton, FL: 2011
|
|
61
|
Kloke J and McKean J: Npsm R package
nonparametric statistical methods using RCRC Press; Boca Raton, FL:
pp. 2872019
|
|
62
|
Winkel EG, Van Winkelhoff AJ, Timmerman
MF, Van der Velden U and Van der Weijden GA: Amoxicillin plus
metronidazole in the treatment of adult periodontitis patients. A
double-blind placebo-controlled study. J Clin Periodontol.
28:296–305. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Heta S and Robo I: The side effects of the
most commonly used group of antibiotics in periodontal treatments.
Med Sci (Basel). 6:E62018.PubMed/NCBI
|
|
64
|
Rodrigues RM, Gonçalves C, Souto R,
Feres-Filho EJ, Uzeda M and Colombo AP: Antibiotic resistance
profile of the subgingival microbiota following systemic or local
tetracycline therapy. J Clin Periodontol. 31:420–427. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Fiehn NE and Westergaard J:
Doxycycline-resistant bacteria in periodontally diseased
individuals after systemic doxycycline therapy and in healthy
individuals. Oral Microbiol Immunol. 5:219–222. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Feres M, Haffajee AD, Goncalves C, Allard
KA, Som S, Smith C, Goodson JM and Socransky SS: Systemic
doxycycline administration in the treatment of periodontal
infections (II). Effect on antibiotic resistance of subgingival
species. J Clin Periodontol. 26:784–792. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Walker CB: The acquisition of antibiotic
resistance in the periodontal microflora. Periodontol 2000.
10:79–88. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Herrera D, Roldán S, O'Connor A and Sanz
M: The periodontal abscess (II). Short-term clinical and
microbiological efficacy of 2 systemic antibiotic regimes. J Clin
Periodontol. 27:395–404. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Ardila CM, Granada MI and Guzmán IC:
Antibiotic resistance of subgingival species in chronic
periodontitis patients. J Periodontal Res. 45:557–563.
2010.PubMed/NCBI
|
|
70
|
Boda D, Negrei C, Nicolescu F and Balalau
C: Assessment of some oxidative stress parameters in methotrexate
treated psoriasis patients. Farmacia. 62:704–710. 2014.
|
|
71
|
Tamaki N, Tomofuji T, Ekuni D, Yamanaka R,
Yamamoto T and Morita M: Short-term effects of non-surgical
periodontal treatment on plasma level of reactive oxygen
metabolites in patients with chronic periodontitis. J Periodontol.
80:901–906. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Rahman K: Studies on free radicals,
antioxidants, and co-factors. Clin Interv Aging. 2:219–236.
2007.PubMed/NCBI
|
|
73
|
Nakayama K, Terawaki H, Nakayama M,
Iwabuchi M, Sato T and Ito S: Reduction of serum antioxidative
capacity during hemodialysis. Clin Exp Nephrol. 11:218–224. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Kim JH, Baik HW, Yoon YS, Joung HJ, Park
JS, Park SJ, Jang EJ, Park SW, Kim SJ, Kim MJ, et al: Measurement
of antioxidant capacity using the biological antioxidant potential
test and its role as a predictive marker of metabolic syndrome.
Korean J Intern Med (Korean Assoc Intern Med). 29:31–39. 2014.
|
|
75
|
Tamaki N, Tomofuji T, Maruyama T, Ekuni D,
Yamanaka R, Takeuchi N and Yamamoto T: Relationship between
periodontal condition and plasma reactive oxygen metabolites in
patients in the maintenance phase of periodontal treatment. J
Periodontol. 79:2136–2142. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Machida T, Tomofuji T, Ekuni D, Yamane M,
Yoneda T, Kawabata Y, Kataoka K, Tamaki N and Morita M:
Longitudinal relationship between plasma reactive oxygen
metabolites and periodontal condition in the maintenance phase of
periodontal treatment. Dis Markers. 2014:4892922014. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
D'Aiuto F, Nibali L, Parkar M, Patel K,
Suvan J and Donos N: Oxidative stress, systemic inflammation, and
severe periodontitis. J Dent Res. 89:1241–1246. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Boda D: Cellomics as integrative omics for
cancer. Curr Proteomics. 10:237–245. 2013. View Article : Google Scholar
|
|
79
|
Gupta A, Govila V and Saini A: Proteomics
- The research frontier in periodontics. J Oral Biol Craniofac Res.
5:46–52. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Calenic B, Greabu M, Caruntu C, Nicolescu
MI, Moraru L, Surdu-Bob CC, Badulescu M, Anghel A, Logofatu C and
Boda D: Oral keratinocyte stem cells behavior on diamond like
carbon films. Rom Biotechnol Lett. 21:11914–11922. 2016.
|