Introduction
Despite the recent advances in cancer therapy, this
malignancy remains a major source of morbidity and mortality
worldwide. Although some cases of cancer can often be treated
successfully by surgery and/or radiotherapy, chemotherapy remains
the preferred therapy. It is a well-known fact that most of the
existing cytostatic drugs allow for control of tumor growth only at
concentrations that also affect healthy cells, which leads to
undesirable side effects. Thus, it is imperative to find new
products with new action mechanisms, and one of the current
research directions is the use of cytotoxic antimicrobial peptides.
These could be the new tumoricidal molecules to be used in adjuvant
cancer therapy, with the potential to reduce cytostatic drug doses
and their toxic side effects.
Numerous studies have shown tumoricide properties of
some natural peptides known to be antimicrobial (1–3).
Therefore, composition in aminoacids, amfifaticity, cationic charge
and size allow cytotoxic peptides to attach and insert in the
phospholipidic cell membrane layers in order to form transmembrane
pores that will change the membrane permeability and will determine
cell development that will lead to apoptosis (2,3).
Considering all these aspects, our experimental research is aimed
at checking whether the cytotoxic peptides such as defensin and
cathelicidin LL37 have tumoricidal potential and whether the extent
of the effect depends on the nature and concentration of the
peptide used in the cells' living environment, but also on the type
of cell line experimentally used in vitro (4–6).
It is our goal to analyze the biological effect of
these peptides (defensin and cathelicidin LL37) on the tumor cell
lines HT-29 (colorectal carcinoma) and A-549 (human alveolar
carcinoma). In order to determine the modulating mechanism of the
chosen cytotoxic peptides the viability and gene expression of the
molecular targets (AKT, HIF-1α XBP, NRF2, PERK, CHOP, BCL2,
IRE1α, PI3K) were assessed, in the sense of activating or
inhibiting certain genes involved in the survival, growth,
proliferation and apoptosis pathways of tumor cells in the presence
or absence of the studied peptides (5–7).
Materials and methods
Peptides, cell lines and cell
cultures
Beta Defensin 1 human ≥98% (HPLC), 10% acetonitrile,
0.1% TFA in H2O, cationic peptides from Sigma product
no. SRP3011, lot no. 090202, molecular mass 7.8 kDa and
cathelicidin LL37 human ≥95% (HPLC), 10% acetonitrile, 0.1% TFA in
H2O, cationic peptides from GenScript product no.
RP-20332, lot no. PE1081804, molecular mass 4495.1
g.mol−1. Defensin and cathelicidin LL37 are positively
charged, amphiphatic molecules and preferentially bind to anionic
phospholipids from cell membranes with the formation of dynamic
peptide-lipid supramolecular pore and cell permeabilization.
The peptides under survey (Defensin and Cathelicidin
LL37) were freeze-dried in a RPMI-1640 (Sigma-Aldrich) medium (pH
7.35), in a sterile environment. Consecutive peptide dilutions with
RPMI-1640 (Sigma-Aldrich) were used for the two peptides, whereas
the working concentrations were 20 µM, 15 µM, and from 10 to 1 µM,
ten concentrations for 1 to 1 µM in 100 µl/well with an optimal and
constant number of cells by 105 tumor cells/well.
We used two adherent tumor cell lines: HT-29 is a
colorectal carcinoma cell line (ATCC® HTB-38™) and A549
is a human alveolar carcinoma adherent cell line (ATCC®
CCL-185™).
All the tumor cell lines were cultivated in
RPMI-1640 medium (Sigma-Aldrich) with 10% FBS (fetal bovine serum;
Gibco) (1). Triplicate cell lines
(with viability >97%) were prepared for each peptide
concentration. The negative control was represented by target cells
incubated without peptide. In order to prevent the edge effect and
preserve humidity in the plate, only culture medium was pipetted in
the edge columns and lines. Their viability was determined after 48
h of incubation at 37°C, 5% CO2, using the vital dye MTT
[3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] by
MTT Cell Proliferation Assay kit from Cayman, product no. 10009365,
lot no. 0518531. Absorbance was measured at 595 nm by reference at
wavelength of 620 nm with the FilterMax F5 Mode Microplate Reader
spectrophotometer.
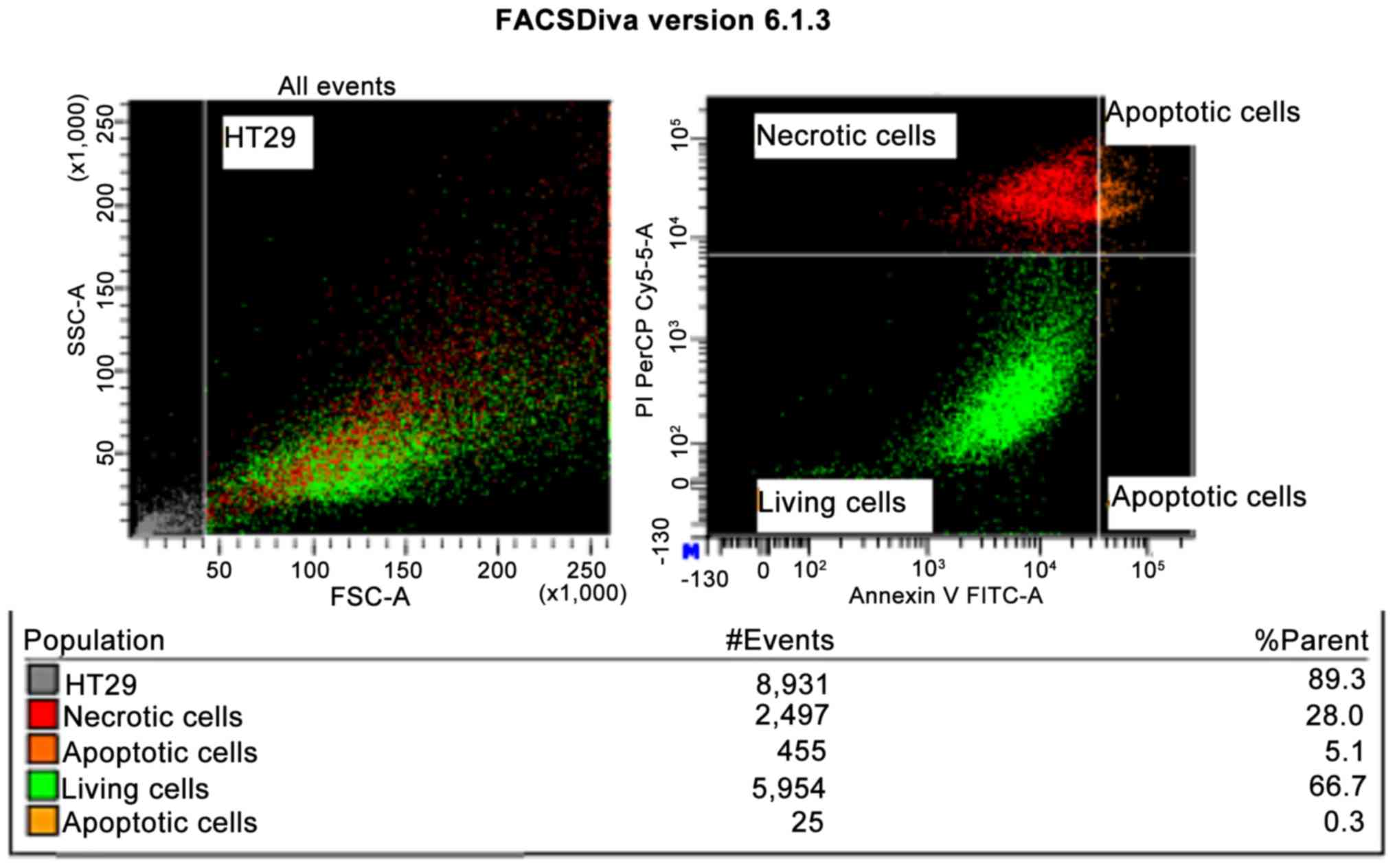
The second method for viability was flow cytometry
using Annexin V and propidium iodide (PI) from EXBIO by
ApoFlowEx® FITC kit product no. ED7044, lot no. 526846.
FACSCanto II cytometer was used for data acquisition and processing
using BD FACSDiva 6.12 program (both from Becton Dickinson
Biosciences) (8).
Molecular biology techniques were used to detect
metabolic changes in tumor cells, by assessing gene expression for
particular molecular targets.
RNA extraction from tumor cell cultures was
performed by automated methods with the RiboZol™ RNA Extraction
Reagent from VWR AMRESCO LLC, lot no. 1587C464. The RNA solution
was stored at −20°C until reverse transcription in cDNA was
performed. This process was accomplished by using the SuperScript
IV RT Enzymes kit from Promega, lot no. 0000361199 with Agilent
SureCycler 8800 equipment. The complementary DNA thus obtained may
be used immediately or stored for a short time at −20°C or for a
longer term at −80°C. The purity of the DNA introduced as matrix is
an important parameter for the success of a PCR reaction.
An in-house method of qRT-PCR with SYBR-Green
(Promega product no. A6001, lot no. 0000322091) was used. This
method requires a melting curve at the end of the amplification
stage, which translates into a slowly and incrementally growing
temperature gradient (1°C approximately every 2–3 sec) starting
from the hybridization temperature of the primers (Table I) up to the temperature of
denaturation of the entire reaction mixture.
 | Table I.Hybridization temperature of the
primers used in our study. |
Table I.
Hybridization temperature of the
primers used in our study.
| No crt. | Primers optimized
for the SYBR-Green method | Hybridization
temperature |
|---|
| XBP | Fw
CCTGGTTGCTGAAGAGGAGG | 88°C |
|
| Rev
CCATGGGGAGATGTTCTGGAG |
|
| CHOP | Fw
TTCTCTGGCTTGGCTGACTG | 66°C |
|
| Rev
CTGCGTATGTGGGATTGAGG |
|
| Nrf2 | Fw
AGTGGATCTGCCAACTACTC | 66°C |
|
| Rev
CATCTACAAACGGGAATGTCTG |
|
| AKT | Fw
TCTATGGCGCTGAGATTGTG | 66°C |
|
| Rev
CTTAATGTGCCCGTCCTTGT |
|
| Bcl2 | Fw
CTGCACCTGACGCCCTTCACC | 72°C |
|
| Rev
CACATGACCCCACCGAACTCAAAGA |
|
| HIF1α | Fw
CACTACCACTGCCACCACTG | 80°C |
|
| Rev
CCTTTTCCTGCTCTGTTTGG |
|
| PERK | Fw
CAGTGGCAATGAGAAGTGGA | 81°C |
|
| Rev
CAGTCAGCAACCGAAACCTT |
|
| GAPDH | Fw
GGGGCTCTCCAGAACATCAT | 88°C |
|
| Rev
AAGTGGTCGTTGAGGGCAAT |
|
| ABL | Fw
TGTGATTATAGCCTAAGACCCGGAGCTTTT | 55°C |
|
| Rev
TTCAGCGGCCAGTAGCATCTGACTT |
|
| IRE1α | Fw
GCCTCTCCCTCAATGGTACA | 88°C |
|
| Rev
TTGGTAGACGCAGACAGTGG |
|
| PI3K | Fw
CTGGAAAGAAGCTGGTTTGG | 85°C |
|
| Rev
CAGGTCATCCCCAGAGTTGT |
|
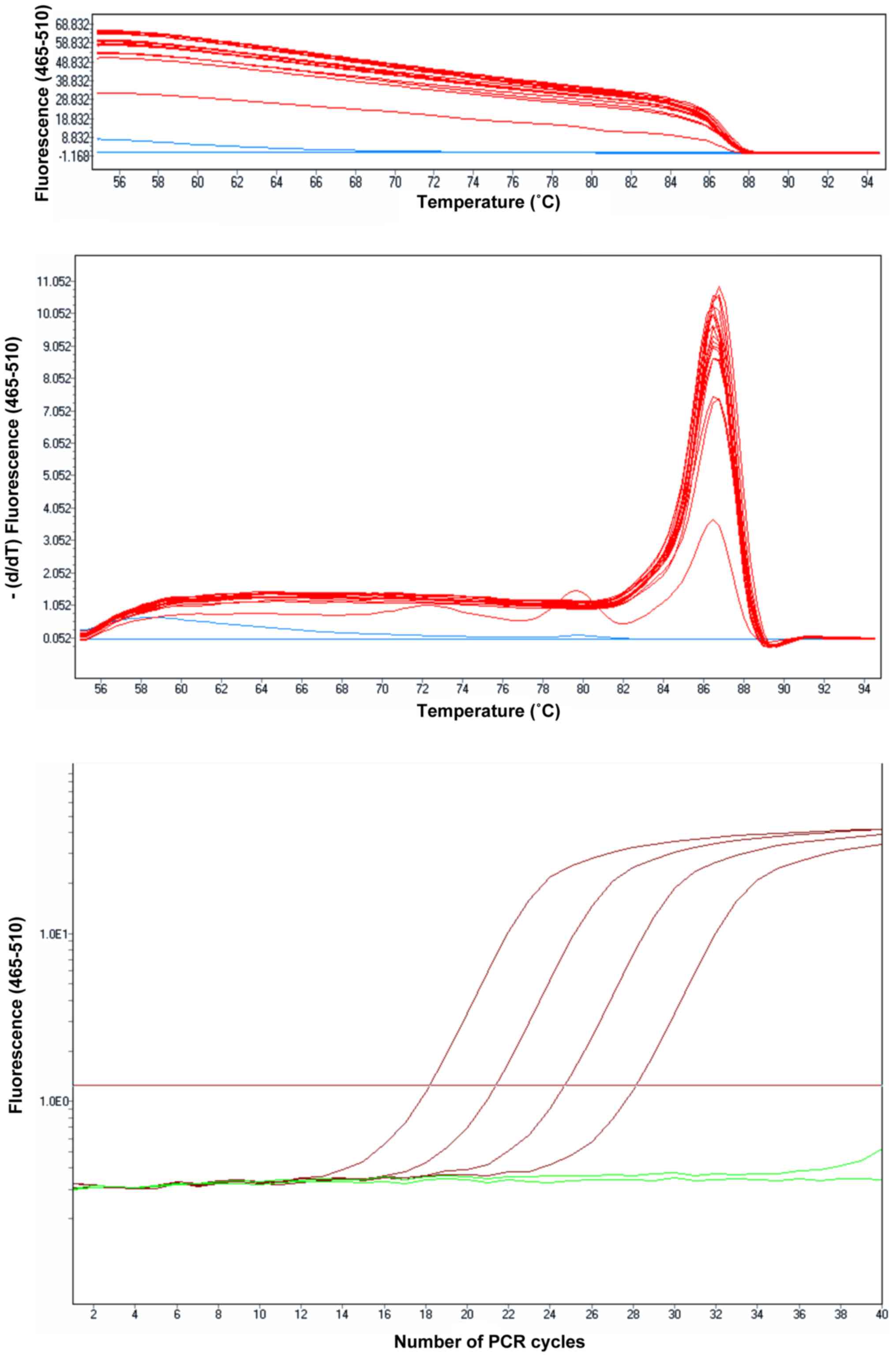
The fluorescence obtained by melting the reaction
products were read in each denaturation stage. Each product
resulting in the amplification reaction thus produces a melting
peak. Depending on the melting temperature of the product(s) and
the number of peaks obtained, the specificity of the reaction may
be interpreted (Fig. 1).
The desired amplicons of different concentrations
were obtained through RT-PCR amplification, which were compared to
the concentration of GAPDH extracted from each cell line used in
the experiment. Results were reported as percentages of increase or
decrease of the gene expression compared to the control, as
compared to the reference gene (GAPDH): % gene = number of
amplicon genes/µl/reference gene (GAPDH) ×100.
Statistical method
For the study we used GraphPad Prism 5, t-test and
Excel method of calculation for gene expression percent. Data
analysis results are presented as the mean ± S.E.M. Means of 2
continuous normally distributed variables were compared by
independent samples Student's t-test. P<0.05 was considered to
indicate a statistically significant difference.
Results
Determination of the cytotoxicity of
defensin and cathelicidin LL37
For the HT-29 tumor line, when the defensin
concentration was 4 µM, the MTT colorimetric technique revealed
intense cytotoxicity (P<0.001), which was also demonstrated by
flow cytometry, when a 33.4% apoptosis was obtained after 48 h
incubation (Fig. 1). Cytotoxicity
was also significant when incubation occurred at the high 15 µM and
20 µM concentrations. This was revealed by both the MTT method
(P<0.001) and by the flow cytometry method, with 57% apoptosis
at a defensin concentration of 15 µM, and only 10% for the control
(cells that were not incubated with the peptide).
For the A549 tumor line, when the defensin
concentration was 4 µM, the MTT colorimetric technique revealed
intensive statistically significant cytotoxicity (P<0.001),
which was also demonstrated by flow cytometry, when a significant
52% apoptosis was obtained after 48 h incubation (Fig. 2). Cytotoxicity was also intensive
when incubation occurred at the high 15 µM and 20 µM
concentrations. This was revealed by both the MTT method
(P<0.001) and by the flow cytometry method, with 32% apoptosis
and 10% cell mortality rate.
Addition of cathelicidin LL37, after 48 h
incubation, by flow cytometry revealed significant apoptosis only
for the high peptide concentrations (15 µM). Of these cells 83.67%
were in late apoptosis compared to the incubation of these cells at
4 µM concentrations, for which the behavior of the cells was
similar to that of the negative control.
Gene expression changes in the
selected molecular targets, under defensin action
The experimental findings achieved for the HT-29 and
A549 tumor cell lines, used molecular biology techniques to
determine the gene expression of some molecular targets involved in
the metabolic reprogramming of the tumor cell both in the presence
and in the absence of peptides with tumoricidal potential (defensin
and cathelicidin LL37). These molecular targets refer to genes
involved in the pro-apoptotic pathway (CHOP, XBP1, IRE1α,
PERK) and anti-apoptotic pathway (BCL2), as well as to
genes that stimulate cell proliferation (NRF2) and survival
(AKT, HIFα, PIK3).
The analysis of the findings revealed a significant
increase (80%) in the CHOP gene expression in the HT29 line
incubated with 4 µM defensin for 48 h (HT4), compared to the
control (HT0), and 2% increase for the A549 line incubated with 4
µM defensin for 48 h (A4), compared to control (A0). The increase
in CHOP gene expression was 16% in non-peptide cells 24 h after
incubation, and there was no change in the cells incubated with 15
µM peptide for 24 h.
The analysis of these findings revealed a
significant increase (302.67%) in the AKT gene expression in
the HT29 line incubated with 15 µM defensin for 48 h (HT15),
compared to the control (HT0), for which the increase reached
92.04% compared to the ABL reference gene. The increase for
the A549 line incubated with defensin was 76.48% (A15) compared to
A0.
For the NRF gene expression in the HT29 line,
a 2-fold decrease in gene expression in the 15 µM defensin-treated
tumor over the untreated line was observed. Also, approximately
fourfold decrease was observed in the A549 line treated with 4 µM
defensin incubated for 48 h (A4), compared to the control (A0).
The XBP gene expression presented a highly
significant 45-fold increase in the gene expression in the 4 µM
defensin-treated HT29 line and a 15-fold increase for the 15 µM
defensin concentration compared to the untreated line. Furthermore,
the increase was 248.32% in the A549 line incubated with 15 µM for
48 h (A15), compared to the control (A0, for which the increase was
94.2% compared to the ABL reference gene), showing, a 3-fold
increase in the gene expression in XBP.
The findings for the PERK gene expression
revealed a 2-fold decrease in the gene expression only in the A549
line incubated with 4 µM concentrated peptide, and showed no
expression whatsoever at higher peptide concentrations. Gene
expression is absent for the HT29 defensin-incubated line compared
to the control that expressed this gene.
Gene expression changes in the
selected molecular targets for HT29 and A549, under cathelicidin
LL37 action
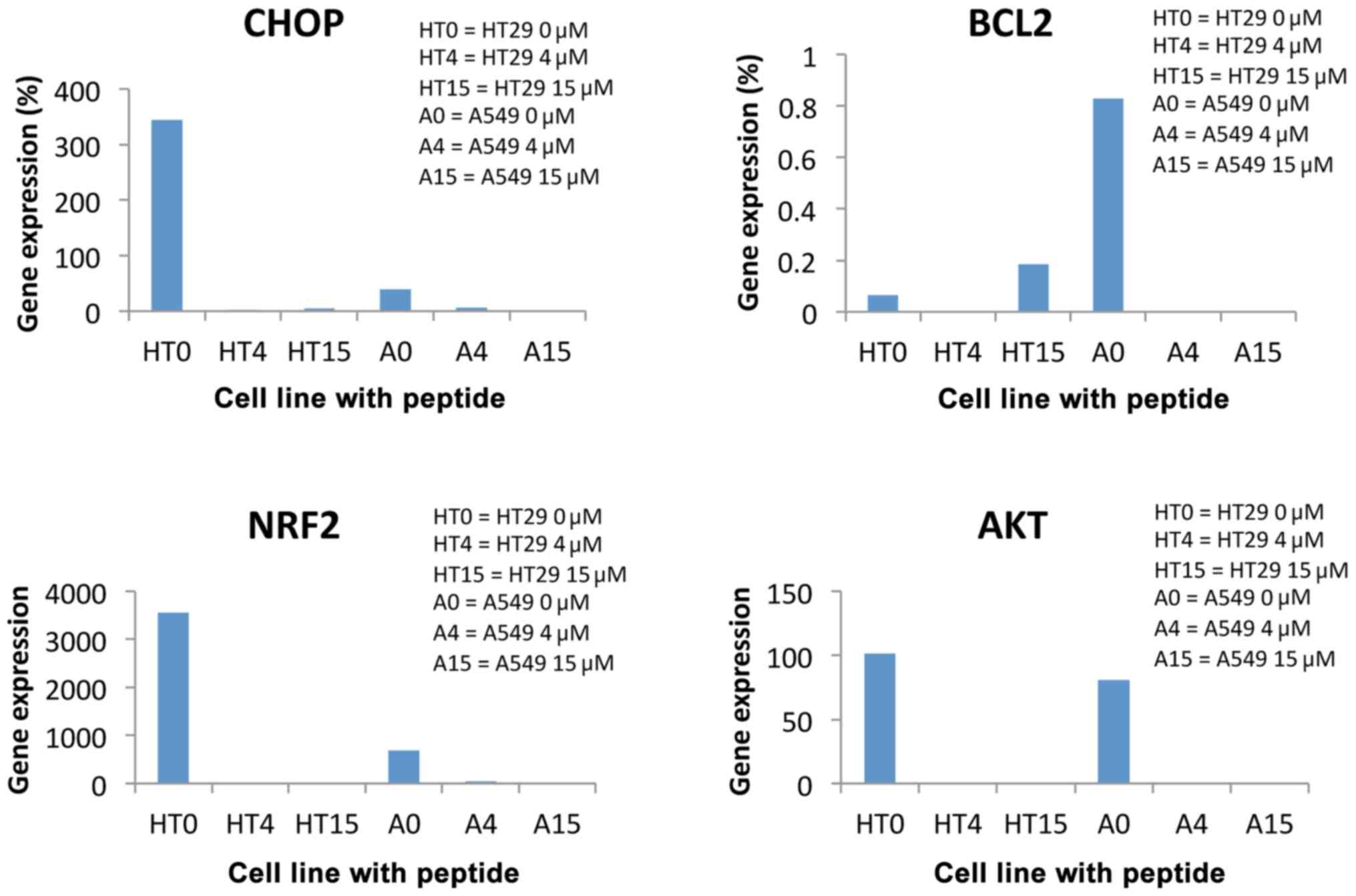
CHOP gene expression for the HT29 cell line
decreased 1.5-fold over the control and 300-fold as a percentage
relative to the reference gene ABL at 4 µM cathelicidin LL37
concentration. It also decreased significantly against the control
at 15 µM concentration. This indicates that under toxic conditions,
the CHOP gene that is involved in the pro-apoptotic pathway of the
cell decreases its expression and thus has an anti-apoptotic
effect. Compared to line A549, for which CHOP gene expression is
3-fold higher at 4 µM and only 2-fold higher at 15 µM. This
indicates that, at lower concentrations of cytotoxic peptide, the
neoplastic cell evolves towards apoptosis faster than at high
concentrations (Fig. 3).
The BCL2 gene expression is involved in the
anti-apoptotic pathway of the cell, i.e., in supporting its
survival and therefore, in toxic conditions, its gene expression
should decrease (Fig. 3).
When cathelicidin LL37 occurred in its living
environment, the HT29 line showed a 4.5-fold increase at 4 µM
concentrations and a 16-fold increase at 15 µM concentration of the
gene expression compared to the control, which means improved cell
defense. As for the A549 line, a significant 13-fold decrease was
noted in gene expression at 4 µM and a 19-fold decrease at 15 µM,
showing poorer cell defense. This proves that cathelicidin LL37 has
a tumoricidal effect on the A549 cell line.
The manner in which cathelicidin LL37 influenced
cell proliferation was determined by assessing the expression of
the NRF2 gene, which presented a 274-fold decrease at 4 µM
concentration and total suppression at 15 µM concentration, in the
HT29 line. Therefore, although it triggers an improvement in cell
defense by modifying pro- and anti-apoptotic gene expression, this
peptide significantly reduces or blocks tumor cell proliferation.
For the A549 line, a similar evolution was noted, as NRF2
gene expression decreased 58-fold at 4 µM concentration and 46-fold
at 15 µM concentration (Fig. 3).
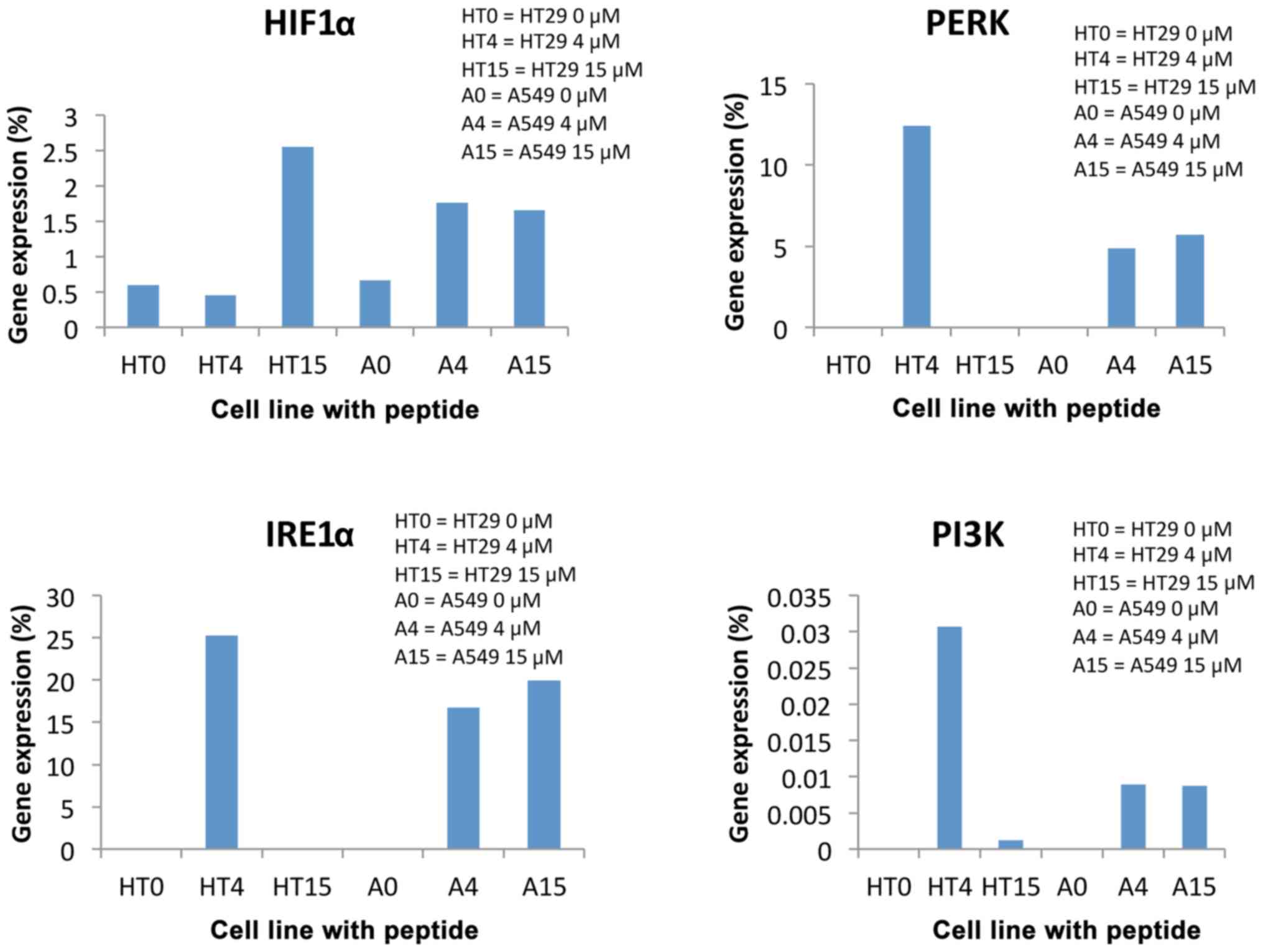
AKT gene expression decreased dramatically in
both cell lines, namely over 80 times regardless of the peptide
concentration (Fig. 4). This meant
that cell survival and proliferation was intensely inhibited
regardless of the peptide concentration, for both cell lines.
Our findings also revealed the presence of RE stress
in the tumor cells. Expression of IRE1α and PI3K genes was
not high for control tumor cells, only for peptide-incubated
cathelicidin LL37, which indicates the presence of stress in the
peptide-incubated tumor cells (Fig.
4).
Discussion
Our study was aimed at determining the tumoricidal
potential of defensin and cathelicidin LL37 by measuring cell
viability by flow cytometry for peptide concentrations which
triggered significant apoptosis as evaluated by the MTT method.
Defensin concentrations to test viability by flow cytometry at 4 µM
(P<0.01) and 15–20 µM (P<0.001) were used. The findings
achieved for the two cell lines HT-29 and A549 are different under
defensin action; at 15 µM concentration, cell apoptosis showed a
2-fold increase in the colorectal carcinoma line compared to the
alveolar carcinoma line. For cathelicidin LL37, the viability of
both tumor cell lines was significantly influenced, and toxicity
was significant (P<0.01).
Defensin, a cationic peptide, increased CHOP
gene expression in both tumor cell lines, which indicates the
increase of apoptotic protein synthesis as well as oxidative
stress, also emphasized by augmentation of XBP gene
expression. The findings achieved for the two cell lines are
significantly different under the action of defensin at 15 µM
concentrations, cell apoptosis was 2-fold higher in the colorectal
carcinoma line as compared to the alveolar carcinoma line. This
explains the increase of XBP gene expression in the HT29
cells, whose fight for survival was fiercer. ER stress may be a
potential target for the development of new cancer therapy able to
reduce adaptation to hypoxia, inflammation and angiogenesis of
tumor cells, so that resistance to cytostatic therapy may be
prevented (9–14).
On the other hand, CHOP has been shown to induce
tumor cell death by stimulating the synthesis of pro-apoptotic
proteins and by stimulating oxidative processes in stress-exposed
cancer cells (15). Under ER stress,
cancer cell increases COX2 expression via the NF-κB pathway, which
plays an important anti-apoptotic role. Also, NF-κB pathway
activation plays an important pro-inflammatory role through CHOP,
and stimulates IL-8 synthesis as is the case in human epithelial
cells (16–19).
Metabolic and inflammatory changes are important in
the carcinogenesis process as they increase protein assembly and ER
activity, leading to ER stress. The stress response of ER is
cytoprotective and is involved in cancer cell growth and adaptation
to the aggressiveness of its environment (20,21).
ER-located pancreatic ER kinase (PERK) is involved in
carcinogenesis. PERK gene expression decreased significantly
in the A549 line. The decrease or inhibition of the protein
synthesis of some unfolded protein response (UPR) components such
as PERK may be potential targets in cancer therapy.
Gene expression increased for members of the
BCL2 family that have an anti-apoptotic role has been
associated with resistance to chemotherapy in various cancers
(22). In our study, at low defensin
concentrations (4 µM) BCL2 decreased in both tumor cell
lines, thus proving a cytostatic effect by stimulating apoptosis.
In the HT29 line, defensin blocks BCL2 gene expression and
determines a decreased anti-apoptotic protein synthese at both 15
µM and 4 µM concentrations.
NRF2 gene expression (molecular marker for
cell proliferation) is significantly inhibited in both the HT29 and
A549 lines, the effect being a drastic limitation of cell
multiplication (cytostatic effect). The A549 cells were noted
(23) to turn glutamine in the
culture medium into glutathione under NRF2 action, thereby
accelerating the proliferation of A549 cells. NRF2 may be a
molecular target for cancer therapy by molecules that would inhibit
NRF2 activity in tumor cells, such as defensin (24,25).
HIF1α gene expression strongly decreased in
both tumor lines. HIF1α plays an important role in tumor
growth as it mediates tumor angiogenesis, proliferation and
invasion, by regulating the expression and activity of glycolytic
enzymes. Therefore, blocking HIF1α expression by defensin
may constitute a new and promising therapeutic option for the
treatment of tumors (26).
In the investigation carried out by us, AKT
gene expression increased significantly. The findings achieved for
the two cell lines HT-29 and A549 are significantly different under
defensin action. At 15 µM concentrations, cell apoptosis was 2-fold
higher for the colorectal carcinoma line compared to alveolar
carcinoma line. This explains AKT gene expression increase
in HT29 cells. AKT is a serine/threonine kinase, which,
activated by phosphorylation, leads to the decrease of the
synthesis of certain proteins with an effector role, so that
AKT becomes an important molecular target in cancer
therapy.
NRF2 plays a protective role in the tumor
cells as it is involved in resistance to cytostatic drugs and tumor
progression. Also, increased NRF2 gene expression is
associated with poor prognosis and multiple chemotherapy
resistance.
Cathelicidin LL37 incubation of the HT-29 and A549
tumor cell lines allowed the determination of molecular markers
used for toxicity monitoring and pro-apoptotic pathway assessment,
by CHOP gene expression and anti-apoptotic pathway
expression, respectively, in the BCL2 gene. CHOP gene
expression decrease and BCL2 increase in the HT29 line under
the action of cathelicidin LL37 indicates tumor cell pro-apoptosis
inhibition. Cell proliferation, tracked by NRF2 gene
expression, decreased significantly for both cell lines under the
action of the LL37 peptide. The greatly inhibited cell growth and
survival was highlighted by AKT gene expression
decrease.
IRE1α gene expression that stimulate cancer
progression, as well as PI3K gene expression (stimulate the
occurrence of membrane receptors on the surface of the tumor cells
for various growth factors) only increased for cathelicidin
LL37-incubated tumor cells indicating the presence of ER stress in
tumor cells and also resistance to therapy.
Therefore, exposure of the two tumor cell lines
(HT29, A549) to defensin or cathelicidin LL37 for 48 h was shown to
be cytotoxic at low peptide concentrations (4 µM).
In conclusion, the research conducted so far has
demonstrated that defensin and cathelicidin LL37 have cytostatic
effects on the HT29 and A549 tumor cell lines and allowed
identification of molecular markers able to assess the growth,
proliferation, survival, pro-apoptosis and anti-apoptosis pathways,
as well as the methods of assessment and monitoring of tumor cell
cytotoxicity by high-performance molecular biology techniques. In
order to monitor the cytostatic effect of defensin on tumor cells,
the following genes may be molecular targets for testing: i)
CHOP, BCL2 (by apoptosis activation); ii) XBP (by
oxidative stress augmentation in the cancer cell); iii) PERK,
AKT (by protein synthesis inhibition) and iv) HIF1a,
NRF2 (role in tumor proliferation and invasion). In order to
monitor the therapeutic effect of cathelicidin LL37 on tumor cells,
the following genes may be molecular targets for testing: i)
CHOP, BCL2 (by apoptosis activation); ii) AKT (by
protein synthesis inhibition) and iii) NRF2 (role in tumor
proliferation and invasion). The research conducted so far has
proven that: i) defensin (strong) and cathelicidin LL37 (moderate)
have cytostatic effects on the HT29 and A549 tumor cell lines and
ii) allowed the identification of molecular markers able to assess
the growth, proliferation, survival, pro-apoptosis and
anti-apoptosis pathways; iii) methods of assessment and monitoring
of tumor cell cytotoxicity by high-performance molecular biology
techniques.
Acknowledgements
The authors thank Organizing Institution of
University Doctoral Studies (IOSUD) of the University of Medicine
and Pharmacy ‘Grigore T. Popa’ (Iasi, Romania), for the financial
support to the PhD students: Teodor Ștefanache, Bogdan Mihail
Diaconescu (2014–2018).
Funding
Partial funding by UMF ‘Grigore T. Popa’ (Iasi,
Romania) was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
MB and NF provided the study concepts and guaranteed
the integrity of the entire study. TȘ, BMD, DJ were responsible for
the study design, for literature research and statistical analysis.
DJ and MLD were responsible for experimental studies and for data
acquisition. MC, CB, ER interpreted the data and revised the work
for important intellectual content. CR and OB analyzed the data of
the study and prepared the discusions and conclusions of the study.
CR, OB and DJ were responsible for manuscript preparation, editing
and review. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that the research was conducted
in the absence of any commercial or financial relationships that
could be construed as a potential conflict of interest.
References
|
1
|
Hilchie AL, Vale R, Zemlak TS and Hoskin
DW: Generation of a hematologic malignancy-selective membranolytic
peptide from the antimicrobial core (RRWQWR) of bovine
lactoferricin. Exp Mol Pathol. 95:192–198. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sato H and Feix JB: Peptide-membrane
interactions and mechanisms of membrane destruction by amphipathic
α-helical antimicrobial peptides. Biochim Biophys Acta.
1758:1245–1256. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Koczulla AR and Bals R: Antimicrobial
peptides: Current status and therapeutic potential. Drugs.
63:389–406. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Homayouni-Tabrizi M, Asoodeh A, Soltani M
and Forghanifard MM: Antimicrobial peptide Brevinin-2R induces the
secretion of a pro-inflamatory cytokine in HepG2 cells. J Bas Res
Med Sci. 2:23–29. 2015.
|
|
5
|
Karadag R, Bayram N, Oguztuzun S,
Bayramlar H, Bozer B, Simsek G and Rapuano CJ: An investigation of
human beta-defensins and cathelicidin expression in patients with
pterygium. Arq Bras Oftalmol. 80:277–280. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhuravel E, Shestakova T, Efanova O,
Yusefovich Y, Lytvin D, Soldatkina M and Pogrebnoy P: Human
beta-defensin-2 controls cell cycle in malignant epithelial cells:
In vitro study. Exp Oncol. 33:114–120. 2011.PubMed/NCBI
|
|
7
|
He M, Zhang H, Li Y, Wang G, Tang B, Zhao
J, Huang Y and Zheng J: Cathelicidin-derived antimicrobial peptides
inhibit zika virus through direct inactivation and interferon
pathway. Front Immunol. 9:7222018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Vermes I, Haanen C and Reutelingsperger C:
Flow cytometry of apoptotic cell death. J Immunol Methods.
243:167–190. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Schleicher SM, Moretti L, Varki V and Lu
B: Progress in the unraveling of the endoplasmic reticulum
stress/autophagy pathway and cancer: Implications for future
therapeutic approaches. Drug Resist Updat. 13:79–86. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Voiculescu VM, Caruntu C, Solomon I, Lupu
M, Ilie MA, Boda D, Constantin C and Neagu M: Squamous cell
carcinoma: Biomarkers and potential therapeutic targetsHuman Skin
Cancers-Pathways, Mechanisms, Targets and Treatments. Blumenberg M:
IntechOpen; London: pp. 135–159. 2018
|
|
11
|
Neagu M, Caruntu C, Constantin C, Boda D,
Zurac S, Spandidos DA and Tsatsakis AM: Chemically induced skin
carcinogenesis: Updates in experimental models (Review). Oncol Rep.
35:2516–2528. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Boda D: Cellomics as integrative omics for
cancer. Curr Proteomics. 10:237–245. 2013. View Article : Google Scholar
|
|
13
|
Neagu M, Constantin C, Tanase C and Boda
D: Patented biomarker panels in early detection of cancer. Recent
Pat Biomark. 1:10–24. 2011. View Article : Google Scholar
|
|
14
|
Kraskiewicz H and FitzGerald U:
InterfERing with endoplasmic reticulum stress. Trends Pharmacol
Sci. 33:53–63. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zinszner H, Kuroda M, Wang X, Batchvarova
N, Lightfoot RT, Remotti H, Stevens JL and Ron D: CHOP is
implicated in programmed cell death in response to impaired
function of the endoplasmic reticulum. Genes Dev. 12:982–995. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Park SH, Choi HJ, Yang H, Do KH, Kim J,
Lee DW and Moon Y: Endoplasmic reticulum stress-activated C/EBP
homologous protein enhances nuclear factor-kappaB signals via
repression of peroxisome proliferator-activated receptor gamma. J
Biol Chem. 285:35330–35339. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lupu M, Caruntu A, Caruntu C, Papagheorghe
LML, Ilie MA, Voiculescu V, Boda D, Constantin C, Tanase C, Sifaki
M, Drakoulis N, Mamoulakis C, Tzanakakis G, Neagu M, Spandidos DA,
Izotov B and Tsatsakis A: Neuroendocrine factors: The missing link
in non melanoma skin cancer. Oncol Rep. 38:1327–13401. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Boda D, Docea AO, Calina D, Ilie MA,
Caruntu C, Zurac S, Neagu M, Constantin C, Branisteanu DE,
Voiculescu V, et al: Human papilloma virus: Apprehending the link
with carcinogenesis and unveiling new research avenues (Review).
Int J Oncol. 52:637–655. 2018.PubMed/NCBI
|
|
19
|
Solomon I, Voiculescu VM, Caruntu C, Lupu
M, Popa A, Ilie MA, Albulescu R, Caruntu A, Tanase C, Constantin C,
et al: Neuroendocrine factors and head and neck squamous cell
carcinoma: An affair to remember. Dis Markers. 2018:97878312018.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Healy SJ, Gorman AM, Mousavi-Shafaei P,
Gupta S and Samali A: Targeting the endoplasmic reticulum-stress
response as an anticancer strategy. Eur J Pharmacol. 625:234–246.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Cioplea M, Caruntu C, Zurac S, Bastian A,
Sticlaru L, Cioroianu A, Boda D, Jugulete G, Nichita L and Popp C:
Dendritic cell distribution in mycosis fungoides vs. inflammatory
dermatosis and other T-cell skin lymphoma. Oncol Lett.
17:4055–4059. 2019.PubMed/NCBI
|
|
22
|
Del Poeta G, Venditti A, Del Principe MI,
Maurillo L, Buccisano F, Tamburini A, Cox MC, Franchi A, Bruno A,
Mazzone C, et al: Amount of spontaneous apoptosis detected by
Bax/Bcl-2 ratio predicts outcome in acute myeloid leukemia (AML).
Blood. 101:2125–2131. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Mitsuishi Y, Taguchi K, Kawatani Y,
Shibata T, Nukiwa T, Aburatani H, Yamamoto M and Motohashi H: Nrf2
redirects glucose and glutamine into anabolic pathways in metabolic
reprogramming. Cancer Cell. 22:66–79. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ren D, Villeneuve NF, Jiang T, Wu T, Lau
A, Toppin HA and Zhang DD: Brusatol enhances the efficacy of
chemotherapy by inhibiting the Nrf2-mediated defense mechanism.
Proc Natl Acad Sci USA. 108:1433–1438. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Magesh S, Chen Y and Hu L: Small molecule
modulators of Keap1-Nrf2-ARE pathway as potential preventive and
therapeutic agents. Med Res Rev. 32:687–726. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kong D, Park EJ, Stephen AG, Calvani M,
Cardellina JH, Monks A, Fisher RJ, Shoemaker RH and Melillo G:
Echinomycin, a small-molecule inhibitor of hypoxia-inducible
factor-1 DNA-binding activity. Cancer Res. 65:9047–9055. 2005.
View Article : Google Scholar : PubMed/NCBI
|