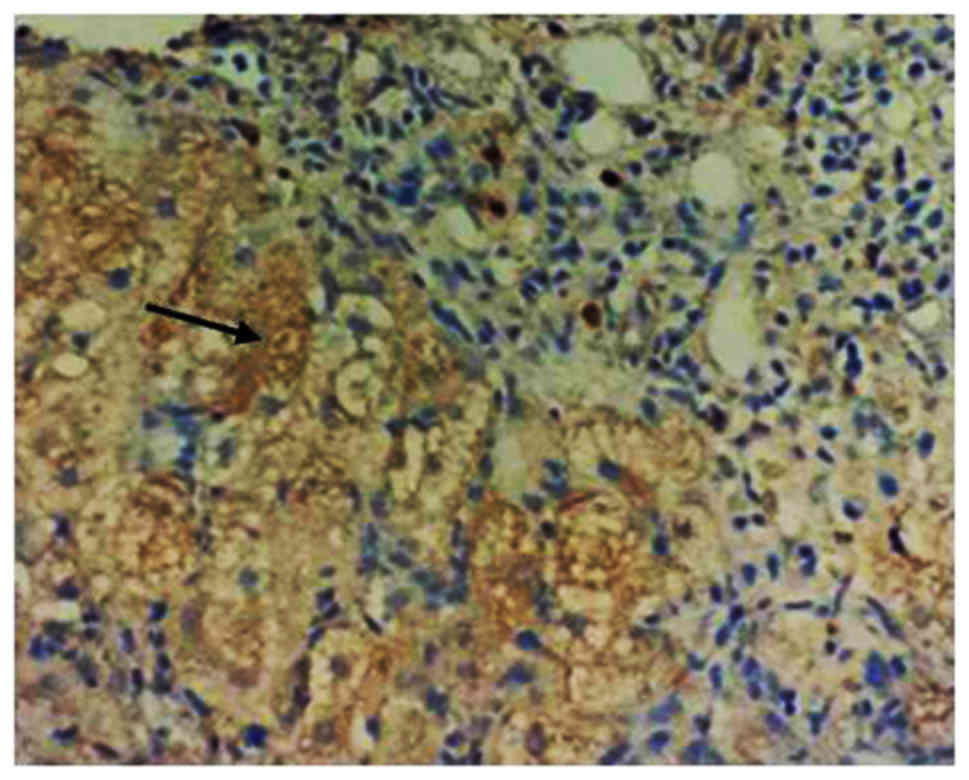
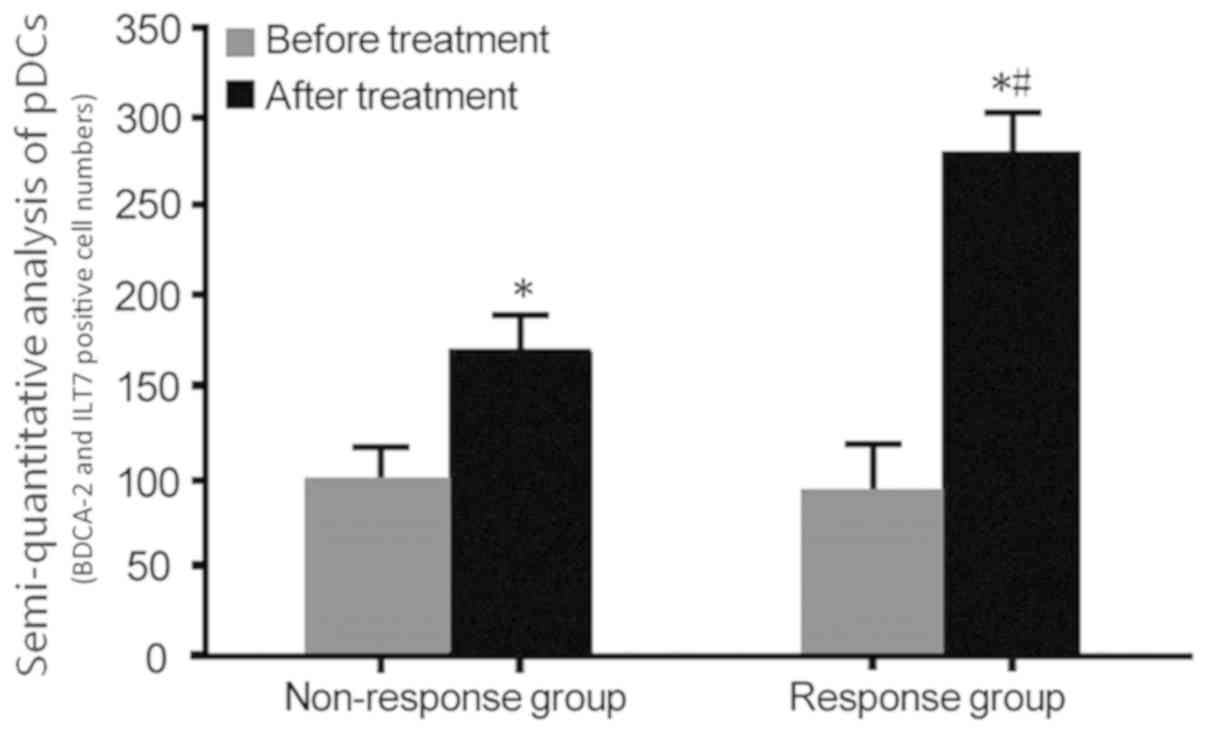
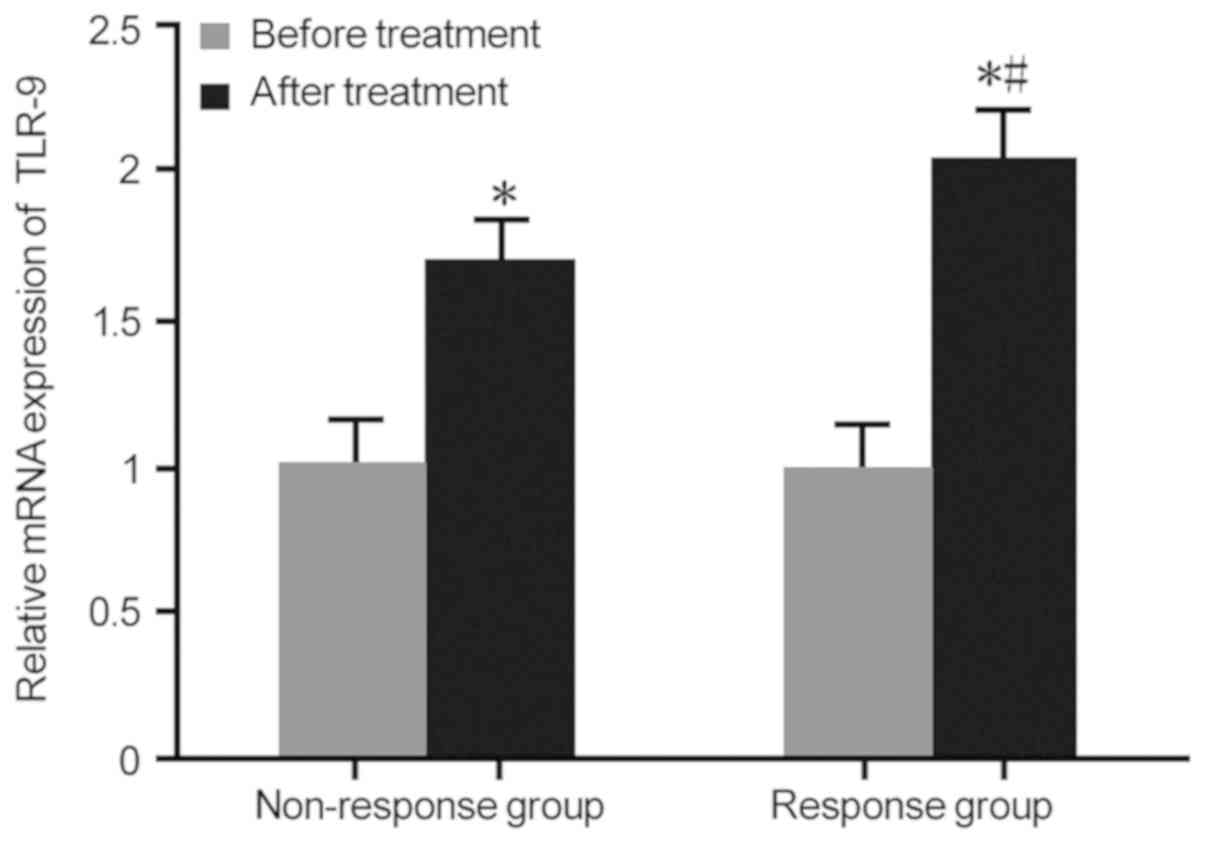
|
1
|
Tang X, Yan L, Li H, Du L, Shi Y, Huang F
and Tang H: Increased expression of phosphoenolpyruvate
carboxykinase cytoplasmic isoform by hepatitis B virus X protein
affects hepatitis B virus replication. J Med Virol. 91:258–264.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Yambasu EE, Reid A, Owiti P, Manzi M,
Murray MJS and Edwin AK: Hidden dangers-prevalence of blood borne
pathogens, hepatitis B, C, HIV and syphilis, among blood donors in
Sierra Leone in 2016: Opportunities for improvement: A
retrospective, cross-sectional study. Pan Afr Med J. 30:442018.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Herrero-Fernández I, Rosado-Sánchez I,
Genebat M, Tarancón-Díez L, Rodríguez-Méndez MM, Pozo-Balado MM,
Lozano C, Ruiz-Mateos E, Leal M and Pacheco YM: Improved CD4 T cell
profile in HIV-infected subjects on maraviroc-containing therapy is
associated with better responsiveness to HBV vaccination. J Transl
Med. 16:2382018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chen M, Du D, Zheng W, Liao M, Zhang L,
Liang G and Gong M: Small hepatitis delta antigen selectively binds
to target mRNA in hepatic cells: A potential mechanism by which
hepatitis D virus down-regulates glutathione S-transferase P1 and
induces liver injury and hepatocarcinogenesis. Biochem Cell Biol.
97:130–139. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Howell J, Pedrana A, Cowie BC, Doyle J,
Getahun A, Ward J, Gane E, Cunningham C, Wallace J, Lee A, et al:
Aiming for the elimination of viral hepatitis in Australia, New
Zealand, the Pacific Islands and Territories: Where are we now and
barriers to meeting WHO targets by 2030. J Gastroenterol Hepatol.
34:40–48. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Li MR, Zheng HW, Ma SM, Liu YY, Qie LX, Li
JQ, Wang DH, Sun XL, Ren GF, Zheng YH, et al: Correlations between
serum hepatitis B surface antigen and hepatitis B core antibody
titers and liver fibrosis in treatment-naive CHB patients. J Chin
Med Assoc. 81:1052–1059. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yang J, Yang G, He H, Ning L, Liu Z, Fu Q,
Chen H, Deng H, Wang Z and Luo K: Association of characteristics of
HBV quasispecies with hepatitis B surface antigen seroconversion
after pegylated interferon-α-2a treatment in child patients.
Antivir Ther. 23:567–574. 2018. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lutterkort GL, Wranke A, Hengst J,
Yurdaydin C, Stift J, Bremer B, Hardtke S, Keskin O, Idilman R,
Manns MP, et al: Viral dominance patterns in chronic hepatitis
delta determine early response to interferon alpha therapy. J Viral
Hepat. 25:1384–1394. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Cao WH, Li MH, Pan CQ, Lu Y, Zhang L, Ran
CP, Wu SL, Hua WH, Liu SA, Shen G, et al: Quantitation of
plasmacytoid dendritic cells in chronic hepatitis B patients with
HBeAg positivity during PEG-IFN and entecavir therapy. J Interferon
Cytokine Res. 38:197–205. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Karamitros T, Papatheodoridis G,
Paraskevis D, Hatzakis A, Mbisa JL, Georgopoulou U, Klenerman P and
Magiorkinis G: Impact of interferon-α receptor-1 promoter
polymorphisms on the transcriptome of the hepatitis B
virus-associated hepatocellular carcinoma. Front Immunol.
9:7772018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sepulveda-Toepfer JA, Pichler J, Fink K,
Sevo M, Wildburger S, Mudde-Boer LC, Taus C and Mudde GC:
TLR9-mediated activation of dendritic cells by CD32 targeting for
the generation of highly immunostimulatory vaccines. Hum Vaccin
Immunother. 15:179–188. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tomasello E, Naciri K, Chelbi R, Bessou G,
Fries A, Gressier E, Abbas A, Pollet E, Pierre P, Lawrence T, et
al: Molecular dissection of plasmacytoid dendritic cell activation
in vivo during a viral infection. EMBO J. 37:e988362018. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wimmers F, Subedi N, van Buuringen N,
Heister D, Vivié J, Beeren-Reinieren I, Woestenenk R, Dolstra H,
Piruska A, Jacobs JF, et al: Single-cell analysis reveals that
stochasticity and paracrine signaling control interferon-alpha
production by plasmacytoid dendritic cells. Nat Commun. 9:33172018.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Frank MJ, Reagan PM, Bartlett NL, Gordon
LI, Friedberg JW, Czerwinski DK, Long SR, Hoppe RT, Janssen R,
Candia AF, et al: In situ vaccination with a TLR 9 agonist and
local low dose radiation induces systemic responses in untreated
indolent lymphoma. Cancer Discov. 8:1258–1269. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Atreya R, Reinisch W, Peyrin-Biroulet L,
Scaldaferri F, Admyre C, Knittel T, Kowalski J, Neurath MF and
Hawkey C: Clinical efficacy of the Toll-like receptor 9 agonist
cobitolimod using patient-reported-outcomes defined clinical
endpoints in patients with ulcerative colitis. Dig Liver Dis.
50:1019–1029. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Xu J, Lee JW, Park SK, Lee SB, Yoon YH,
Yeon SH, Rha KS, Choi JA, Song CH and Kim YM: Toll-like receptor 9
ligands increase type I interferon induced B-cell activating factor
expression in chronic rhinosinusitis with nasal polyposis. Clin
Immunol. 197:19–26. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Gibbert K, Schlaak JF, Yang D and Dittmer
U: IFN-α subtypes: Distinct biological activities in anti-viral
therapy. Br J Pharmacol. 168:1048–1058. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hou J, Wang G, Wang F, Cheng J, Ren H,
Zhuang H, Sun J, Li L, Li J, Meng Q, et al: Guideline of prevention
and treatment for chronic hepatitis B (2015 update). J Clin Transl
Hepatol. 5:297–318. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhu S, Zhang H, Dong Y, Wang L, Xu Z, Liu
W, Gan Y, Tang H, Chen D, Wang F and Zhao P: Antiviral therapy in
hepatitis B virus-infected children with immune-tolerant
characteristics: A pilot open-label randomized study. J Hepatol.
68:1123–1128. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Xi Y, Troy NM, Anderson D, Pena OM, Lynch
JP, Phipps S, Bosco A and Upham JW: Critical role of plasmacytoid
dendritic cells in regulating gene expression and innate immune
responses to human rhinovirus-16. Front Immunol. 8:13512017.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Cédile O, Jørgensen LØ, Frank I,
Wlodarczyk A and Owens T: The chemokine receptor CCR2 maintains
plasmacytoid dendritic cell homeostasis. Immunol Lett. 192:72–78.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ainola M, Porola P, Takakubo Y, Przybyla
B, Kouri VP, Tolvanen TA, Hänninen A and Nordström DC: Activation
of plasmacytoid dendritic cells by apoptotic particles-mechanism
for the loss of immunological tolerance in Sjögren's syndrome. Clin
Exp Immunol. 191:301–310. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ruben JM, García-Romo GS, Breman E, van
der Kooij S, Redeker A, Arens R and van Kooten C: Human
plasmacytoid dendritic cells acquire phagocytic capacity by TLR9
ligation in the presence of soluble factors produced by renal
epithelial cells. Kidney Int. 93:355–364. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Murayama G, Furusawa N, Chiba A, Yamaji K,
Tamura N and Miyake S: Enhanced IFN-α production is associated with
increased TLR7 retention in the lysosomes of palasmacytoid
dendritic cells in systemic lupus erythematosus. Arthritis Res
Ther. 19:2342017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Torigoe M, Sakata K, Ishii A, Iwata S,
Nakayamada S and Tanaka Y: Hydroxychloroquine efficiently
suppresses inflammatory responses of human class-switched memory B
cells via Toll-like receptor 9 inhibition. Clin Immunol. 195:1–7.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Han N, Zhang Z, Jv H, Hu J, Ruan M and
Zhang C: Culture supernatants of oral cancer cells induce impaired
IFN-α production of pDCs partly through the down-regulation of
TLR-9 expression. Arch Oral Biol. 93:141–148. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
White MP, Webster G, Leonard F and La
Flamme AC: Innate IFN-γ ameliorates experimental autoimmune
encephalomyelitis and promotes myeloid expansion and PDL-1
expression. Sci Rep. 8:2592018. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Reizis B, Bunin A, Ghosh HS, Lewis KL and
Sisirak V: Plasmacytoid dendritic cells: Recent progress and open
questions. Annu Rev Immunol. 29:163–83. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lebensztejn DM, Sobaniec-Lotowska ME,
Kaczmarski M, Voelker M and Schuppan D: Matrix-derived serum
markers in monitoring liver fibrosis in children with chronic
hepatitis B treated with interferon alpha. World J Gastroenterol.
12:3338–3343. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lebensztejn DM, Sobaniec-Łotowska ME,
Bauer M, Kaczmarski M, Voelker M and Schuppan D: Serum fibrosis
markers as predictors of an antifibrotic effect of interferon alfa
in children with chronic hepatitis B. Eur J Gastroenterol Hepatol.
17:843–848. 2005. View Article : Google Scholar : PubMed/NCBI
|