Introduction
Breast cancer is a common gynecologic cancer
worldwide (1). Low expression of
human epidermal growth factor receptor 2 (HER2), progesterone
receptor (PR) and estrogen receptor (ER) are the main
characteristics of TNBC (2). TNBC
accounts for 10–15% of breast carcinomas, which constitute ~80% of
all ‘basal-like tumors’ (3). There
are many risk factors for TNBC, including the lack of
breastfeeding, high parity, high body mass index and young age at
menarche (4). The majority of tumor
exhibiting a BRCA1-mutation belong to the TNBC subtype
(5). Compared with other subtypes of
breast cancer, TNBC has a poor prognosis and tends to recur more
frequently (6). Although a previous
study showed that TNBC is sensitive to chemotherapy, sensitive
patients only represent a minority of all patients with TNBC
(7). Although the survival rate of
breast cancer has increased significantly in recent years, there is
still no effective treatment for TNBC (8). Currently, breast cancer treatments
target ER, PR or HER2, and it is therefore essential to identify
novel biomarkers that may predict tumor progression and that can be
used as potential therapeutic targets (9–12).
MicroRNAs (miRNAs) are a class of small non-coding
RNAs that can regulate gene expression by triggering translation
repression or RNA degradation of the target mRNAs (13). Dysregulation of miRNAs or the
expression of mutant miRNAs in human diseases including cancer,
suggest that miRNAs may act as oncogenes or tumor suppressors
(14–18). Among the differentially expressed
miRNAs, miRNA (miR)-155-5p, miR-21-3p, miR-181a-5p, miR-181b-5p and
miR-183-5p are significantly upregulated in TNBC, whereas
miR-10b-5p, miR-451a, miR-125b-5p, miR-31-5p, miR-195-5p and
miR-130a-3p are downregulated (7).
In addition, miRNAs that act as metastasis suppressors in breast
cancer include miR-17/20, miR-22, miR-30, miR-31, miR-126, miR-145,
miR-146, miR-205, miR-206 and let-7 (19). However, the mechanism underlying
miR-31-5p function in TNBC remains unclear. miR-31 is involved in
many biological processes, including bone formation (20), embryonic development (21) and myogenesis (22). In addition, miR-31 has been reported
to promote spermatogenesis and to facilitate embryonic implantation
(23,24). Dysregulation of miR-31 has also been
found in various human diseases, including cancer and autoimmune
diseases (21). Repression of miR-31
was identified in breast cancer, suggesting that miR-31 may serve
as a tumor suppressor (25). miR-31
inhibition has been identified in leukemia patients, and it was
shown that miR-31 can inhibit NF-κB signaling by suppressing
mitogen-activated protein kinase kinase kinase 14 (26). Similarly, patients with
hepatocellular carcinoma exhibit downregulated levels of miR-31
(27). By contrast, miR-31 was also
found to be upregulated in certain types of cancer; in colorectal
cancer, miR-31 acts as an oncogenic miRNA (28,29). In
lung cancer, miR-31 directly regulates tumor-suppressing genes,
such as protein phosphatase 2 regulatory subunit B α, large tumor
suppressor kinase 2 and BRCA1 associated protein 1 (30,31).
Although previous studies have revealed important
roles for miR-31 in different cancer types (20–22,32), the
role of miR-31 in TNBC remains unclear. The aim of the present
study was to investigate the role of miR-31 in TNBC by
overexpressing or silencing miR-31 in TNBC cell lines.
Materials and methods
Cell line and cell culture
The human TNBC cell line MDA-MB-231 was obtained
from The Cell Bank of the Chinese Academy of Sciences and cultured
in DMEM (HyClone; GE Healthcare Life Sciences) containing 10% FBS,
100 U/ml penicillin and 100 µg/ml streptomycin (all Gibco; Thermo
Fisher Scientific, Inc.). Cells were incubated at 37°C in a
humidified incubator with 5% CO2.
Cells were transfected with miR-31-5p mimics or
miR-31-5p inhibitors for 48 h at 37°C, and then treated with 50 µM
Taxol (TAX; Aladdin Biochemical Technology, Co., Ltd.) for 24 h, 50
µM cisplatin (DDP; Sigma-Aldrich; Merck KGaA) for 24 h or 20 µM
LY294002 (Selleck Chemicals), an antagonist of the AKT pathway, for
48 h at 37°C, respectively. Following treatments, cells were used
for the following experiments.
miR-31-5p mimic and inhibitor
The miR-31-5p mimic and miR-31-5p inhibitor were
used to overexpress or repress the expression level of miR-31-5p.
The hsa-miR-31-5p mimic (final concentration, 5 nM), inhibitor
(final concentration, 50 nM) and negative controls (NCs; final
concentration, 5 nM) were synthesized by GenePharma. The sequences
of the miR-31-5p mimic, inhibitor and NC are presented in Table I. Cells were plated in six-well
plates and transfected with the mimic, inhibitor and NC using
Lipofectamine 2000 (Invitrogen; Thermo Fisher Scientific, Inc.)
according to the manufacturer's protocol. Cells were harvested for
further experimentation after 48 h.
 | Table I.Primer, mimic and inhibitor
sequences. |
Table I.
Primer, mimic and inhibitor
sequences.
|
Oligonucleotide | Sequences
(5′-3′) |
|---|
| miR-31-5p
Primers | F:
ACACTCCAGCTGGGAGGCAAGATGCTGGC |
|
| R:
TGGTGTCGTGGAGTCG |
| U6 Primers | F: CTCGCTT
CGGCAGCACA |
|
| R:
AACGCTTCACGAATTTGCGT |
| miR-31-5p
mimic |
AGGCAAGAUGCUGGCAUAGCU |
| miR-31-5p mimic
NC |
UUGUACUACACAAAAGUACUG |
| miR-31-5p
inhibitor |
AGCUAUGCCAGCAUCUUGCCU |
| miR-31-5p inhibitor
NC |
CAGUACUUUUGUGUAGUACAA |
Reverse transcription-quantitative PCR
(RT-qPCR)
Treated cells were rinsed twice with PBS, and RNA
was extracted using TRIzol Reagent (Invitrogen; Thermo Fisher
Scientific, Inc.) according to the manufacturer's protocol. cDNA
was synthesized using a Moloney murine leukemia virus reverse
transcriptase kit (Promega Corporation) on an ABI 7300 system
(Applied Biosystems; Thermo Fisher Scientific, Inc.) using the
following protocol: 37°C for 60 min, 85°C for 5 min and 4°C for 5
min. RT-qPCR was performed using a SYBR Green qPCR kit (Thermo
Fisher Scientific, Inc.) and an ABI 7300 system using the following
protocol: 95°C for 10 min, 40 cycles of 95°C for 15 sec and 60°C
for 45 sec, 95°C for 15 sec, 60°C for 1 min, and 4°C for 10 min.
Gene expression levels were normalized to the expression level of
U6 and calculated using the 2−∆∆Cq method (33). The sequences of the primers used for
the RT-qPCR analysis are presented in Table I.
Cell proliferation
A Cell Counting Kit-8 assay (CCK-8; Beyotime
Institute of Biotechnology) was used to examine cell proliferation.
Treated cells were plated into 96-well plate (2,000 cells/well).
After 24, 48 and 72 h of all treatments, the medium was replaced
with DMEM containing 10% CCK-8 solution and the cells were
incubated for 1 h at 37°C. Following incubation, the absorbance was
detected using an automatic microplate reader (Bio-Rad
Laboratories, Inc.) at a wavelength of 450 nm. Experiments were
performed in triplicate.
Cell apoptosis
An Annexin V/propidium iodide (PI) apoptosis
detection kit (BD Biosciences) was used to measure the cell
apoptosis according to the manufacturer's protocol. Cells were
plated into six-well plates and then transfected and/or treated
with various drugs. Cells were rinsed twice with cold PBS and
incubated in the staining buffer, which was included in the kit.
Then, the cells were stained with Annexin V and PI for 15 min at
37°C in the dark and then evaluated by flow cytometry (Accuri C6;
BD Biosciences). Experiments were performed in triplicate.
Western blot analysis
Whole protein was extracted from MDA-MB-231 cells
after two washes with cold PBS using RIPA lysis buffer (Beyotime
Institute of Biotechnology). Protein concentration was measured by
BCA kit (Bio-Rad Laboratories, Inc.). A total of 20 µg protein from
each group was and then separated by SDS-PAGE on 10% gels, and
after separation the proteins were transferred to nitrocellulose
membranes (EMD Millipore). After blocking with PBST containing 5%
BSA (Beyotime Institute of Biotechnology), the blots were incubated
with the appropriate primary antibodies at 4°C overnight and
horseradish peroxidase-conjugated secondary antibodies (cat. no.
A0216; 1:1,000 dilution; Beyotime Institute of Biotechnology) at
room temperature for 1 h. The primary antibodies used (dilution,
1:1,000) were the following: Anti-P-glycoprotein (P-gp; cat. no.
ab103477), anti-Bcl-2 (cat. no. ab32124), and anti-Bax (cat. no.
ab32503; all Abcam), anti-AKT (cat. no. 4685), anti-phosphorylated
(p)-AKT (cat. no. 4060) and anti-GAPDH (cat. no. 5174; all Cell
Signaling Technologies, Inc.). Signals were detected using an ECL
kit (EMD Millipore). Bands were analyzed with ImageJ 5.0 (National
Institutes of Health) and GAPDH was used as the loading
control.
Statistics
All the experiments were performed ≥3 times. Data
are presented as the mean ± SD. The statistical differences between
the control and treatment groups were determined using ANOVA
followed by a Student-Newman-Keuls test. Data were analyzed using
GraphPad Prism 6.0 (GraphPad Software, Inc.). P<0.05 was
considered to indicate a statistically significant difference.
Results
Overexpression of miR-31 promotes
apoptosis and inhibits cell proliferation in TNBC cells
To investigate the role of miR-31-5p in TNBC,
MDA-MB-231 and MDA-MB-468 cell lines were selected to examine the
effects of altering the expression level of miR-31-5p. The
miR-31-5p mimic and miR-31-5p inhibitor were transfected into
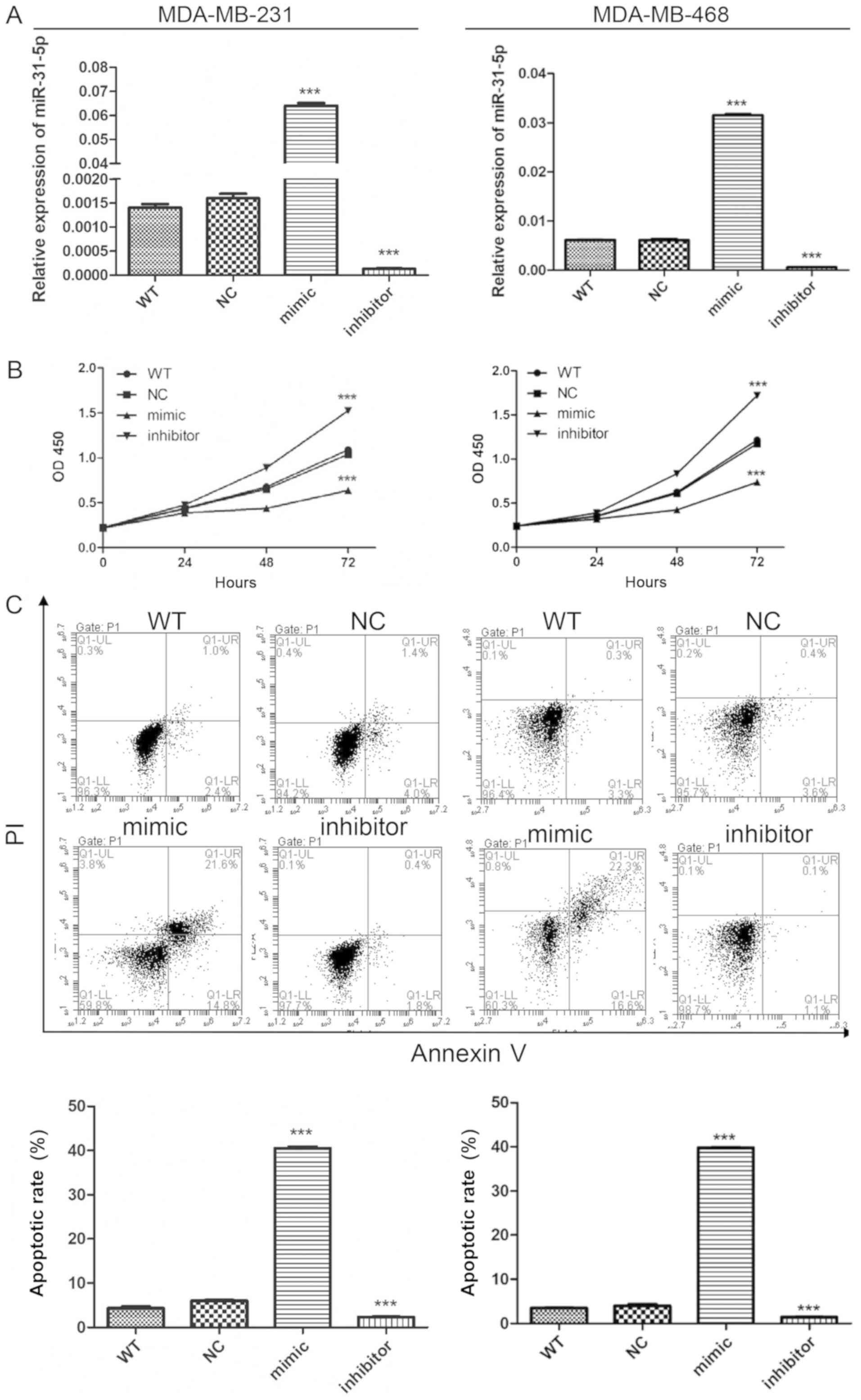
MDA-MB-231 and MDA-MB-468 cells. RT-qPCR was used to detect the
efficiency of miR-31-5p overexpression and inhibition. miR-31-5p
was dramatically increased and reduced after miR-31-5p mimic and
inhibitor transfection, respectively (Fig. 1A). Following the determination of the
transfection efficiencies, the effects of miR-31-5p on cell
proliferation were examined. Cell Counting Kit-8 assay was
performed to measure cell proliferation and cytotoxicity. Cells
transfected with miR-31-5p mimic proliferated more slowly than
cells transfected with miR-NC (Fig.
1B). The miR-31-5p inhibitor transfection exhibited opposite
effects, as this transfection promoted cell proliferation in
MDA-MB-231 and MDA-MB-468 cells. To explore the mechanism
underlying miR-31-p function, cells were stained with Annexin-V,
and flow cytometry was performed to detect the effects of miR-31 on
cell apoptosis. An increased number of PI and Annexin-V double
positive cells were found in the group transfected with miR-31-5p
mimic, suggesting an increase in cell apoptosis. miR-31-5p
inhibitor reduced apoptosis in MDA-MB-231 and MDA-MB-468 cells, in
line with the aforementioned results (Fig. 1C). Collectively, the present data
suggested that overexpression of miR-31-5p promoted apoptosis and
inhibited cell proliferation in MDA-MB-231 and MDA-MB-468 cells. By
contrast, inhibition of miR-31-5p expression exhibited the opposite
effects on cell proliferation and apoptosis.
miR-31 overexpression increases the
sensitivity of TNBC to TAX and DDP treatment
TNBC is an aggressive cancer with a high risk of
relapse within the first 3–5 years after the completion of adjuvant
chemotherapy treatments (34). The
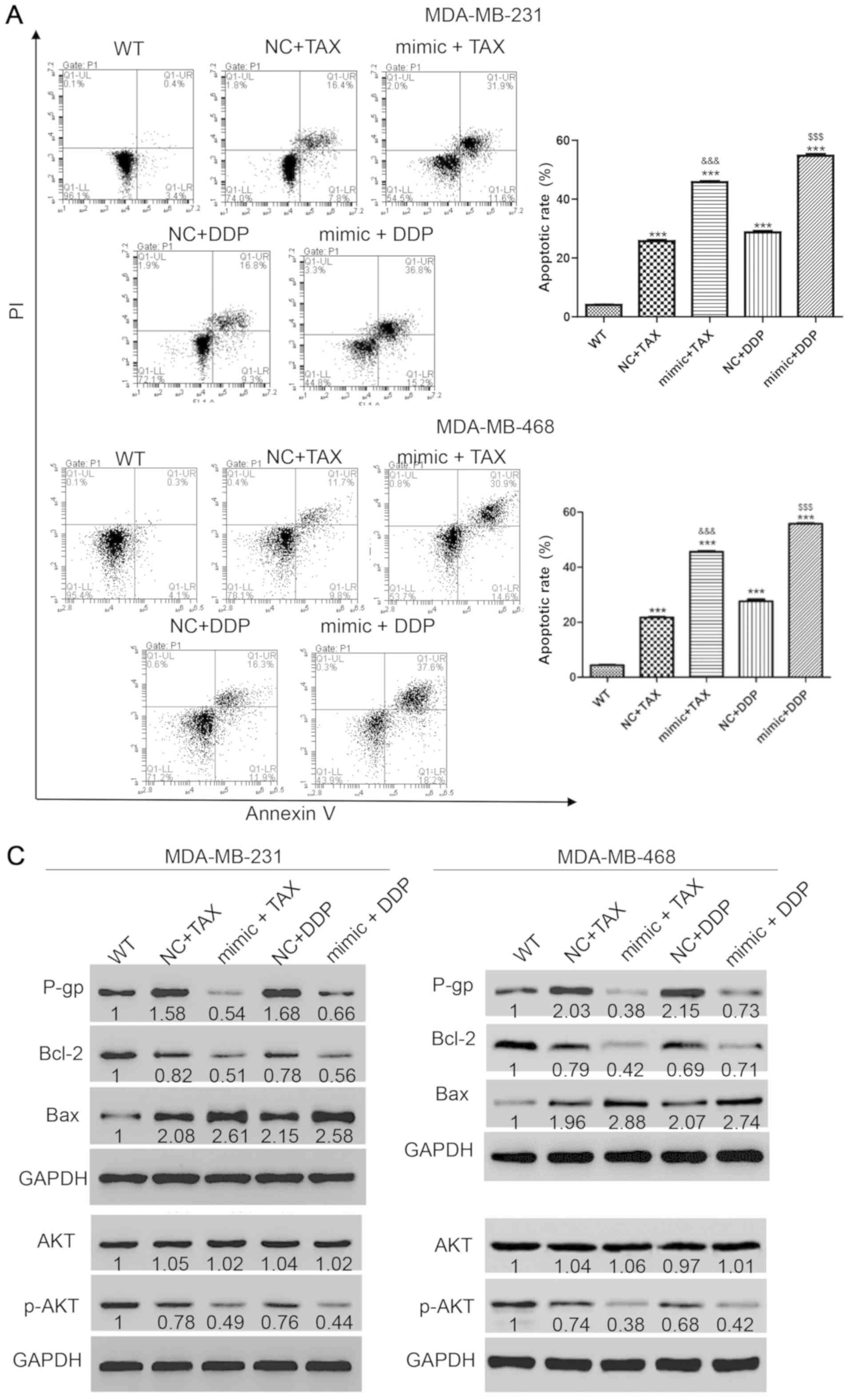
potential role of miR-31-5p in chemotherapy resistance was then
investigated. First, the appropriate concentration of each drug was
examined. Treatment with TAX and DDP at 50 µmol/l induced cell
apoptosis but did not induce cell death in a large portion of the
cell population. After transfection with miR-31-5p mimic for 48 h,
50 µmol/l TAX or DDP were used to treat cells for 48 h. Then, the
harvested cells were stained with PI and annexin-V and flow
cytometry was performed to detect cell apoptosis. Single treatment
with TAX or DDP increased apoptosis, but the effects were increased
when combined with miR-31-5p mimic transfection (apoptotic rates:
TAX, 25.7±0.61%; TAX + mimic, 45.8±0.52%; DDP, 28.74±0.74%; and DDP
+ mimic, 54.8±0.87%) (Fig. 2A).
Then, the underlying mechanisms of miR-31-5p-mediated
chemoresistance were investigated. Following various treatments,
cells were harvested and western blotting was performed to detect
the expression levels of apoptosis-related proteins, including an
apoptosis suppressor (Bcl-2) (35)
and a positive regulator of apoptosis (Bax) (36). The protein expression level of P-gp,
which is negatively associated with chemotherapy sensitivity
(37), was also detected. As
presented in Fig. 2B, P-gp
expression increased with single treatment with TAX or DDP, but
significantly decreased following drug treatments combined with
miR-31 mimic transfection. Bcl-2 was decreased after treatment with
TAX or DDP and the effects were more significant in cells
transfected with miR-31 mimic. Consistently, monotherapy with TAX
or DDP significantly increased the protein expression levels of
Bax, and the increase was greater following miR-31 mimics
transfection. However, the levels of p-AKT, a tumor proliferation
marker (38), showed opposite trends
compared with Bax, suggesting decreased proliferation and increased
apoptosis following miR-31 mimics transfection (Fig. 2C). Collectively, the synergistic
effect of miR-31-5p and TAX or DDP promoted apoptosis in TNBC
cells.
Inhibition of miR-31-5p decreases the
sensitivity of TNBC to TAX and DDP treatment
To further validate the present findings, cells were
transfected with miR-31-5p inhibitor, and flow cytometry and
western blotting were performed. The inhibition of miR-31-5p
slightly reduced the apoptotic rates induced by single treatment
with TAX or DDP (apoptosis rates: TAX, 23.8±0.46%; TAX + inhibitor,
16.2±0.46%; DDP, 28.37±1.12%; and DDP + inhibitor, 16.63±0.4%)
(Fig. 3A). To study the mechanism of
miR-31-5p, markers of apoptosis and proliferation were detected by
western blotting. Both P-gp and Bcl-2 were significantly increased
after combined chemotherapy and transfection of miR-31-5p inhibitor
(Fig. 3B). Consistently, treatment
with TAX or DDP and transfection of the inhibitor dramatically
decreased the levels of Bax. However, the amplification marker
p-AKT showed contrasting trends with Bax, suggesting increased
proliferation but decreased apoptosis after transfection of
miR-31-5p inhibitor (Fig. 3B).
Collectively, the present results suggested that inhibition of
miR-31-5p reduced the apoptotic rates induced by TAX and DDP,
indicating a decreased sensitivity to chemotherapy in TNBC.
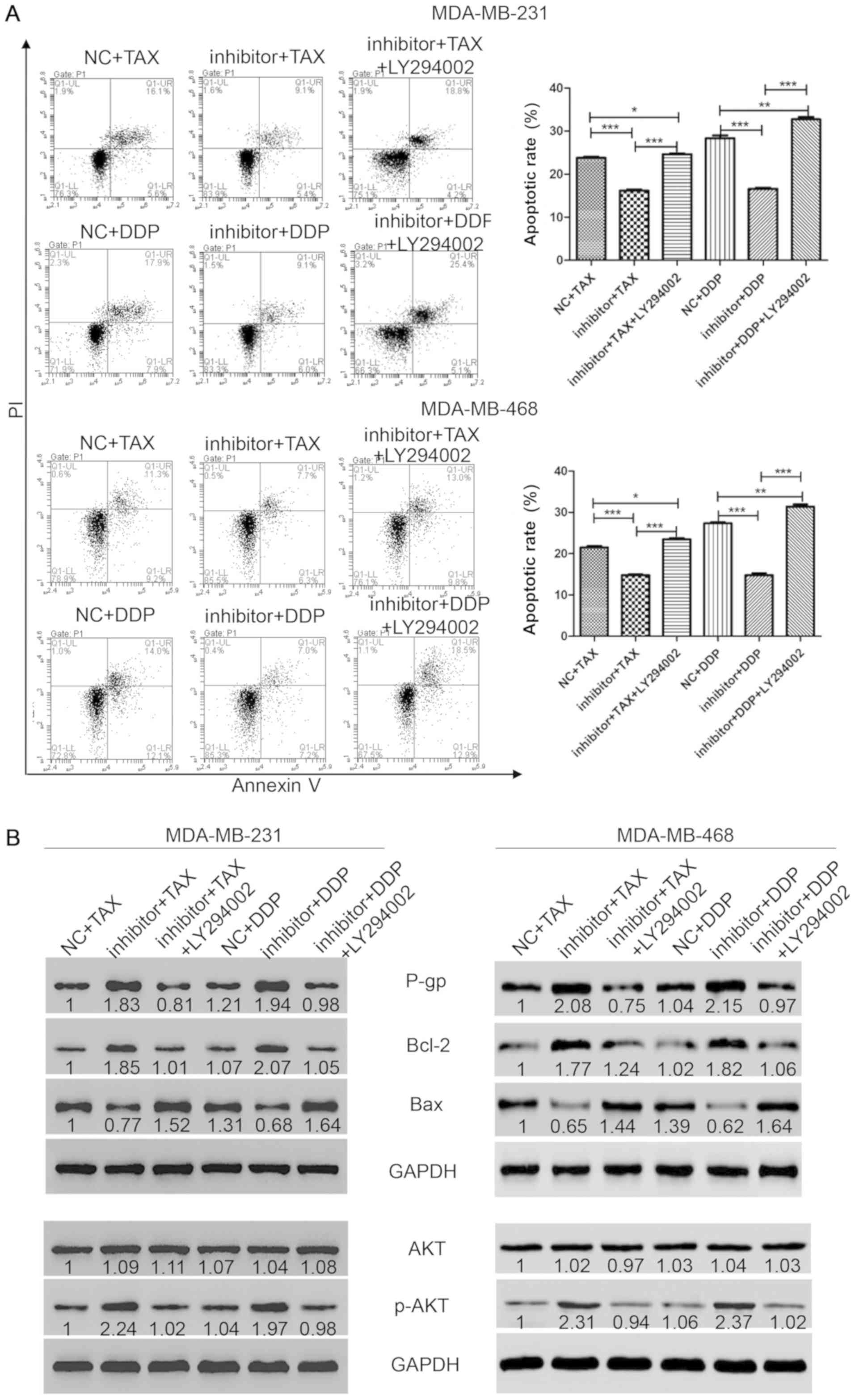 | Figure 3.Inhibition of AKT pathway
reestablishes the sensitivity to chemotherapy in TNBC cells. (A)
Flow cytometry was performed to measure cell apoptosis after
staining with annexin-V and PI. (B) Western blotting was performed
to detect the protein levels of P-gp, Bcl-2, Bax, AKT and p-AKT.
*P<0.05, **P<0.01, ***P<0.001. NC, negative control; TAX,
Taxol; DCC, cisplatin; miR, microRNA; p-, phosphorylated; P-gp,
P-glycoprotein; PI, propidium iodide. |
Suppression of the AKT pathway
restores sensitivity to chemotherapy in TNBC
The present results suggested that treatment with
TAX or DDP with or without the transfection of miR-31-5p mimic or
inhibitor altered the activity of the AKT pathway. To further
investigate the role of the AKT pathway in chemotherapy resistance
of TNBC, the AKT inhibitor LY294002 was used to block the AKT
pathway, and the effects on chemotherapy resistance in TNBC cells
were investigated. First, cells were transfected with miR-31-5p
inhibitor for 48 h and treated with TAX or DDP combined with AKT
inhibitor LY294002 (20 µM) for 48 h. Then, flow cytometry was
performed to detect cell viability. As shown in Fig. 3A, cells treated with a monotherapy of
TAX or DDP showed a slight increase of cell apoptosis, as indicated
by the number of cells positive for both PI and Annexin-V
(apoptotic rates: TAX, 23.8±0.46%; DDP, 28.37±1.12%). However, the
apoptosis induced by chemotherapy could be attenuated by the
inhibition of miR-31-5p following transfection of miR-31-5p
inhibitor (apoptotic rates: TAX + inhibitor, 16.2±0.46%; DDP +
inhibitor, 16.63±0.4%). Interestingly, LY294002 treatment could
restore sensitivity to TAX or DDP (apoptotic rates: TAX + inhibitor
+ LY294002, 24.67±0.32%; DDP + inhibitor + LY294002, 32.73±1%). To
investigate the mechanism underlying miR-31-5p function, western
blotting was performed to detect the protein expression level of
apoptosis and proliferation markers. Treatment with TAX or DDP in
cells transfected with miR-31-5p inhibitor induced an increase in
P-gp expression. Similar results were found for Bcl-2. However, Bax
levels were reduced after treatment with TAX or DDP in cells
transfected with miR-31-5p inhibitor. Interestingly, cells treated
with LY294002 showed increased levels of Bax compared with
monotherapy of TAX or DDP, indicating higher levels of apoptosis
were found in the LY294002 group (Fig.
3B). In addition, AKT and p-AKT levels were also investigated.
As shown in Fig. 3B, the level of
AKT did not change among groups. However, p-AKT levels increased
significantly compared with single treatments (NC + TAX or NC +
DDP). Consistently, after treatment with LY294002, the expression
level of p-AKT was significantly reduced to the expression level in
the control group. Collectively, the present results suggested that
the apoptosis induced by monotherapy with TAX or DDP could be
attenuated by transfection with miR-31-5p inhibitor, and this
effect was reversed by treatment with LY294002, an AKT
inhibitor.
Discussion
As an essential chemotherapeutic agent, TAX, also
known as paclitaxel, has been used to treat various types of
tumors, including non-small cell lung cancer, prostate cancer and
ovarian cancer (39–41). However, its clinical efficacy is
limited as the chemoresistance of primary tumor cells during the
course of treatment was found in a large proportion of patients
(42,43). For the treatment of a diverse group
of malignancies, including breast, prostate, ovarian, testicular,
cervical, bladder, lung cancer and refractory non-Hodgkin's
lymphomas, DDP is a widely used chemotherapeutic agent (44). However, resistance to TAX and DDP is
found in the late stage of breast cancer and in particular in
TNBC.
It has been reported that AKT, a serine/threonine
kinase, serves a significant role in cell survival following
exposure to apoptotic stimuli (45,46). In
breast cancer, constitutive activation of the PI3K/AKT pathway in
combination with the upregulation of HER2 and/or loss of PTEN
suppressor gene, confers resistance to endocrine therapy including
tamoxifen, and EGFR- and HER2-targeted therapies (47,48). In
the present study, cell proliferation was found to be suppressed
following overexpression of miR-31-5p, and was increased following
miR-31-5p inhibitor transfection. Consistently, cell apoptosis was
promoted by overexpression of miR-31-5p but suppressed by miR-31-5p
inhibition. The present results are in line with prior observations
regarding miR-31-5p, and suggested that miR-31-5p may act as a
tumor suppressor in breast cancer and in particular in TNBC. TAX
and DDP showed no significant inhibitory effects on TNBC cell
proliferation. Cells treated with mimic + TAX or mimic + DDP showed
higher apoptotic rate than cells treated with only TAX or DDP.
The present results indicated that overexpression of
miR-31-5p restored sensitivity to both drugs. Consistently,
inhibition of miR-31-5p blocked the effects of the drugs by
reducing cell apoptosis. Interestingly, the reduction in the
sensitivity to TAX and DDP induced by miR-31-5p inhibition could be
restored by inhibiting AKT using LY294002. The PI3K/AKT/mTOR
pathway is frequently activated in breast cancer, and PIK3
catalytic subunit α was found to be the most commonly mutated gene
in ER-positive breast cancer (9). As
previously reported, the activation of this pathway has been found
to be associated with the resistance to therapies targeting HER2,
and endocrine and cytotoxic therapy in breast cancer (48).
As previously reported, miR-31 induces cell
apoptosis by downregulating the expression level of Bcl-2 by
directly targeting protein kinase C ε (PKCε) in breast cancer cells
(32). PKCε may serve a role in the
increased rates of cell apoptosis induced by miR-31-5p and TAX or
DDP treatment in TNBC. In addition, PKCε may be involved in the
molecular mechanism underlying miR-31-5p-mediated apoptosis
regulation.
The present findings suggested that the AKT pathway
may be involved in the acquisition of chemotherapy resistance.
Therefore, combination of miR-31-5p and AKT inhibitors may enhance
efficacy of chemotherapy in breast cancer, and, in particular, in
TNBC. However, in order to confirm the present results, mechanistic
and clinical studies are required to identify the exact pathways
involved in the increased chemosensitivity caused by miR-31-5p. In
addition, xenograft models and genetically engineered mouse model
may be used in the future to investigate the role of miR-31-5p
in vivo. However, the present findings suggest that it is
necessary to examine the role and the underlying mechanisms of
miR-31-5p in TNBC, and the present results may facilitate the
development of using miR-31-5p to regulate treatments in order to
enhance therapeutic efficacy in the future.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
LD conceived and designed the present study. XS
performed cell culture experiments and wrote the manuscript. JL
performed western blot and RT-qPCR analyses, and analyzed the
data.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Fragomeni SM, Sciallis A and Jeruss JS:
Molecular subtypes and local-regional control of breast cancer.
Surg Oncol Clin N Am. 27:95–120. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Camorani S, Fedele M, Zannetti A and
Cerchia L: TNBC challenge: Oligonucleotide aptamers for new imaging
and therapy modalities. Pharmaceuticals (Basel). 11(pii): E1232018.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Dent R, Trudeau M, Pritchard KI, Hanna WM,
Kahn HK, Sawka CA, Lickley LA, Rawlinson E, Sun P and Narod SA:
Triple-negative breast cancer: Clinical features and patterns of
recurrence. Clin Cancer Res. 13:4429–4434. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
John EM, Hines LM, Phipps AI, Koo J,
Longacre TA, Ingles SA, Baumgartner KB, Slattery ML and Wu AH:
Reproductive history, breast-feeding and risk of triple negative
breast cancer: The Breast Cancer Etiology in Minorities (BEM)
study. Int J Cancer. 142:2273–2285. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Domagala P, Huzarski T, Lubinski J, Gugala
K and Domagala W: Immunophenotypic predictive profiling of
BRCA1-associated breast cancer. Virchows Arc. 458:55–64. 2011.
View Article : Google Scholar
|
|
6
|
Sameni M, Tovar EA, Essenburg CJ,
Chalasani A, Linklater ES, Borgman A, Cherba DM, Anbalagan A, Winn
ME, Graveel CR and Sloane BF: Cabozantinib (XL184) inhibits growth
and invasion of preclinical TNBC models. Clin Cancer Res.
22:923–934. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ouyang M, Li Y, Ye S, Ma J, Lu L, Lv W,
Chang G, Li X, Li Q, Wang S and Wang W: MicroRNA profiling implies
new markers of chemoresistance of triple-negative breast cancer.
PLoS One. 9:e962282014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
O'Shaughnessy J, Osborne C, Pippen JE,
Yoffe M, Patt D, Rocha C, Koo IC, Sherman BM and Bradley C:
Iniparib plus chemotherapy in metastatic triple-negative breast
cancer. N Engl J Med. 364:205–214. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ellis MJ and Perou CM: The genomic
landscape of breast cancer as a therapeutic roadmap. Cancer Discov.
3:27–34. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Altundag K: Is there a role of breast
pathologist in diagnostic challenges of discordances in ER, PR, and
HER2 between primary breast cancer and brain metastasis? J
Neurooncol. 138:2192018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Dackus GMHE, Jozwiak K, Sonke GS, van der
Wall E, van Diest PJ, Hauptmann M, Siesling S and Linn SC: Optimal
adjuvant endocrine treatment of ER+/HER2+ breast cancer patients by
age at diagnosis: A population-based cohort study. Eur J Cancer.
90:92–101. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Jung J, Lee SH, Park M, Youn JH, Shin SH,
Gwak HS and Yoo H: Discordances in ER, PR, and HER2 between primary
breast cancer and brain metastasis. J Neurooncol. 137:295–302.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Di Leva G, Calin GA and Croce CM:
MicroRNAs: Fundamental facts and involvement in human diseases.
Birth Defects Res C Embryo Today. 78:180–189. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Calin GA, Dumitru CD, Shimizu M, Bichi R,
Zupo S, Noch E, Aldler H, Rattan S, Keating M, Rai K, et al:
Frequent deletions and down-regulation of micro-RNA genes miR15 and
miR16 at 13q14 in chronic lymphocytic leukemia. Proc Natl Acad Sci
USA. 99:15524–15529. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Michael MZ, SM OC, van Holst Pellekaan NG,
Young GP and James RJ: Reduced accumulation of specific microRNAs
in colorectal neoplasia. Mol Cancer Res. 1:882–891. 2003.PubMed/NCBI
|
|
16
|
Takamizawa J, Konishi H, Yanagisawa K,
Tomida S, Osada H, Endoh H, Harano T, Yatabe Y, Nagino M, Nimura Y,
et al: Reduced expression of the let-7 microRNAs in human lung
cancers in association with shortened postoperative survival.
Cancer Res. 64:3753–3756. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Metzler M, Wilda M, Busch K, Viehmann S
and Borkhardt A: High expression of precursor microRNA-155/BIC RNA
in children with Burkitt lymphoma. Genes Chromosomes Cancer.
39:167–169. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Eis PS, Tam W, Sun L, Chadburn A, Li Z,
Gomez MF, Lund E and Dahlberg JE: Accumulation of miR-155 and BIC
RNA in human B cell lymphomas. Proc Natl Acad Sci USA.
102:3627–3632. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Singh R and Mo YY: Role of microRNAs in
breast cancer. Cancer Biol Ther. 14:201–212. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Deng Y, Bi X, Zhou H, You Z, Wang Y, Gu P
and Fan X: Repair of critical-sized bone defects with
anti-miR-31-expressing bone marrow stromal stem cells and
poly(glycerol sebacate) scaffolds. Eur Cell Mater. 27:13–25. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Stepicheva NA and Song JL: Function and
regulation of microRNA-31 in development and disease. Mol Reprod
Dev. 83:654–674. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Huang S, Chen Z, Wu W, Wang M, Wang R, Cui
J, Li W and Wang S: MicroRNA-31 promotes arterial smooth muscle
cell proliferation and migration by targeting mitofusin-2 in
arteriosclerosis obliterans of the lower extremitie. Exp Ther Med.
15:633–640. 2018.PubMed/NCBI
|
|
23
|
Munoz X, Mata A, Bassas L and Larriba S:
Altered miRNA signature of developing germ-cells in infertile
patients relates to the severity of spermatogenic failure and
persists in spermatozoa. Sci Rep. 5:179912015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kresowik JD, Devor EJ, Van Voorhis BJ and
Leslie KK: MicroRNA-31 is significantly elevated in both human
endometrium and serum during the window of implantation: A
potential biomarker for optimum receptivity. Biol Reprod.
91:172014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Augoff K, Das M, Bialkowska K, McCue B,
Plow EF and Sossey-Alaoui K: miR-31 is a broad regulator of
β1-integrin expression and function in cancer cells. Mol Cancer
Res. 9:1500–1508. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yamagishi M, Nakano K, Miyake A, Yamochi
T, Kagami Y, Tsutsumi A, Matsuda Y, Sato-Otsubo A, Muto S,
Utsunomiya A, et al: Polycomb-mediated loss of miR-31 activates
NIK-dependent NF-kappaB pathway in adult T cell leukemia and other
cancers. Cancer Cell. 21:121–135. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kim HS, Lee KS, Bae HJ, Eun JW, Shen Q,
Park SJ, Shin WC, Yang HD, Park M, Park WS, et al: MicroRNA-31
functions as a tumor suppressor by regulating cell cycle and
epithelial-mesenchymal transition regulatory proteins in liver
cancer. Oncotarget. 6:8089–8102. 2015.PubMed/NCBI
|
|
28
|
Cottonham CL, Kaneko S and Xu L: miR-21
and miR-31 converge on TIAM1 to regulate migration and invasion of
colon carcinoma cells. J Biol Chem. 285:35293–35302. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Xu RS, Wu XD, Zhang SQ, Li CF, Yang L, Li
DD, Zhang BG, Zhang Y, Jin JP and Zhang B: The tumor suppressor
gene RhoBTB1 is a novel target of miR-31 in human colon cancer. Int
J Oncol. 42:676–682. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Liu CJ, Tsai MM, Hung PS, Kao SY, Liu TY,
Wu KJ, Chiou SH, Lin SC and Chang KW: miR-31 ablates expression of
the HIF regulatory factor FIH to activate the HIF pathway in head
and neck carcinoma. Cancer Res. 70:1635–1644. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yu M, Liang H, Fu Z, Wang X, Liao Z, Zhou
Y, Liu Y, Wang Y, Hong Y, Zhou X, et al: BAP1 suppresses lung
cancer progression and is inhibited by miR-31. Oncotarget.
7:13742–13753. 2016.PubMed/NCBI
|
|
32
|
Korner C, Keklikoglou I, Bender C, Worner
A, Munstermann E and Wiemann S: MicroRNA-31 sensitizes human breast
cells to apoptosis by direct targeting of protein kinase C epsilon
(PKCepsilon). J Biol Chem. 288:8750–8761. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Park JH, Ahn JH and Kim SB: How shall we
treat early triple-negative breast cancer (TNBC): From the current
standard to upcoming immuno-molecular strategies. ESMO Open. 3
(Suppl 1):e0003572018. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Siddiqui WA, Ahad A and Ahsan H: The
mystery of BCL2 family: Bcl-2 proteins and apoptosis: An update.
Arch Toxicol. 89:289–317. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Lin Y, Kokontis J, Tang F, Godfrey B, Liao
S, Lin A, Chen Y and Xiang J: Androgen and its receptor promote
Bax-mediated apoptosis. Mol Cell Biol. 26:1908–1916. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Ge C, Cao B, Feng D, Zhou F, Zhang J, Yang
N, Feng S, Wang G and Aa J: The down-regulation of SLC7A11 enhances
ROS induced P-gp over-expression and drug resistance in MCF-7
breast cancer cells. Sci Rep. 7:37912017. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Paplomata E and O'Regan R: The
PI3K/AKT/mTOR pathway in breast cancer: Targets, trials and
biomarkers. Ther Adv Med Oncol. 6:154–166. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Henley D, Isbill M, Fernando R, Foster JS
and Wimalasena J: Paclitaxel induced apoptosis in breast cancer
cells requires cell cycle transit but not Cdc2 activity. Cancer
Chemother Pharmacol. 59:235–249. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Tan M and Yu D: Molecular mechanisms of
erbB2-mediated breast cancer chemoresistance. Adv Exp Med Biol.
608:119–129. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Orr GA, Verdier-Pinard P, McDaid H and
Horwitz SB: Mechanisms of Taxol resistance related to microtubules.
Oncogene. 22:7280–7295. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Frankel A, Buckman R and Kerbel RS:
Abrogation of taxol-induced G2-M arrest and apoptosis in human
ovarian cancer cells grown as multicellular tumor spheroids. Cancer
Res. 57:2388–2393. 1997.PubMed/NCBI
|
|
43
|
Yin S, Bhattacharya R and Cabral F: Human
mutations that confer paclitaxel resistance. Mol Cancer Ther.
9:327–335. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Tsimberidou AM, Braiteh F, Stewart DJ and
Kurzrock R: Ultimate fate of oncology drugs approved by the us food
and drug administration without a randomized Trial. J Clin Oncol.
27:6243–6250. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Song G, Ouyang G and Bao S: The activation
of Akt/PKB signaling pathway and cell survival. J Cell Mol Med.
9:59–71. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Wang YP, Huang LY, Sun WM, Zhang ZZ, Fang
JZ, Wei BF, Wu BH and Han ZG: Insulin receptor tyrosine kinase
substrate activates EGFR/ERK signalling pathway and promotes cell
proliferation of hepatocellular carcinoma. Cancer Lett. 337:96–106.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Tokunaga E, Kimura Y, Mashino K, Oki E,
Kataoka A, Ohno S, Morita M, Kakeji Y, Baba H and Maehara Y:
Activation of PI3K/Akt signaling and hormone resistance in breast
cancer. Breast Cancer. 13:137–144. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Clark AS, West K, Streicher S and Dennis
PA: Constitutive and inducible Akt activity promotes resistance to
chemotherapy, trastuzumab, or tamoxifen in breast cancer cells. Mol
Cancer Ther. 1:707–717. 2002.PubMed/NCBI
|